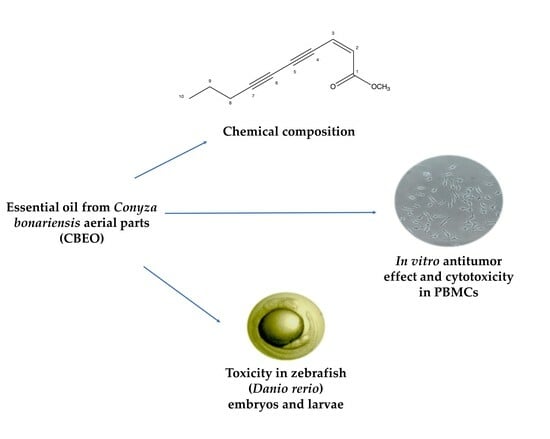
Chemical Composition, In Vitro Antitumor Effect, and Toxicity in Zebrafish of the Essential Oil from Conyza bonariensis (L.) Cronquist (Asteraceae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drugs and Reagents
2.2. Human Tumor Cell Lines
2.3. Human Peripheral Blood Mononuclear Cells (PBMC)
2.4. Zebrafish Embryos
2.5. Botanical Material and Essential Oil Extraction
2.6. Essential Oil Analysis
2.7. Cytotoxicity Assessment of CBEO in Human Cells
2.8. Quantification of Reactive Oxygen Species in Human Tumor Cells
2.9. Evaluation of CBEO Cytotoxicity in the Presence or Absence of N-acetylcysteine (NAC)
2.10. CBEO Toxicity in Zebrafish Model
2.10.1. Acute Toxicity Test Using Zebrafish Embryos
2.10.2. Oxidative Stress Biomarker Enzymes in Zebrafish Larvae
2.11. Statistical Analysis
3. Results
3.1. (Z)-2-lachnophyllum Ester Was the Major Compound in the Chemical Characterization of CBEO
3.2. CBEO Induces Cytotoxicity in Human Cell Lines
3.3. CBEO Induces Less Cytotoxicity in PBMC Cells Than Doxorubicin
3.4. CBEO Induces Oxidative Stress in SK-MEL-28 Cells
3.5. CBEO Cytotoxicity in SK-MEL-28 Cells Is ROS-Dependent
3.6. Embryotoxicity Induced by CBEO in Zebrafish Model
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz, A.; Barbosa, J.; Antunes, P.; Bonifácio, V.D.; Pinto, S.N. A Glimpse into dendrimers integration in cancer imaging and theranostics. Int. J. Mol. Sci. 2023, 24, 5430. [Google Scholar] [CrossRef] [PubMed]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.; Rodrigues, C.M.; Gaspar, M.M.; Reis, C.P. Melanoma management: From epidemiology to treatment and latest advances. Cancers 2022, 14, 4652. [Google Scholar] [CrossRef]
- Wang, L.; Li, P.; Feng, K. EGCG adjuvant chemotherapy: Current status and future perspectives. Eur. J. Med. Chem. 2023, 250, 115197. [Google Scholar] [CrossRef]
- Malyarenko, O.S.; Malyarenko, T.V.; Usoltseva, R.V.; Kicha, A.A.; Ivanchina, N.V.; Ermakova, S.P. Combined radiomodifying effect of fucoidan from the brown alga Saccharina cichorioides and pacificusoside D from the starfish Solaster pacificus in the model of 3D melanoma cells. Biomolecules 2023, 13, 419. [Google Scholar] [CrossRef]
- Rayan, A.; Raiyn, J.; Falah, M. Nature is the best source of anticancer drugs: Indexing natural products for their anticancer bioactivity. PLoS ONE 2017, 12, e0187925. [Google Scholar] [CrossRef]
- Felgueiras, H.P.; Homem, N.C.; Teixeira, M.A.; Ribeiro, A.R.M.; Antunes, J.C.; Amorim, M.T.P. Physical, thermal, and antibacterial effects of active essential oils with potential for biomedical applications loaded onto cellulose acetate/polycaprolactone wet-spun microfibers. Biomolecules 2020, 10, 1129. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.; Ntoutoume, G.M.-A.N.; Martin, P.; Morillo, M. Antibacterial activity and reversal of multidrug resistance of tumor cells by essential oils from fresh leaves, flowers, and stems of Montanoa quadrangularis Schultz Bipontinus (Asteraceae) collected in Mérida—Venezuela. Biomolecules 2021, 11, 605. [Google Scholar] [CrossRef]
