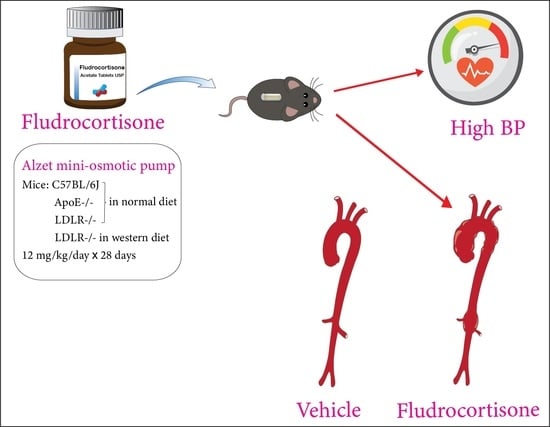
Fludrocortisone Induces Aortic Pathologies in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Mini Osmotic Pump Implantation and Fludrocortisone Infusion
2.3. Systolic Blood Pressure Measurements
2.4. Plasma Collection and Total Cholesterol Measurement
2.5. Measurement of Aortic Diameters
2.6. Statistical Analyses
3. Results
3.1. Fludrocortisone Induced Aortic Pathologies in the Thoracic Aorta of Male C57BL/6J Mice
3.2. Fludrocortisone Induced Aortic Pathologies in Male ApoE −/− Mice and LDLR −/− Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saverino, S.; Falorni, A. Autoimmune Addison’s disease. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101379. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Okamoto, L.E.; Shibao, C.A.; Biaggioni, I. Pharmacologic treatment of orthostatic hypotension. Auton. Neurosci. 2020, 229, 102721. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Mohammadmoradi, S.; Chen, J.Z.; Sawada, H.; Daugherty, A.; Lu, H.S. Renin-angiotensin system and cardiovascular functions. Arterioscler. Thromb. Vasc. Biol. 2018, 38, e108–e116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uijl, E.; Colafella, K.M.M.; Hoorn, E.J.; van Veghel, R.; Zlatev, I.; Kim, J.B.; Huang, S.; Melton, L.; Foster, D.; Danser, A.H.J. Control of antihypertensive effect of small interfering RNA targeting angiotensinogen. Hypertension 2019, 74, AP2031. [Google Scholar] [CrossRef]
- Sawada, H.; Lu, H.S.; Cassis, L.A.; Daugherty, A. Twenty years of studying AngII (angiotensin II)-induced abdominal aortic pathologies in mice: Continuing questions and challenges to provide insight into the human disease. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 277–288. [Google Scholar]
- Daugherty, A.; Manning, M.W.; Cassis, L.A. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J. Clin. Investig. 2000, 105, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Nsengiyumva, V.; Krishna, S.M.; Moran, C.S.; Moxon, J.V.; Morton, S.K.; Clarke, M.W.; Seto, S.W.; Golledge, J. Vitamin D deficiency promotes large rupture-prone abdominal aortic aneurysms and cholecalciferol supplementation limits progression of aneurysms in a mouse model. Clin. Sci. 2020, 134, 2521–2534. [Google Scholar] [CrossRef]
- Aslanidou, L.; Ferraro, M.; Lovric, G.; Bersi, M.R.; Humphrey, J.D.; Segers, P.; Trachet, B.; Stergiopulos, N. Co-localization of microstructural damage and excessive mechanical strain at aortic branches in angiotensin-II-infused mice. Biomech. Modeling Mechanobiol. 2020, 19, 81–97. [Google Scholar] [CrossRef]
- Lu, H.; Howatt, D.A.; Balakrishnan, A.; Moorleghen, J.J.; Rateri, D.L.; Cassis, L.A.; Daugherty, A. Subcutaneous angiotensin II infusion using osmotic pumps induces aortic aneurysms in mice. J. Vis. Exp. 2015, 103, e53191. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Xu, Y.; Lu, H.; Howatt, D.A.; Balakrishnan, A.; Moorleghen, J.J.; Vander Kooi, C.W.; Cassis, L.A.; Wang, J.A.; Daugherty, A. Cys18-Cys137 disulfide bond in mouse angiotensinogen does not affect AngII-dependent functions in vivo. Hypertension 2015, 65, 800–805. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Wu, C.; Howatt, D.A.; Balakrishnan, A.; Moorleghen, J.J.; Chen, X.; Zhao, M.; Graham, M.J.; Mullick, A.E.; Crooke, R.M.; et al. Angiotensinogen exerts effects independent of angiotensin II. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 256–265. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Lu, H.; Howatt, D.A.; Balakrishnan, A.; Moorleghen, J.J.; Sorci-Thomas, M.; Cassis, L.A.; Daugherty, A. Associations of ApoAI and ApoB-containing lipoproteins with AngII-induced abdominal aortic aneurysms in mice. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1826–1834. [Google Scholar] [CrossRef] [Green Version]
- Rateri, D.L.; Davis, F.M.; Balakrishnan, A.; Howatt, D.A.; Moorleghen, J.J.; O’Connor, W.N.; Charnigo, R.; Cassis, L.A.; Daugherty, A. Angiotensin II induces region-specific medial disruption during evolution of ascending aortic aneurysms. Am. J. Pathol. 2014, 184, 2586–2595. [Google Scholar] [CrossRef] [Green Version]
- Robinet, P.; Milewicz, D.M.; Cassis, L.A.; Leeper, N.J.; Lu, H.S.; Smith, J.D. Consideration of sex differences in design and reporting of experimental arterial pathology studies-Statement from ATVB Council. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 292–303. [Google Scholar] [CrossRef] [Green Version]
- Percie du Sert, N.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef]
- Daugherty, A.; Rateri, D.; Hong, L.; Balakrishnan, A. Measuring blood pressure in mice using volume pressure recording, a tail-cuff method. J. Vis. Exp. 2009, 27, e1291. [Google Scholar] [CrossRef] [Green Version]
- Ohno-Urabe, S.; Kukida, M.; Franklin, M.K.; Katsumata, Y.; Su, W.; Gong, M.C.; Lu, H.S.; Daugherty, A.; Sawada, H. Authentication of in situ measurements for thoracic aortic aneurysms in mice. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2117–2119. [Google Scholar] [CrossRef]
- Ito, S.; Lu, H.S.; Daugherty, A.; Sawada, H. Imaging techniques for aortic aneurysms and dissections in mice: Comparisons of ex vivo, in situ and ultrasound approaches. Biomolecules 2022, 12, 339. [Google Scholar] [CrossRef]
- Daugherty, A.; Tall, A.R.; Daemen, M.; Falk, E.; Fisher, E.A.; García-Cardeña, G.; Lusis, A.J.; Owens, A.P., 3rd; Rosenfeld, M.E.; Virmani, R. Recommendation on design, execution, and reporting of animal atherosclerosis studies: A scientific statement from the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e131–e157. [Google Scholar] [CrossRef] [Green Version]
- Kuhlencordt, P.J.; Gyurko, R.; Han, F.; Scherrer-Crosbie, M.; Aretz, T.H.; Hajjar, R.; Picard, M.H.; Huang, P.L. Accelerated atherosclerosis, aortic aneurysm formation and ischemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice. Circulation 2001, 104, 448–454. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Xie, Z.; Daugherty, A.; Cassis, L.A.; Pearson, K.J.; Gong, M.C.; Guo, Z. Mineralocorticoid receptor agonists induce mouse aortic aneurysm formation and rupture in the presence of high salt. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1568–1579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.; Daugherty, A.; Lu, H.S. Updates on approaches for studying atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, e108–e117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassis, L.A.; Gupte, M.; Thayer, S.; Zhang, X.; Charnigo, R.; Howatt, D.A.; Rateri, D.L.; Daugherty, A. ANG II infusion promotes abdominal aortic aneurysms independent of increased blood pressure in hypercholesterolemic mice. Am. J. Physiol.-Heart Circ. Physiol. 2009, 296, H1660–H1665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, F.M.; Rateri, D.L.; Balakrishnan, A.; Howatt, D.A.; Strickland, D.K.; Muratoglu, S.C.; Haggerty, C.M.; Fornwalt, B.K.; Cassis, L.A.; Daugherty, A. Smooth muscle cell deletion of low-density lipoprotein receptor-related protein 1 augments angiotensin II-induced superior mesenteric arterial and ascending aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 155–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owens, A.P., 3rd; Subramanian, V.; Moorleghen, J.J.; Guo, Z.; McNamara, C.A.; Cassis, L.A.; Daugherty, A. Angiotensin II induces a region-specific hyperplasia of the ascending aorta through regulation of inhibitor of differentiation 3. Circ. Res. 2010, 106, 611–619. [Google Scholar] [CrossRef]
- Mori, T.; Katayama, Y.; Kawamata, T.; Hirayama, T. Improved efficiency of hypervolemic therapy with inhibition of natriuresis by fludrocortisone in patients with aneurysmal subarachnoid hemorrhage. J. Neurosurg. 1999, 91, 947–952. [Google Scholar] [CrossRef]
