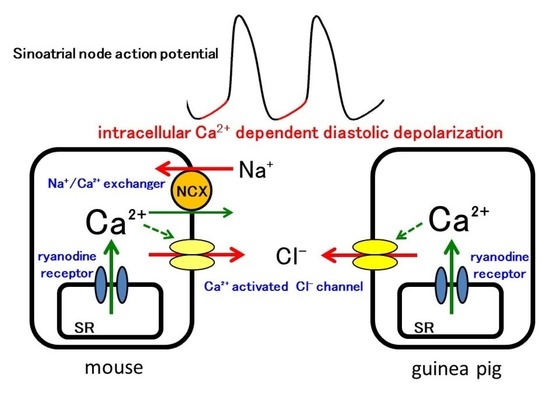
Intracellular Ca2+-Mediated Mechanisms for the Pacemaker Depolarization of the Mouse and Guinea Pig Sinus Node Tissue
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DiFrancesco, D. The role of the funny current in pacemaker activity. Circ. Res. 2010, 106, 434–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, H.; Komikado, C.; Namekata, I.; Nakamura, H.; Suzuki, M.; Tsuneoka, Y.; Shigenobu, K.; Takahara, A. Species difference in the contribution of T-type calcium current to cardiac pacemaking as revealed by R(−)-efonidipine. J. Pharmacol. Sci. 2008, 107, 99–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, H.; Namekata, I.; Ogawa, T.; Tsuneoka, Y.; Komikado, C.; Takahara, A.; Iida-Tanaka, N.; Izumi-Nakaseko, H.; Tsuru, H.; Adachi-Akahane, S. Effects of S(+)-efonidipine on the rabbit sinus node action potential and calcium channel subunits CaV 1.2, CaV 1.3 and CaV 3.1. Eur. J. Pharmacol. 2010, 649, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Himeno, Y.; Toyoda, F.; Satoh, H.; Amano, A.; Cha, C.Y.; Matsuura, H.; Noma, A. Minor contribution of cytosolic Ca2+ transients to the pacemaker rhythm in guinea pig sinoatrial node cells. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, 251–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toyoda, F.; Ding, W.G.; Matsuura, H. Heterogeneous functional expression of the sustained inward Na+ current in guinea pig sinoatrial node cells. Pflug. Arch. 2018, 470, 481–490. [Google Scholar] [CrossRef]
- Satoh, H. Sino-atrial nodal cells of mammalian hearts: Ionic currents and gene expression of pacemaker ionic channels. J. Smooth Muscle Res. 2003, 39, 175–193. [Google Scholar] [CrossRef] [Green Version]
- Ono, K.; Iijima, T. Cardiac T-type Ca2+ channels in the heart. J. Mol. Cell. Cardiol. 2010, 48, 65–70. [Google Scholar] [CrossRef]
- Maltsev, V.A.; Vinogradova, T.M.; Lakatta, E.G. The emergence of a general theory of the initiation and strength of the heartbeat. J. Pharmacol. Sci. 2006, 100, 338–369. [Google Scholar] [CrossRef] [Green Version]
- Sirenko, S.G.; Yang, D.; Maltseva, L.A.; Kim, M.S.; Lakatta, E.G.; Maltsev, V.A. Spontaneous, local diastolic subsarcolemmal calcium releases in single, isolated guinea-pig sinoatrial nodal cells. PLoS ONE 2017, 12, e0185222. [Google Scholar] [CrossRef] [Green Version]
- Sanders, L.; Rakovic, S.; Lowe, M.; Mattick, P.A.; Terrar, D.A. Fundamental importance of Na+-Ca2+ exchange for the pacemaking mechanism in guinea-pig sino-atrial node. J. Physiol. 2006, 571, 639–649. [Google Scholar] [CrossRef]