- Yeh, T.-H.; Lin, J.-Y. Acorus gramineusand and Euodia ruticarpa steam distilled essential oils exert anti-inflammatory effects through decreasing Th1/Th2 and pro-/anti-inflammatory cytokine secretion ratios in vitro. Biomolecules 2020, 10, 338. [Google Scholar] [CrossRef]
- Pavithra, P.S.; Mehta, A.; Verma, R.S. Essential oils: From prevention to treatment of skin cancer. Drug Discov. Today 2019, 24, 644–655. [Google Scholar] [CrossRef]
- Hakkim, F.L.; Bakshi, H.A.; Khan, S.; Nasef, M.; Farzand, R.; Sam, S.; Rashan, L.; Al-Baloshi, M.S.; Anwar, S.S.; Hasson, A.; et al. Frankincense essential oil suppresses melanoma cancer through down regulation of Bcl-2/Bax cascade signaling and ameliorates heptotoxicity via phase I and II drug metabolizing enzymes. Oncotarget 2019, 10, 3472–3490. [Google Scholar] [CrossRef]
- Marques, M.P.; Neves, B.G.; Varela, C.; Zuzarte, M.; Gonçalves, A.C.; Dias, M.I.; Amaral, J.S.; Barros, L.; Magalhães, M.; Cabral, C. Essential oils from Côa Valley Lamiaceae species: Cytotoxicity and antiproliferative effect on glioblastoma cells. Pharmaceutics 2023, 15, 341. [Google Scholar] [CrossRef]
- Ghavam, M. Heracleum persicum Desf. ex Fisch., CA Mey. & Avé-Lall. fruit essential oil: Content, antimicrobial activity and cytotoxicity against ovarian cancer cell line. BMC Complement. Med. Ther. 2023, 23, 87. [Google Scholar]
- An, Z.; Feng, X.; Sun, M.; Wang, Y.; Wang, H.; Gong, Y. Chamomile essential oil: Chemical constituents and antitumor activity in MDA-MB-231 cells through PI3K/Akt/mTOR signaling pathway. Chem. Biodivers. 2023, 20, e202200523. [Google Scholar] [CrossRef]
- Cappelli, G.; Giovannini, D.; Vilardo, L.; Basso, A.; Iannetti, I.; Massa, M.; Ruberto, G.; Muir, R.; Pastore, C.; D’Agnano, I.; et al. Cinnamomum zeylanicum blume essential oil inhibits metastatic melanoma cell proliferation by triggering an incomplete tumour cell stress response. Int. J. Mol. Sci. 2023, 24, 5698. [Google Scholar] [CrossRef]
- Tohidi, B.; Rahimmalek, M.; Trindade, H. Review on essential oil, extracts composition, molecular and phytochemical properties of Thymus species in Iran. Ind. Crops Prod. 2019, 134, 89–99. [Google Scholar] [CrossRef]
- Baptista-Silva, S.; Borges, S.; Ramos, O.L.; Pintado, M.; Sarmento, B. The progress of essential oils as potential therapeutic agents: A review. J. Essent. Oil Res. 2020, 32, 279–295. [Google Scholar] [CrossRef]
- Opiyo, S.A.; Njoroge, P.W.; Kuria, K.M. Chemical composition and biological activity of extracts from Conyza species. J. Appl. Chem. 2023, 16, 61–71. [Google Scholar]