- Hasan, D.; Lindsay, K.W.; Wijdicks, E.F.; Murray, G.D.; Brouwers, P.J.; Bakker, W.H.; van Gijn, J.; Vermeulen, M. Effect of fludrocortisone acetate in patients with subarachnoid hemorrhage. Stroke 1989, 20, 1156–1161. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, I.; Hironaka, Y.; Nishimura, F.; Takeshima, Y.; Matsuda, R.; Yamada, S.; Motoyama, Y.; Park, Y.S.; Nakase, H. Early inhibition of natriuresis suppresses symptomatic cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage. Cerebrovasc. Dis. 2013, 35, 131–137. [Google Scholar] [CrossRef]
- Zavatta, G.; Di Dalmazi, G.; Pizzi, C.; Bracchetti, G.; Mosconi, C.; Balacchi, C.; Pagotto, U.; Vicennati, V. Larger ascending aorta in primary aldosteronism: A 3-year prospective evaluation of adrenalectomy vs. medical treatment. Endocrine 2019, 63, 470–475. [Google Scholar] [CrossRef]






| Strain | Description | Sex | Persistent ID | URL |
|---|---|---|---|---|
| C57BL/6J | C57BL/6J | M | 000664 | https://www.jax.org/strain/000664 (accessed on 22 May 2022) |
| ApoE −/− | B6.129P2-Apoetm1Unc/J (>N10 to C57BL/6J) | M | 002052 | https://www.jax.org/strain/002052 (accessed on 22 May 2022) |
| LDLR −/− | B6.129S7-Ldlrtm1Her/J (>N10 to C57BL/6J) | M | 002207 | https://www.jax.org/strain/002207 (accessed on 22 May 2022) |
| Item | Application |
|---|---|
| IACUC Protocol | # 2018-2968, approved by the University of Kentucky IACUC. |
| Sex | This study examined aortic pathologies only in male mice because of the higher prevalence in male mice [14]. |
| Inclusion criteria | Body weight > 20 g and 7–9 weeks of age |
| Exclusion criteria |
|
| Sample size | n = 5 for vehicle, and n = 15 for fludrocortisone infusion in each experiment |
| Power analysis (prospective) | Not performed |
| Endpoint |
|
| Randomization | DLAR staff placed study mice randomly in cages (n = 5/cage) upon arrival. |
| Blinding | All experimental data were verified by an independent investigator blinded to the study group information. |
| Statistical analysis | SigmaPlot version 14.5 (SYSTAT Software Inc., Palo Alto, CA, USA) |
| Statistical method | Continuous variables between groups were analyzed using Mann–Whitney rank sum test. The correlation between ascending aortic diameters and systolic blood pressure was analyzed using Spearman rank order correlation analysis. |
| Data availability | All raw data and analytical methods are available from the corresponding authors upon appropriate request. |
| Mouse Strain | Diet | Aortic Pathologies (n of Mice) | |||
|---|---|---|---|---|---|
| Asc/Arch | Asc/Arch + Desc | Asc/Arch + Suprarenal | Desc + Suprarenal | ||
| C57BL/6J | Normal | 3 | 1 | 0 | 0 |
| ApoE −/− | Normal | 2 | 0 | 0 | 0 |
| LDLR −/− | Normal | 4 | 0 | 1 | 0 |
| LDLR −/− | Western | 2 | 0 | 1 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, D.; Wu, C.; Chen, H.; Liang, C.-L.; Howatt, D.A.; Franklin, M.K.; Moorleghen, J.J.; Tyagi, S.C.; Uijl, E.; Danser, A.H.J.; et al. Fludrocortisone Induces Aortic Pathologies in Mice. Biomolecules 2022, 12, 825. https://doi.org/10.3390/biom12060825
Ye D, Wu C, Chen H, Liang C-L, Howatt DA, Franklin MK, Moorleghen JJ, Tyagi SC, Uijl E, Danser AHJ, et al. Fludrocortisone Induces Aortic Pathologies in Mice. Biomolecules. 2022; 12(6):825. https://doi.org/10.3390/biom12060825
Chicago/Turabian StyleYe, Dien, Congqing Wu, Hui Chen, Ching-Ling Liang, Deborah A. Howatt, Michael K. Franklin, Jessica J. Moorleghen, Samuel C. Tyagi, Estrellita Uijl, A. H. Jan Danser, and et al. 2022. "Fludrocortisone Induces Aortic Pathologies in Mice" Biomolecules 12, no. 6: 825. https://doi.org/10.3390/biom12060825
APA StyleYe, D., Wu, C., Chen, H., Liang, C. -L., Howatt, D. A., Franklin, M. K., Moorleghen, J. J., Tyagi, S. C., Uijl, E., Danser, A. H. J., Sawada, H., Daugherty, A., & Lu, H. S. (2022). Fludrocortisone Induces Aortic Pathologies in Mice. Biomolecules, 12(6), 825. https://doi.org/10.3390/biom12060825