- Mangoni, M.E.; Nargeot, J. Properties of the hyperpolarization-activated current (If) in isolated mouse sino-atrial cells. Cardiovasc. Res. 2001, 52, 51–64. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, H.; Nishimaru, K.; Aikawa, T.; Hirayama, W.; Tanaka, Y.; Shigenobu, K. Effect of SEA0400, a novel inhibitor of sodium-calcium exchanger, on myocardial ionic currents. Br. J. Pharmacol. 2002, 135, 1096–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, H.; Namekata, I.; Takeda, K.; Kazama, A.; Shimizu, Y.; Moriwaki, R.; Hirayama, W.; Sato, A.; Kawanishi, T.; Shigenobu, K. Unique excitation-contraction characteristics of mouse myocardium as revealed by SEA0400, a specific inhibitor of Na+-Ca2+ exchanger. Naunyn Schmiedebergs Arch. Pharmacol. 2005, 371, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Shimada, H.; Namekata, I.; Kawanishi, T.; Iida-Tanaka, N.; Shigenobu, K. Involvement of the Na+/Ca2+ exchanger in ouabain-induced inotropy and arrhythmogenesis in guinea-pig myocardium as revealed by SEA0400. J. Pharmacol. Sci. 2007, 103, 241–246. [Google Scholar] [CrossRef] [Green Version]
- Namekata, I.; Nakamura, H.; Shimada, H.; Tanaka, H.; Shigenobu, K. Cardioprotection without cardiosuppression by SEA0400, a novel inhibitor of Na+-Ca2+ exchanger, during ischemia and reperfusion in guinea-pig myocardium. Life Sci. 2005, 77, 312–324. [Google Scholar] [CrossRef]
- Torrente, A.G.; Zhang, R.; Zaini, A.; Giani, J.F.; Kang, J.; Lamp, S.T.; Philipson, K.D.; Goldhaber, J.I. Burst pacemaker activity of the sinoatrial node in sodium-calcium exchanger knockout mice. Proc. Natl. Acad. Sci. USA 2015, 112, 9769–9774. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Rasmussen, T.P.; Li, Y.; Kutschke, W.; Koval, O.M.; Wu, Y.; Wu, Y.; Hall, D.D.; Joiner, M.L.; Wu, X.Q.; et al. Genetic inhibition of Na+-Ca2+ exchanger current disables fight or flight sinoatrial node activity without affecting resting heart rate. Circ. Res. 2013, 112, 309–317. [Google Scholar] [CrossRef] [Green Version]
- Kojima, A.; Ito, Y.; Kitagawa, H.; Matsuura, H.; Nosaka, S. Direct negative chronotropic action of desflurane on sinoatrial node pacemaker activity in the guinea pig heart. Anesthesiology 2014, 120, 1400–1413. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Wu, Y.; Mohler, P.J.; Anderson, M.E.; Song, L.S. Local control of Ca2+-induced Ca2+ release in mouse sinoatrial node cells. J. Mol. Cell. Cardiol. 2009, 47, 706–715. [Google Scholar] [CrossRef] [Green Version]