- Adande, K.; Eloh, K.; Simalou, O.; Bakaï, M.F.; Caboni, P. Chemical composition of different extracts of Conyza bonariensis: Insecticidal and nematicidal activities. Am. J. Anal. Chem. 2023, 14, 95–120. [Google Scholar] [CrossRef]
- Elgamal, A.M.; Ahmed, R.F.; Abd-ElGawad, A.M.; El Gendy, A.E.N.G.; Elshamy, A.I.; Nassar, M.I. Chemical profiles, anticancer, and anti-aging activities of essential oils of Pluchea dioscoridis (L.) DC. and Erigeron bonariensis L. Plants 2021, 10, 667. [Google Scholar] [CrossRef] [PubMed]
- Araujo, L.; Moujir, L.M.; Rojas, J.; Rojas, L.; Carmona, J.; Rondón, M. Chemical Composition and Biological Activity of Conyza bonariensis Essential Oil Collected in Mérida, Venezuela. Nat. Prod. Commun. 2013, 8, 1175–1178. [Google Scholar] [CrossRef]
- Piasecki, B.; Biernasiuk, A.; Skiba, A.; Skalicka-Woźniak, K.; Ludwiczuk, A. Composition, anti-MRSA activity and toxicity of essential oils from Cymbopogon species. Molecules 2021, 26, 7542. [Google Scholar] [CrossRef] [PubMed]
- Maia, J.G.S.; Silva, M.H.L.; Zoghbi, M.D.G.B.; Andrade, E.H.A. Composition of the essential oils of Conyza bonariensis (L.) Cronquist. J. Essent. Oil Res. 2020, 14, 325–326. [Google Scholar] [CrossRef]
- Santana, P.M.; Miranda, M.; Gutiérrez, Y.; García, G.; Orellana, T.; Orellana-Manzano, A. Anti-inflammatory and antimitotic effect of the alcoholic extract and chemical composition of the oil from Conyza bonariensis (L.) Cronquist (deer shinbone) leaves. Rev. Cuba. Plantas Med. 2011, 16, 13–23. [Google Scholar]
- Urdampilleta, J.D.; Amat, A.G.; Bidau, C.J.; Koslobsky, N.K. Biosystematic and chemosystematic studies in five South American species of Conyza (Asteraceae). Bol. Soc. Argent. Bot. 2005, 40, 2. [Google Scholar]
- Benzarti, A.; Hammami, S.; Piras, A.; Falconieri, D.; Elmokni, R.; M’henni, M.F.; Mighri, Z. Effects of different ecological conditions and extraction techniques on the quality of volatile oils from flaxleaf fleabane (Erigeron bonariensis L.). J. Med. Plant Res. 2013, 7, 3059–3065. [Google Scholar]
- Barbosa, L.C.; Paula, V.F.; Azevedo, A.S.; Silva, E.A.; Nascimento, E.A. Essential oil composition from some plant parts of Conyza bonariensis (L.) Cronquist. Flavour Fragr. J. 2005, 20, 39–41. [Google Scholar] [CrossRef]
- Mabrouk, S.; Elaissi, A.; Jannet, H.B.; Harzallah-Skhiri, F. Chemical composition of essential oils from leaves, stems, flower heads and roots of Conyza bonariensis L. from Tunisia. Nat. Prod. Res. 2011, 25, 77–84. [Google Scholar] [CrossRef]
- Lisboa, T.; Silva, D.; Duarte, S.; Ferreira, R.; Andrade, C.; Lopes, A.L.; Ribeiro, J.; Farias, D.; Moura, R.; Reis, M.; et al. Toxicity and antitumor activity of a thiophene–acridine hybrid. Molecules 2020, 25, 64. [Google Scholar] [CrossRef]
- National Health Surveillance Agency (ANVISA). Farmacopeia Brasileira, 6th ed.; National Health Surveillance Agency: Brasília, Brazil, 2019.
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Duarte, S.S.; Silva, D.K.F.; Lisboa, T.M.H.; Gouveia, R.G.; Andrade, C.C.N.; Sousa, V.M.; Ferreira, R.C.; Moura, R.O.; Gomes, J.N.S.; Silva, P.M.; et al. Apoptotic and antioxidant effects in HCT-116 colorectal carcinoma cells by a spiro-acridine compound, AMTAC-06. Pharmacol. Rep. 2022, 74, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Hasui, M.; Hirabayashi, Y.; Kobayashi, Y. Simultaneous measurement by flow cytometry of phagocytosis and hydrogen peroxide production of neutrophils in whole blood. J. Immunol. Methods 1989, 117, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Jávega, B.; Herrera, G.; Martínez-Romero, A.; O’Connor, J.E. Flow cytometry of oxygen and oxygen-related cellular stress. Oxygen 2023, 3, 222–255. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development (OECD). Guidelines for the Testing of Chemicals: Fish Embryo Acute Toxicity (FET); Test No. 236; OECD: Paris, France, 2013; pp. 1–22. [Google Scholar]
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University Press: Cambridge, UK, 1971. [Google Scholar]
- Martins, R.X.; Vieira, L.; Souza, J.A.C.R.; Silva, M.G.F.; Muniz, M.S.; Souza, T.; Queiroga, F.R.; Machado, M.R.F.; Silva, P.M.; Farias, D. Exposure to 2,4-D herbicide induces hepatotoxicity in zebrafish larvae. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 248, 109110. [Google Scholar] [CrossRef]
- Domingues, I.; Oliveira, R.; Lourenço, J.; Grisolia, C.K.; Mendo, S.; Soares, A.M.V.M. Biomarkers as a tool to assess effects of chromium (VI): Comparison of responses in zebrafish early life stages and adults. Comp. Biochem. 2010, 152, 338–345. [Google Scholar] [CrossRef]
- Sairazi, N.S.M.; Sirajudeen, K.N.S. Natural products and their bioactive compounds: Neuroprotective potentials against neurodegenerative diseases. Evid. Based Complement. Altern. Med. 2020, 2020, 6565396. [Google Scholar]
- Marino, S.; Ahmad, U.; Ferreira, M.I.; Alvino, A. Evaluation of the effect of irrigation on biometric growth, physiological response, and essential oil of Mentha spicata (L.). Water 2019, 11, 2264. [Google Scholar] [CrossRef]
- Stefanakis, M.K.; Papaioannou, C.; Lianopoulou, V.; Philotheou-Panou, E.; Giannakoula, A.E.; Lazari, D.M. Seasonal variation of aromatic plants under cultivation conditions. Plants 2022, 11, 2083. [Google Scholar] [CrossRef]
- Li, Y.; Zidorn, C. Seasonal variations of natural products in european herbs. J. Food Process Eng. 2022, 21, 1549–1575. [Google Scholar] [CrossRef]
- Amaral, W.; Deschamps, C.; Biasi, L.A.; Bizzo, H.R.; Machado, M.P.; Silva, L.E. Yield and chemical composition of the essential oil of species of the Asteraceae family from Atlantic Forest, South of Brazil. J. Essent. Oil Res. 2018, 30, 278–284. [Google Scholar] [CrossRef]
- Musembei, R.; Kiplimo, J.J. Chemical composition and antibacterial activity of essential oil from Kenyan Conyza bonariensis (L.) Cronquist. Sci. Lett. 2017, 5, 180–185. [Google Scholar]
- Hoi, T.M.; Huong, L.T.; Chinh, H.V.; Hau, D.V.; Satyal, P.; Tai, T.A.; Dai, D.N.; Hung, N.H.; Hien, V.T.; Setzer, W.N. Essential oil compositions of three invasive Conyza species collected in Vietnam and their larvicidal activities against Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus. Molecules 2020, 25, 4576. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, G.A.; Braga, S.D.P.; Albuquerque, T.M.R.; Tavares, J.F.; Vieira, W.A.D.S.; Câmara, M.P.S.; Souza, E.L. Antifungal effects of Conyza bonariensis (L.) Cronquist essential oil against pathogenic Colletotrichum musae and its incorporation in gum Arabic coating to reduce anthracnose development in banana during storage. J. Appl. Microbiol. 2021, 132, 547–561. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Prado, V.M.J.; Jesus, R.A.; Oliveira, J.M.A.; Blank, A.F.; Bezerra, D.P.; Soares, M.B.P.; Silva, V.R.; Santos, L.S.; Cardoso, C.L.; Vilela, A.F.L.; et al. Biological studies and chromatograms aided by chemometric analysis in evaluation of seasonality and extraction method of Croton grewioides extracts. Rev. Bras. Bot. 2022, 45, 607–618. [Google Scholar] [CrossRef]
- Lis, A.; Mielczarek, J.; Kalemba, D.; Nazaruk, J. Chemical composition of the essential oil from the herb of Erigeron annuus (L.). Pers. J. Essent. Oil Res. 2008, 20, 229–232. [Google Scholar] [CrossRef]
- Nazaruk, J.; Kalemba, D. Chemical composition of the essential oils from the roots of Erigeron acris L. and Erigeron annuus (L.) Pers. Molecules 2009, 14, 2458–2465. [Google Scholar] [CrossRef]
- Minteguiaga, M.; Umpierrez, N.; González, A.; Dellacassa, E.; Catalán, C.A. New C9-polyacetylenes from the essential oil of the highly endangered species Baccharis palustris Heering (Asteraceae). Phytochem. Lett. 2022, 48, 106–113. [Google Scholar] [CrossRef]
- Tzakou, O.; Vagias, C.; Gani, A.; Yannitsaros, A. Volatile constituents of essential oils isolated at different growth stages from three Conyza species growing in Greece. Flavour Fragr. J. 2005, 20, 425–428. [Google Scholar] [CrossRef]
- Sharaf, S.; Ziaee, A.; Dahmardeh, H. What are the outcomes of hospice care for cancer patients? A systematic review. Support. Cancer Ther. 2023, 31, 64. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, Y.; Zhu, Y.; Zhou, B.; Ren, C.; Liang, S.; Guo, Y. α-Pinene induces apoptotic cell death via caspase activation in human ovarian cancer cells. Med. Sci. Monit. 2019, 25, 6631. [Google Scholar] [CrossRef]
- Machado, T.Q.; Felisberto, J.R.S.; Guimarães, E.F.; Queiroz, G.A.D.; Fonseca, A.C.C.D.; Ramos, Y.J.; Robbs, B.K. Apoptotic effect of β-pinene on oral squamous cell carcinoma as one of the major compounds from essential oil of medicinal plant Piper rivinoides Kunth. Nat. Prod. Res. 2022, 36, 1636–1640. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Wang, Y.U.; Leng, Q.; Sun, Y.U.; Hoffman, R.M.; Jin, H. The anti-oxidant monoterpene p-cymene reduced the occurrence of colorectal cancer in a hyperlipidemia rat model by reducing oxidative stress and expression of inflammatory cytokines. Anticancer Res. 2021, 41, 1213–1218. [Google Scholar] [CrossRef]
- Ye, Z.; Liang, Z.; Mi, Q.; Guo, Y. Limonene terpenoid obstructs human bladder cancer cell (T24 cell line) growth by inducing cellular apoptosis, caspase activation, G2/M phase cell cycle arrest and stops cancer metastasis. J. BUON 2020, 25, 280–285. [Google Scholar]
- Mandal, D.; Patel, P.; Verma, S.K.; Sahu, B.R.; Parija, T. Proximal discrepancy in intrinsic atomic interaction arrests G2/M phase by inhibiting Cyclin B1/CDK1 to infer molecular and cellular biocompatibility of d-limonene. Sci. Rep. 2022, 12, 18184. [Google Scholar] [CrossRef]
- Cao, W.; Tian, R.; Pan, R.; Sun, B.; Xiao, C.; Chen, Y.; Lei, S. Terpinen-4-ol inhibits the proliferation and mobility of pancreatic cancer cells by downregulating Rho-associated coiled-coil containing protein kinase 2. Bioengineered 2022, 13, 8643–8656. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Murata, S.; Ito, H.; Iwasaki, K.; Villareal, M.O.; Zheng, Y.W.; Ohkohchi, N. Terpinen-4-ol inhibits colorectal cancer growth via reactive oxygen species. Oncol. Lett. 2017, 14, 2015–2024. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wen, J.M.; Du, C.J.; Hu, S.M.; Chen, J.X.; Zhang, S.G.; Ding, K.F. Thymol inhibits bladder cancer cell proliferation via inducing cell cycle arrest and apoptosis. Biochem. Biophys. Res. Commun. 2017, 491, 530–536. [Google Scholar] [CrossRef]
- Elbe, H.; Yigitturk, G.; Cavusoglu, T.; Uyanikgil, Y.; Ozturk, F. Apoptotic effects of thymol, a novel monoterpene phenol, on different types of cancer. Bratisl. Lek. Listy 2020, 121, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Moradipour, A.; Dariushnejad, H.; Ahmadizadeh, C.; Lashgarian, H.E. Dietary flavonoid carvacrol triggers the apoptosis of human breast cancer MCF-7 cells via the p53/Bax/Bcl-2 axis. Med. Oncol. 2022, 40, 46. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yuan, J.; Hao, J.; Wen, Y.; Lv, Y.; Chen, L.; Yang, X. α-Humulene inhibits hepatocellular carcinoma cell proliferation and induces apoptosis through the inhibition of Akt signaling. Food Chem. Toxicol. 2019, 134, 110830. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Prasad, S.; Yuan, W.; Li, S.; Aggarwal, B.B. Identification of a novel compound (β-sesquiphellandrene) from turmeric (Curcuma longa) with anticancer potential: Comparison with curcumin. Investig. New Drugs 2015, 33, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Xiu, Z.; Zhu, Y.; Han, J.; Li, Y.; Yang, X.; Yang, G.; Jin, N. Caryophyllene oxide induces ferritinophagy by regulating the NCOA4/FTH1/LC3 pathway in hepatocellular carcinoma. Front. Pharmacol. 2022, 13, 930958. [Google Scholar] [CrossRef]
- Jo, H.W.; Kim, M.M. β-Caryophyllene oxide inhibits metastasis by downregulating MMP-2, p-p38 and p-ERK in human fibrosarcoma cells. J. Food Biochem. 2022, 46, e14468. [Google Scholar] [CrossRef]
- Delgado, C.; Mendez-Callejas, G.; Celis, C. Caryophyllene oxide, the active compound isolated from leaves of Hymenaea courbaril L. (Fabaceae) with antiproliferative and apoptotic effects on PC-3 androgen-independent prostate cancer cell line. Molecules 2021, 26, 6142. [Google Scholar] [CrossRef]
- Satyal, P.; Chhetri, B.K.; Dosoky, N.S.; Shrestha, S.; Poudel, A.; Setzer, W.N. Chemical composition of Blumea lacera essential oil from Nepal. Biological activities of the essential oil and (Z)-lachnophyllum ester. Nat. Prod. Commun. 2015, 10, 1934578X1501001028. [Google Scholar] [CrossRef]
- Sobrinho, A.C.N.; Fontenelle, R.O.D.S.; Souza, E.B.D.; Morais, S.M.D. Antifungal and antioxidant effect of the lachnophyllum ester, isolated from the essential oil of Baccharis trinervis (Lam.) Pers., against dermatophytes fungi. Rev. Bras. Saúde Prod. Anim. 2022, 22, 1–11. [Google Scholar] [CrossRef]
- Kumara, V.; Mathelaa, C.S.; Tewaria, G.; Pandeyb, A.K. Biopesticide potential of (7R)-trans, trans-nepetalactone and cis-lachnophyllum ester in control of mustard aphid, Lipaphis erysimi (Kalt.). J. Teknol. 2015, 77, 19–24. [Google Scholar] [CrossRef]
- Kimura, Y.; Mori, M.; Suzuki, A.; Kobayashi, A. Isolation and identification of two nematicidal substances from roots of Erigeron philadelphicus L. and nematicidal activities of their related compounds. Agric. Biol. Chem. 1981, 45, 2915–2917. [Google Scholar] [CrossRef]
- Van der Zanden, S.; Qiao, X.; Neefjes, J. New insights into the activities and toxicities of the old anticancer drug doxorubicin. FEBS J. 2021, 288, 6095–6111. [Google Scholar] [CrossRef] [PubMed]
- Ihsan, S.A.; Wang, M.; Zaki, A.A.; Khan, S.I.; Khan, I.A. Chemical analysis and biological activities of Salvia lavandulifolia Vahl. essential oil. Chem. Anal. 2017, 7, 71–78. [Google Scholar]
- Aranha, E.S.P.; Azevedo, S.G.; Reis, G.G.; Lima, E.S.; Machado, M.B.; Vasconcellos, M.C. Essential oils from Eugenia spp.: In vitro antiproliferative potential with inhibitory action of metalloproteinases. Ind. Crops Prod. 2019, 141, 111736. [Google Scholar] [CrossRef]
- Sousa, M.H.; Morgan, J.M.; Cesca, K.; Flach, A.; Moura, N.F. Cytotoxic activity of Cunila angustifolia essential oil. Chem. Biodivers. 2020, 17, e1900656. [Google Scholar] [CrossRef] [PubMed]
- Tabanca, N.; Nalbantsoy, A.; Kendra, P.E.; Demirci, F.; Demirci, B. Chemical characterization and biological activity of the mastic gum essential oils of Pistacia lentiscus var. chia from Turkey. Molecules 2020, 25, 2136. [Google Scholar] [CrossRef]
- Salama, Y.; Jaradat, N.; Hattori, K.; Heissig, B. Aloysia citrodora essential oil inhibits melanoma cell growth and migration by targeting HB-EGF-EGFR signaling. Int. J. Mol. Sci. 2021, 22, 8151. [Google Scholar] [CrossRef]
- Abarca, J.M.H.; Chávez, A.J.P. Malignant nail melanoma in a case report. J. Pharm. Negat. 2023, 14, 67–72. [Google Scholar]
- Arnold, M.; Singh, D.; Laversanne, M.; Vignat, J.; Vaccarella, S.; Meheus, F.; Cust, A.E.; Vries, E.; Whiteman, D.C.; Bray, F. Global burden of cutaneous melanoma in 2020 and projections to 2040. JAMA Dermatol. 2022, 158, 495–503. [Google Scholar] [CrossRef]
- Sangthong, S.; Promputtha, I.; Pintathong, P.; Chaiwut, P. Chemical constituents, antioxidant, anti-tyrosinase, cytotoxicity, and anti-melanogenesis activities of Etlingera elatior (Jack) leaf essential oils. Molecules 2022, 27, 3469. [Google Scholar] [CrossRef]
- Azadi, S.; Osanloo, M.; Zarenezhad, E.; Farjam, M.; Jalali, A.; Ghanbariasad, A. Nano-scaled emulsion and nanogel containing Mentha pulegium essential oil: Cytotoxicity on human melanoma cells and effects on apoptosis regulator genes. BMC Complement. Med. Ther. 2023, 23, 6. [Google Scholar] [CrossRef]
- Betsou, F.; Gaignaux, A.; Ammerlaan, W.; Norris, P.J.; Stone, M. Biospecimen science of blood for peripheral blood mononuclear cell (PBMC) functional applications. Curr. Pathobiol. Rep. 2019, 7, 17–27. [Google Scholar] [CrossRef]
- Hołota, M.; Magiera, J.; Michlewska, S.; Kubczak, M.; Olmo, N.S.; García-Gallego, S.; Ortega, P.; Mata, F.J.; Ionov, M.; Bryszewska, M. In vitro anticancer properties of copper metallodendrimers. Biomolecules 2019, 9, 155. [Google Scholar] [CrossRef]
- Ciftci, H.; Sever, B.; Bayrak, N.; Yıldız, M.; Yıldırım, H.; Tateishi, H.; Otsuka, M.; Fujita, M.; TuYuN, A.F. In vitro cytotoxicity evaluation of plastoquinone analogues against colorectal and breast cancers along with in silico insights. Pharmaceuticals 2022, 15, 1266. [Google Scholar] [CrossRef] [PubMed]
- Mektrirat, R.; Yano, T.; Okonogi, S.; Katip, W.; Pikulkaew, S. Phytochemical and safety evaluations of volatile terpenoids from Zingiber cassumunar Roxb. on mature carp peripheral blood mononuclear cells and embryonic zebrafish. Molecules 2020, 25, 613. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.O.; Orlando, J.B.; Pires, C.L.; Hiruma-Lima, C.A.; Gaivão, I.M.; Perazzo, F.F.; Maistro, E.L. Genotoxicity induced by nerol, an essential oil present in citric plants using human peripheral blood mononuclear cells (PBMC) and HepG2/C3A cells as a model. J. Toxicol. Environ. Health 2021, 84, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Yazdanian, M.; Rostamzadeh, P.; Alam, M.; Abbasi, K.; Tahmasebi, E.; Tebyaniyan, H.; Ranjbar, R.; Seifalian, A.; