Influence of Inhibition of COX-2-Dependent Lipid Metabolism on Regulation of UVB-Induced Keratinocytes Apoptosis by Cannabinoids
Abstract
:1. Introduction
2. Materials and Methods
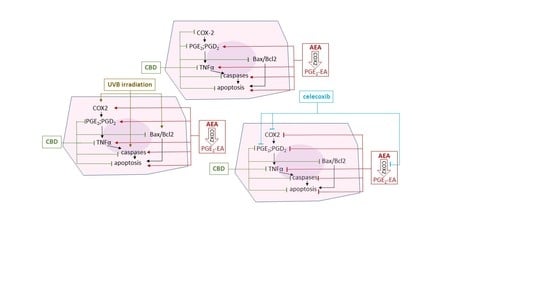
- Control—cells cultured for 24 in standard medium without (A) or with 20 µM celecoxib (B);
- CBD—cells were pre-cultured with 4 µM CBD and then cultured for 24 in standard medium without (A) or with 20 µM celecoxib (B);
- AEA—cells were pre-cultured with 4 µM AEA and then cultured for 24 in standard medium without (A) or with 20 µM celecoxib (B);
- UVB—cells were irradiated with UVB (60 mJ/cm2), and then cultured for 24 in standard medium without (A) or with 20 µM celecoxib (B);
- CBD + UVB—cells were pre-cultured for 24 h with 4 µM CBD, next irradiated with UVB (60 mJ/cm2), and then cultured for 24 h in medium supplemented with 4 µM CBD. The last 24 h culturing was carried out in medium without (A) or with 20 µM celecoxib (B);
- AEA + UVB—cells were pre-cultured for 24 h with 4 µM with AEA, irradiated with UVB (60 mJ/cm2), and then cultured for 24 h in medium supplemented with 4 µM AEA. The last 24 h culturing was carried out in medium without (A) or with 20 µM celecoxib (B).
2.1. Assessment of Apoptosis Regulators
2.2. Assessment of the Number of Cells Undergoing Apoptosis
2.3. Measurement of Cyclooxygenase Activity
2.4. Determination of Prostaglandins Level in Culture Media
2.5. Statistical Analysis
3. Results
4. Discussion
4.1. Effect of UVB on Keratinocyte Apoptosis
4.2. Effect of Cannabinoids on Apoptosis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of Apoptosis in Health and Disease: The Balancing Act of BCL-2 Family Proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef] [PubMed]
- D’Arcy, M.S. Cell Death: A Review of the Major Forms of Apoptosis, Necrosis and Autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as Anticancer Mechanism: Function and Dysfunction of Its Modulators and Targeted Therapeutic Strategies. Aging (Albany NY) 2016, 8, 603–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webster, J.D.; Vucic, D. The Balance of TNF Mediated Pathways Regulates Inflammatory Cell Death Signaling in Healthy and Diseased Tissues. Front. Cell Dev. Biol. 2020, 8, 365. [Google Scholar] [CrossRef]
- Powell, N.; Pantazi, E.; Pavlidis, P.; Tsakmaki, A.; Li, K.; Yang, F.; Parker, A.; Pin, C.; Cozzetto, D.; Minns, D.; et al. Interleukin-22 Orchestrates a Pathological Endoplasmic Reticulum Stress Response Transcriptional Programme in Colonic Epithelial Cells. Gut 2020, 69, 578–590. [Google Scholar] [CrossRef] [Green Version]
- Grunnet, L.G.; Aikin, R.; Tonnesen, M.F.; Paraskevas, S.; Blaabjerg, L.; Størling, J.; Rosenberg, L.; Billestrup, N.; Maysinger, D.; Mandrup-Poulsen, T. Proinflammatory Cytokines Activate the Intrinsic Apoptotic Pathway in Beta-Cells. Diabetes 2009, 58, 1807–1815. [Google Scholar] [CrossRef] [Green Version]
- Biernacki, M.; Skrzydlewska, E. Metabolism of endocannabinoids. Postępy Hig. I Med. Doświadczalnej 2016, 70, 830–843. [Google Scholar] [CrossRef]
- Liaras, K.; Fesatidou, M.; Geronikaki, A. Thiazoles and Thiazolidinones as COX/LOX Inhibitors. Molecules 2018, 23, 685. [Google Scholar] [CrossRef] [Green Version]
- Sangiovanni, E.; Fumagalli, M.; Pacchetti, B.; Piazza, S.; Magnavacca, A.; Khalilpour, S.; Melzi, G.; Martinelli, G.; Dell’Agli, M. Cannabis sativa L. Extract and Cannabidiol Inhibit in Vitro Mediators of Skin Inflammation and Wound Injury. Phytother Res. 2019, 33, 2083–2093. [Google Scholar] [CrossRef]
- Petrovici, A.R.; Simionescu, N.; Sandu, A.I.; Paraschiv, V.; Silion, M.; Pinteala, M. New Insights on Hemp Oil Enriched in Cannabidiol: Decarboxylation, Antioxidant Properties and In Vitro Anticancer Effect. Antioxidants 2021, 10, 738. [Google Scholar] [CrossRef]
- Sorrentino, G.; Comel, A.; Mantovani, F.; Del Sal, G. Regulation of Mitochondrial Apoptosis by Pin1 in Cancer and Neurodegeneration. Mitochondrion 2014, 19, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism Pathways of Arachidonic Acids: Mechanisms and Potential Therapeutic Targets. Sig. Transduct Target 2021, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-C.; Mackie, K. Review of the Endocannabinoid System. Biol. Psychiatry Cogn. Neurosci. Neuroimag. 2021, 6, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; Di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S.; et al. Cannabinoids for Medical Use: A Systematic Review and Meta-Analysis. JAMA 2015, 313, 2456–2473. [Google Scholar] [CrossRef] [PubMed]
- Turcotte, C.; Blanchet, M.-R.; Laviolette, M.; Flamand, N. The CB2 Receptor and Its Role as a Regulator of Inflammation. Cell Mol. Life Sci. 2016, 73, 4449–4470. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Zhao, D.; Zhuang, J.; Zhang, F.; Xu, C. Caspase-8 and Caspase-9 Functioned Differently at Different Stages of the Cyclic Stretch-Induced Apoptosis in Human Periodontal Ligament Cells. PLoS ONE 2016, 11, e0168268. [Google Scholar] [CrossRef]
- Jeong, S.-J.; Dasgupta, A.; Jung, K.-J.; Um, J.-H.; Burke, A.; Park, H.U.; Brady, J.N. PI3K/AKT Inhibition Induces Caspase-Dependent Apoptosis in HTLV-1-Transformed Cells. Virology 2008, 370, 264–272. [Google Scholar] [CrossRef] [Green Version]
- Kisková, T.; Mungenast, F.; Suváková, M.; Jäger, W.; Thalhammer, T. Future Aspects for Cannabinoids in Breast Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 1673. [Google Scholar] [CrossRef] [Green Version]
- Mecha, M.; Torrao, A.S.; Mestre, L.; Carrillo-Salinas, F.J.; Mechoulam, R.; Guaza, C. Cannabidiol Protects Oligodendrocyte Progenitor Cells from Inflammation-Induced Apoptosis by Attenuating Endoplasmic Reticulum Stress. Cell Death Dis. 2012, 3, e331. [Google Scholar] [CrossRef] [Green Version]
- Ruhaak, L.R.; Felth, J.; Karlsson, P.C.; Rafter, J.J.; Verpoorte, R.; Bohlin, L. Evaluation of the Cyclooxygenase Inhibiting Effects of Six Major Cannabinoids Isolated from Cannabis Sativa. Biol. Pharm. Bull. 2011, 34, 774–778. [Google Scholar] [CrossRef] [Green Version]
- Wójcik, P.; Žarković, N.; Gęgotek, A.; Skrzydlewska, E. Involvement of Metabolic Lipid Mediators in the Regulation of Apoptosis. Biomolecules 2020, 10, 402. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Martínez, E.; Martín-Ruiz, A.; Martín, P.; Calvo, V.; Provencio, M.; García, J.M. CB2 Cannabinoid Receptor Activation Promotes Colon Cancer Progression via AKT/GSK3β Signaling Pathway. Oncotarget 2016, 7, 68781–68791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moriwaki, K.; Luz, N.F.; Balaji, S.; De Rosa, M.J.; O’Donnell, C.L.; Gough, P.J.; Bertin, J.; Welsh, R.M.; Chan, F.K.-M. The Mitochondrial Phosphatase PGAM5 Is Dispensable for Necroptosis but Promotes Inflammasome Activation in Macrophages. J. Immunol. 2016, 196, 407–415. [Google Scholar] [CrossRef] [Green Version]
- Kondreddy, V.K.R.; Kamatham, A.N. Celecoxib, a COX-2 Inhibitor, Synergistically Potentiates the Anti-Inflammatory Activity of Docosahexaenoic Acid in Macrophage Cell Line. Immunopharmacol. Immunotoxicol. 2016, 38, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Pucci, M.; Pasquariello, N.; Battista, N.; Di Tommaso, M.; Rapino, C.; Fezza, F.; Zuccolo, M.; Jourdain, R.; Finazzi Agrò, A.; Breton, L.; et al. Endocannabinoids Stimulate Human Melanogenesis via Type-1 Cannabinoid Receptor. J. Biol. Chem. 2012, 287, 15466–15478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desjardins, P.; Conklin, D. NanoDrop Microvolume Quantitation of Nucleic Acids. J. Vis. Exp. 2010, 45, 2565. [Google Scholar] [CrossRef] [Green Version]
- Baranowska-Kuczko, M.; Kozłowska, H.; Kloza, M.; Kusaczuk, M.; Harasim-Symbor, E.; Biernacki, M.; Kasacka, I.; Malinowska, B. Vasoprotective Endothelial Effects of Chronic Cannabidiol Treatment and Its Influence on the Endocannabinoid System in Rats with Primary and Secondary Hypertension. Pharmaceuticals 2021, 14, 1120. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, W.; Zhang, J.; Li, H. Role of JNK and ERK1/2 MAPK Signaling Pathway in Testicular Injury of Rats Induced by Di-N-Butyl-Phthalate (DBP). Biol. Res. 2019, 52, 41. [Google Scholar] [CrossRef]
- Kulmacz, R.J.; Lands, W.E. Requirements for Hydroperoxide by the Cyclooxygenase and Peroxidase Activities of Prostaglandin H Synthase. Prostaglandins 1983, 25, 531–540. [Google Scholar] [CrossRef]
- Smith, C.J.; Zhang, Y.; Koboldt, C.M.; Muhammad, J.; Zweifel, B.S.; Shaffer, A.; Talley, J.J.; Masferrer, J.L.; Seibert, K.; Isakson, P.C. Pharmacological Analysis of Cyclooxygenase-1 in Inflammation. PNAS 1998, 95, 13313–13318. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Xiao, L.; Park, G.; Wang, X.; Azim, A.C.; Christman, J.W.; van Breemen, R.B. An Improved LC-MS-MS Method for the Quantification of Prostaglandins E2 and D2 Production in Biological Fluids. Anal. Biochem. 2008, 372, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Lam, P.M.W.; Marczylo, T.H.; El-Talatini, M.; Finney, M.; Nallendran, V.; Taylor, A.H.; Konje, J.C. Ultra Performance Liquid Chromatography Tandem Mass Spectrometry Method for the Measurement of Anandamide in Human Plasma. Anal. Biochem. 2008, 380, 195–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velasco, G.; Sánchez, C.; Guzmán, M. Anticancer Mechanisms of Cannabinoids. Curr. Oncol. 2016, 23, S23–S32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, C.; Ramirez, D.A.; McGrath, S.; Gustafson, D.L. Cannabidiol Induces Apoptosis and Perturbs Mitochondrial Function in Human and Canine Glioma Cells. Front. Pharmacol. 2021, 12, 2081. [Google Scholar] [CrossRef] [PubMed]
- Lukhele, S.T.; Motadi, L.R. Cannabidiol Rather than Cannabis Sativa Extracts Inhibit Cell Growth and Induce Apoptosis in Cervical Cancer Cells. BMC Complementary Altern. Med. 2016, 16, 335. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.-K.; Jang, B.-C. UVB-Induced Anti-Survival and pro-Apoptotic Effects on HaCaT Human Keratinocytes via Caspase- and PKC-Dependent Downregulation of PKB, HIAP-1, Mcl-1, XIAP and ER Stress. Int. J. Mol. Med. 2014, 33, 695–702. [Google Scholar] [CrossRef] [Green Version]
- Muzaffer, U.; Paul, V.I.; Prasad, N.R.; Karthikeyan, R. Juglans Regia L. Protects against UVB Induced Apoptosis in Human Epidermal Keratinocytes. Biochem. Biophys. Rep. 2018, 13, 109–115. [Google Scholar] [CrossRef]
- Lee, C.-H.; Wu, S.-B.; Hong, C.-H.; Yu, H.-S.; Wei, Y.-H. Molecular Mechanisms of UV-Induced Apoptosis and Its Effects on Skin Residential Cells: The Implication in UV-Based Phototherapy. Int. J. Mol. Sci. 2013, 14, 6414–6435. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, J.; Pofahl, R.; Haase, I. Epidermal Rac1 Regulates the DNA Damage Response and Protects from UV-Light-Induced Keratinocyte Apoptosis and Skin Carcinogenesis. Cell Death Dis. 2017, 8, e2664. [Google Scholar] [CrossRef] [Green Version]
- Niture, S.K.; Jaiswal, A.K. Nrf2 Protein Up-Regulates Antiapoptotic Protein Bcl-2 and Prevents Cellular Apoptosis. J. Biol. Chem. 2012, 287, 9873–9886. [Google Scholar] [CrossRef] [Green Version]
- Aydin, Y.; Chedid, M.; Chava, S.; Danielle Williams, D.; Liu, S.; Hagedorn, C.H.; Sumitran-Holgersson, S.; Reiss, K.; Moroz, K.; Lu, H.; et al. Activation of PERK-Nrf2 Oncogenic Signaling Promotes Mdm2-Mediated Rb Degradation in Persistently Infected HCV Culture. Sci. Rep. 2017, 7, 9223. [Google Scholar] [CrossRef] [Green Version]
- Calvaruso, G.; Pellerito, O.; Notaro, A.; Giuliano, M. Cannabinoid-Associated Cell Death Mechanisms in Tumor Models (Review). Int. J. Oncol. 2012, 41, 407–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, K.H.; Jung, J.H.; Kim, J.E.; Kang, H. Prostaglandin D2-Mediated DP2 and AKT Signal Regulate the Activation of Androgen Receptors in Human Dermal Papilla Cells. Int. J. Mol. Sci. 2018, 19, 556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jendrossek, V. Targeting Apoptosis Pathways by Celecoxib in Cancer. Cancer Lett. 2013, 332, 313–324. [Google Scholar] [CrossRef]
- Nagamachi, M.; Sakata, D.; Kabashima, K.; Furuyashiki, T.; Murata, T.; Segi-Nishida, E.; Soontrapa, K.; Matsuoka, T.; Miyachi, Y.; Narumiya, S. Facilitation of Th1-Mediated Immune Response by Prostaglandin E Receptor EP1. J. Exp. Med. 2007, 204, 2865–2874. [Google Scholar] [CrossRef] [PubMed]
- Boniface, K.; Bak-Jensen, K.S.; Li, Y.; Blumenschein, W.M.; McGeachy, M.J.; McClanahan, T.K.; McKenzie, B.S.; Kastelein, R.A.; Cua, D.J.; de Waal Malefyt, R. Prostaglandin E2 Regulates Th17 Cell Differentiation and Function through Cyclic AMP and EP2/EP4 Receptor Signaling. J. Exp. Med. 2009, 206, 535–548. [Google Scholar] [CrossRef] [Green Version]
- Razali, N.; Hohjoh, H.; Inazumi, T.; Maharjan, B.D.; Nakagawa, K.; Konishi, M.; Sugimoto, Y.; Hasegawa, H. Induced Prostanoid Synthesis Regulates the Balance between Th1- and Th2-Producing Inflammatory Cytokines in the Thymus of Diet-Restricted Mice. Biol. Pharm. Bull. 2020, 43, 649–662. [Google Scholar] [CrossRef] [Green Version]
- Bernard, M.P.; Phipps, R.P. Inhibition of Cyclooxygenase-2 Impairs the Expression of Essential Plasma Cell Transcription Factors and Human B-Lymphocyte Differentiation. Immunology 2010, 129, 87–96. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Kang, D.; Yang, D.; Tang, Y. Activation of PGE2/EP2 and PGE2/EP4 Signaling Pathways Positively Regulate the Level of PD-1 in Infiltrating CD8+ T Cells in Patients with Lung Cancer. Oncol. Lett. 2018, 15, 552–558. [Google Scholar] [CrossRef] [Green Version]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Patel, V.; Saito, K.; Matsumoto, S.; Kashiwaya, Y.; Horváth, B.; Mukhopadhyay, B.; Becker, L.; et al. Cannabidiol Attenuates Cardiac Dysfunction, Oxidative Stress, Fibrosis, Inflammatory and Cell Death Signaling Pathways in Diabetic Cardiomyopathy. J. Am. Coll. Cardiol. 2010, 56, 2115–2125. [Google Scholar] [CrossRef] [Green Version]
- Iuvone, T.; Esposito, G.; Esposito, R.; Santamaria, R.; Di Rosa, M.; Izzo, A.A. Neuroprotective Effect of Cannabidiol, a Non-Psychoactive Component from Cannabis Sativa, on Beta-Amyloid-Induced Toxicity in PC12 Cells. J. Neurochem. 2004, 89, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-J.; Lu, Y.; Wang, H.-W.; Zhang, H.; Wang, S.-R.; Xu, W.-Y.; Fu, H.-L.; Yao, X.-Y.; Yang, F.; Yuan, H.-B. Activation of Endocannabinoid Receptor 2 as a Mechanism of Propofol Pretreatment-Induced Cardioprotection against Ischemia-Reperfusion Injury in Rats. Oxid. Med. Cell. Longev. 2017, 2017, 2186383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.-Y.; Chang, A.-C.; Wang, C.-C.; Kuo, F.-H.; Lee, C.-Y.; Liu, D.-Z.; Jan, T.-R. Cannabidiol Induced a Contrasting Pro-Apoptotic Effect between Freshly Isolated and Precultured Human Monocytes. Toxicol. Appl. Pharmacol. 2010, 246, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Sultan, A.S.; Marie, M.A.; Sheweita, S.A. Novel Mechanism of Cannabidiol-Induced Apoptosis in Breast Cancer Cell Lines. Breast 2018, 41, 34–41. [Google Scholar] [CrossRef]
- Costa, M.A.; Fonseca, B.M.; Teixeira, N.A.; Correia-da-Silva, G. The Endocannabinoid Anandamide Induces Apoptosis in Cytotrophoblast Cells: Involvement of Both Mitochondrial and Death Receptor Pathways. Placenta 2015, 36, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, P.; Gęgotek, A.; Žarković, N.; Skrzydlewska, E. Disease-Dependent Antiapoptotic Effects of Cannabidiol for Keratinocytes Observed upon UV Irradiation. Int. J. Mol. Sci. 2021, 22, 9956. [Google Scholar] [CrossRef]
- Cannabinoid 2 Receptor Agonist—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/cannabinoid-2-receptor-agonist (accessed on 9 June 2022).
- Gupta, B.; Hornick, M.G.; Briyal, S.; Donovan, R.; Prazad, P.; Gulati, A. Anti-Apoptotic and Immunomodulatory Effect of CB2 Agonist, JWH133, in a Neonatal Rat Model of Hypoxic-Ischemic Encephalopathy. Front. Pediatr. 2020, 8, 65. [Google Scholar] [CrossRef]
- Ramer, R.; Heinemann, K.; Merkord, J.; Rohde, H.; Salamon, A.; Linnebacher, M.; Hinz, B. COX-2 and PPAR-γ Confer Cannabidiol-Induced Apoptosis of Human Lung Cancer Cells. Mol. Cancer Ther. 2013, 12, 69–82. [Google Scholar] [CrossRef] [Green Version]
- Kuc, C.; Jenkins, A.; Van Dross, R.T. Arachidonoyl Ethanolamide (AEA)-Induced Apoptosis Is Mediated by J-Series Prostaglandins and Is Enhanced by Fatty Acid Amide Hydrolase (FAAH) Blockade. Mol. Carcinog. 2012, 51, 139–149. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, B.M.; Correia-da-Silva, G.; Teixeira, N.A. The Endocannabinoid Anandamide Induces Apoptosis of Rat Decidual Cells through a Mechanism Involving Ceramide Synthesis and P38 MAPK Activation. Apoptosis 2013, 18, 1526–1535. [Google Scholar] [CrossRef]
- Rajesh, M.; Mukhopadhyay, P.; Haskó, G.; Liaudet, L.; Mackie, K.; Pacher, P. Cannabinoid-1 Receptor Activation Induces Reactive Oxygen Species-Dependent and -Independent Mitogen-Activated Protein Kinase Activation and Cell Death in Human Coronary Artery Endothelial Cells. Br. J. Pharmacol. 2010, 160, 688–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlosser, M.; Löser, H.; Siegmund, S.V.; Montesinos-Rongen, M.; Bindila, L.; Lutz, B.; Barrett, D.A.; Sarmad, S.; Ortori, C.A.; Grau, V.; et al. The Endocannabinoid, Anandamide, Induces Cannabinoid Receptor-Independent Cell Death in Renal Proximal Tubule Cells. Cell Biol. 2017, 6, 35–55. [Google Scholar] [CrossRef] [Green Version]
- Pokrywka, M.; Góralska, J.; Solnica, B. Cannabinoids—A New Weapon against Cancer? Postepy Hig. Med. Dosw. (Online) 2016, 70, 1309–1320. [Google Scholar] [CrossRef]
- Salazar, M.; Carracedo, A.; Salanueva, Í.J.; Hernández-Tiedra, S.; Lorente, M.; Egia, A.; Vázquez, P.; Blázquez, C.; Torres, S.; García, S.; et al. Cannabinoid Action Induces Autophagy-Mediated Cell Death through Stimulation of ER Stress in Human Glioma Cells. J. Clin. Investig. 2009, 119, 1359–1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soliman, E.; Henderson, K.L.; Danell, A.S.; Van Dross, R. Arachidonoyl-Ethanolamide Activates Endoplasmic Reticulum Stress-Apoptosis in Tumorigenic Keratinocytes: Role of Cyclooxygenase-2 and Novel J-Series Prostamides. Mol. Carcinog. 2015, 55, 117–130. [Google Scholar] [CrossRef]
- Ansari, K.M.; Sung, Y.M.; He, G.; Fischer, S.M. Prostaglandin Receptor EP2 Is Responsible for Cyclooxygenase-2 Induction by Prostaglandin E 2 in Mouse Skin. Carcinogenesis 2007, 28, 2063–2068. [Google Scholar] [CrossRef] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójcik, P.; Biernacki, M.; Domian, N.; Žarković, N.; Skrzydlewska, E. Influence of Inhibition of COX-2-Dependent Lipid Metabolism on Regulation of UVB-Induced Keratinocytes Apoptosis by Cannabinoids. Biomolecules 2022, 12, 842. https://doi.org/10.3390/biom12060842
Wójcik P, Biernacki M, Domian N, Žarković N, Skrzydlewska E. Influence of Inhibition of COX-2-Dependent Lipid Metabolism on Regulation of UVB-Induced Keratinocytes Apoptosis by Cannabinoids. Biomolecules. 2022; 12(6):842. https://doi.org/10.3390/biom12060842
Chicago/Turabian StyleWójcik, Piotr, Michał Biernacki, Natalia Domian, Neven Žarković, and Elżbieta Skrzydlewska. 2022. "Influence of Inhibition of COX-2-Dependent Lipid Metabolism on Regulation of UVB-Induced Keratinocytes Apoptosis by Cannabinoids" Biomolecules 12, no. 6: 842. https://doi.org/10.3390/biom12060842
APA StyleWójcik, P., Biernacki, M., Domian, N., Žarković, N., & Skrzydlewska, E. (2022). Influence of Inhibition of COX-2-Dependent Lipid Metabolism on Regulation of UVB-Induced Keratinocytes Apoptosis by Cannabinoids. Biomolecules, 12(6), 842. https://doi.org/10.3390/biom12060842