- Magee, W.P.; Deshmukh, G.; Deninno, M.P.; Sutt, J.C.; Chapman, J.G.; Tracey, W.R. Differing cardioprotective efficacy of the Na+/Ca2+ exchanger inhibitors SEA0400 and KB-R7943. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, 903–910. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, T.; Takahashi, K.; Onishi, M.; Suzuki, T.; Tanaka, Y.; Ota, T.; Yoshida, S.; Nakaike, S.; Matsuda, T.; Baba, A. Effects of SEA0400, a novel inhibitor of the Na+/Ca2+ exchanger, on myocardial stunning in anesthetized dogs. Eur. J. Pharmacol. 2004, 505, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Namekata, I.; Tsuneoka, Y.; Takahara, A.; Shimada, H.; Sugimoto, T.; Takeda, K.; Nagaharu, M.; Shigenobu, K.; Kawanishi, T.; Tanaka, H. Involvement of the Na+/Ca2+ exchanger in the automaticity of guinea-pig pulmonary vein myocardium as revealed by SEA0400. J. Pharmacol. Sci. 2009, 110, 111–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namekata, I.; Tanaka, Y.; Ohmori, T.; Tsuneoka, Y.; Hamaguchi, S.; Tanaka, H.; Tanaka, H. Cell morphology and early-phase Ca2+ transients of guinea-pig pulmonary vein cardiomyocytes compared with atrial and ventricular cardiomyocytes. Bioimages 2019, 27, 1–12. [Google Scholar]
- Verkerk, A.O.; Wilders, R.; Zegers, J.G.; van Borren, M.M.; Ravesloot, J.H.; Verheijck, E.E. Ca2+-activated Cl− current in rabbit sinoatrial node cells. J. Physiol. 2002, 540, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Wang, Y.; Peng, H.; He, F.; Zhu, L.; Huang, H.; Huang, X.; Lu, X.; Tan, X. A newly identified missense mutation in CLCA2 is associated with autosomal dominant cardiac conduction block. Gene 2019, 714, 143990. [Google Scholar] [CrossRef]
- Turner, D.; Kang, C.; Mesirca, P.; Hong, J.; Mangoni, M.E.; Glukhov, A.V.; Sah, R. Electrophysiological and Molecular Mechanisms of Sinoatrial node mechanosensitivity. Front. Cardiovasc. Med. 2021, 8, 662410. [Google Scholar] [CrossRef]
- Lei, M.; Jones, S.A.; Liu, J.; Lancaster, M.K.; Fung, S.S.; Dobrzynski, H.; Camelliti, P.; Maier, S.K.; Noble, D.; Boyett, M.R. Requirement of neuronal- and cardiac-type sodium channels for murine sinoatrial node pacemaking. J. Physiol. 2004, 559, 835–843. [Google Scholar] [CrossRef]






| BAPTA | Ryanodine | |||
|---|---|---|---|---|
| Mouse | Guinea Pig | Mouse | Guinea Pig | |
| Firing rate (bpm) | 423.7 ± 10.1 | 241.4 ± 16.1 | 453.6 ± 10.0 | 248.7 ± 11.7 |
| 396.0 ± 6.4 * | 213.1 ± 12.2 * | 414.3 ± 13.9 * | 237.4 ± 12.6 * | |
| Cycle length (ms) | 141.9 ± 3.4 | 254.2 ± 17.0 | 132.5 ± 2.9 | 241.3 ± 13.4 |
| 151.7 ± 2.4 * | 286.7 ± 17.2 * | 145.5 ± 4.7 * | 256.7 ± 13.6 * | |
| Maximum diastolic potential (mV) | −60.6 ± 1.4 | −62.9 ± 1.2 | −58.8 ± 1.2 | −63.2 ± 1.3 |
| −60.0 ± 1.7 | −58.9 ± 2.1 * | −55.9 ± 1.7 | −60.9 ± 1.7 | |
| Slope of pacemaker depolarization (mV/s) | 192.0 ± 26.2 | 147.4 ± 14.7 | 223.5 ± 25.8 | 138.2 ± 10.4 |
| 130.0 ± 17.7 * | 104.8 ± 9.8 * | 110.0 ± 24.5 * | 119.2 ± 9.1 * | |
| Threshold potential (mV) | −50.4 ± 1.6 | −49.0 ± 1.6 | −46.9 ± 1.9 | −52.6 ± 1.9 |
| −51.6 ± 1.5 | −46.4 ± 2.1 * | −45.8 ± 3.0 | −49.1 ± 2.3 | |
| Maximum rate of rise (V/s) | 10.3 ± 2.6 | 9.0 ± 2.2 | 8.7 ± 2.7 | 18.7 ± 9.4 |
| 9.0 ± 2.2 | 8.1 ± 1.9 | 7.8 ± 2.2 | 15.6 ± 7.8 | |
| Peak potential (mV) | 0.9 ± 2.5 | 18.9 ± 1.1 | 4.6 ± 1.2 | 18.3 ± 1.7 |
| −0.9 ± 3.2 | 17.7 ± 1.5 | 4.2 ± 1.0 | 17.8 ± 1.6 | |
| Duration at 50% repolarization (ms) | 30.3 ± 1.7 | 81.8 ± 2.0 | 28.3 ± 0.9 | 75.4 ± 5.9 |
| 30.2 ± 1.4 | 85.6 ± 6.3 | 28.9 ± 1.4 | 77.4 ± 6.4 | |
| SEA0400 (1 μM) | SEA0400 (10 μM) | |||
|---|---|---|---|---|
| Mouse | Guinea Pig | Mouse | Guinea Pig | |
| Firing rate (bpm) | 449.7 ± 24.9 | 239.0 ± 9.2 | 428.2 ± 27.2 | 239.4 ± 8.9 |
| 396.8 ± 29.0 * | 240.1 ± 9.2 | 363.8 ± 17.7 * | 237.4 ± 11.6 | |
| Cycle length (ms) | 135.2 ± 8.1 | 253.0 ± 10.1 | 142.1 ± 8.0 | 252.0 ± 9.0 |
| 155.0 ± 12.2 * | 251.8 ± 9.7 | 166.4 ± 7.6 * | 255.1 ± 12.3 | |
| Maximum diastolic potential (mV) | −55.8 ± 1.8 | −62.6 ± 2.5 | −59.2 ± 1.0 | −61.7 ± 2.1 |
| −51.7 ± 1.9 * | −62.1 ± 2.4 | −55.9 ± 1.3 * | −59.1 ± 3.0 | |
| Slope of pacemaker depolarization (mV/s) | 217.0 ± 22.2 | 148.3 ± 16.2 | 215.4 ± 26.6 | 172.5 ± 6.0 |
| 173.2 ± 22.9 * | 150.8 ± 13.8 | 161.0 ± 20.9 * | 177.0 ± 16.3 | |
| Threshold potential (mV) | −46.0 ± 1.2 | −49.8 ± 2.7 | −48.6 ± 1.2 | −48.3 ± 2.5 |
| −41.9 ± 1.4 * | −48.7 ± 2.5 | −45.5 ± 1.3 * | −43.9 ± 3.1 | |
| Maximum rate of rise (V/s) | 6.7 ± 0.6 | 7.0 ± 1.5 | 7.9 ± 1.6 | 5.8 ± 1.5 |
| 5.6 ± 0.7 * | 7.7 ± 2.2 | 4.5 ± 0.8 * | 4.8 ± 1.1 | |
| Peak potential (mV) | 4.8 ± 0.7 | 12.0 ± 2.1 | 4.7 ± 1.6 | 11.9 ± 1.6 |
| −0.5 ± 1.3 * | 12.8 ± 1.8 | −11.2 ± 3.0 * | 12.0 ± 1.6 | |
| Duration at 50% repolarization (ms) | 33.5 ± 2.1 | 80.4 ± 5.4 | 31.3 ± 2.2 | 85.4 ± 6.2 |
| 35.5 ± 1.5 | 79.8 ± 4.4 | 36.2 ± 2.2 | 83.6 ± 5.3 | |
| Low Na+ | ||
|---|---|---|
| Mouse | Guinea Pig | |
| Firing rate (bpm) | 498.4 ± 17.1 | 232.7 ± 9.5 |
| 455.5 ± 20.5 * | 232.7 ± 9.9 | |
| Cycle length (ms) | 121.1 ± 4.0 | 260.2 ± 12.0 |
| 133.1 ± 5.9 * | 260.5 ± 12.6 | |
| Maximum diastolic potential (mV) | −60.4 ± 1.1 | −63.2 ± 0.9 |
| −56.9 ± 0.9 * | −64.0 ± 1.6 | |
| Slope of pacemaker depolarization (mV/s) | 251.4 ± 25.3 | 166.3 ± 17.4 |
| 206.7 ± 30.7 * | 187.3 ± 20.4 | |
| Threshold potential (mV) | −49.8 ± 1.1 | −48.2 ± 1.1 |
| −47.6 ± 1.0 * | −48.1 ± 1.8 | |
| Maximum rate of rise (V/s) | 8.6 ± 1.1 | 4.0 ± 0.7 |
| 5.6 ± 0.6 * | 3.6 ± 0.6 * | |
| Peak potential (mV) | 1.9 ± 1.3 | 11.0 ± 1.2 |
| −5.1 ± 2.4 * | 8.3 ± 2.4 | |
| Duration at 50% repolarization (ms) | 28.7 ± 1.3 | 89.3 ± 3.5 |
| 32.2 ± 1.3 * | 88.8 ± 4.2 | |
| Niflumic Acid | ||
|---|---|---|
| Mouse | Guinea Pig | |
| Firing rate (bpm) | 444.5 ± 18.1 | 236.6 ± 14.1 |
| 418.4 ± 21.0 * | 205.0 ± 12.5 * | |
| Cycle length (ms) | 136.1 ± 5.5 | 258.4 ± 15.9 |
| 145.3 ± 7.6 * | 298.0 ± 17.7 * | |
| Maximum diastolic potential (mV) | −61.0 ± 1.1 | −62.7 ± 3.1 |
| −60.6 ± 1.8 | −61.8 ± 2.8 | |
| Slope of pacemaker depolarization (mV/s) | 221.5 ± 40.9 | 152.2 ± 20.2 |
| 173.8 ± 35.7 * | 104.8 ± 12.4 * | |
| Threshold potential (mV) | −50.5 ± 1.7 | −48.8 ± 3.2 |
| −51.7 ± 2.5 | −49.0 ± 3.5 | |
| Maximum rate of rise (V/s) | 14.5 ± 6.5 | 8.4 ± 2.1 |
| 14.7 ± 6.8 | 6.9 ± 1.3 | |
| Peak potential (mV) | 5.8 ± 0.8 | 19.4 ± 4.0 |
| 5.2 ± 1.6 | 17.8 ± 3.4 | |
| Duration at 50% repolarization (ms) | 31.3 ± 1.2 | 78.5 ± 2.6 |
| 32.1 ± 1.0 | 74.3 ± 3.2 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Namekata, I.; Jitsukata, K.; Fukuda, A.; Odaka, R.; Hamaguchi, S.; Tanaka, H. Intracellular Ca2+-Mediated Mechanisms for the Pacemaker Depolarization of the Mouse and Guinea Pig Sinus Node Tissue. Biomolecules 2022, 12, 377. https://doi.org/10.3390/biom12030377
Namekata I, Jitsukata K, Fukuda A, Odaka R, Hamaguchi S, Tanaka H. Intracellular Ca2+-Mediated Mechanisms for the Pacemaker Depolarization of the Mouse and Guinea Pig Sinus Node Tissue. Biomolecules. 2022; 12(3):377. https://doi.org/10.3390/biom12030377
Chicago/Turabian StyleNamekata, Iyuki, Kento Jitsukata, Ayumi Fukuda, Ryosuke Odaka, Shogo Hamaguchi, and Hikaru Tanaka. 2022. "Intracellular Ca2+-Mediated Mechanisms for the Pacemaker Depolarization of the Mouse and Guinea Pig Sinus Node Tissue" Biomolecules 12, no. 3: 377. https://doi.org/10.3390/biom12030377
APA StyleNamekata, I., Jitsukata, K., Fukuda, A., Odaka, R., Hamaguchi, S., & Tanaka, H. (2022). Intracellular Ca2+-Mediated Mechanisms for the Pacemaker Depolarization of the Mouse and Guinea Pig Sinus Node Tissue. Biomolecules, 12(3), 377. https://doi.org/10.3390/biom12030377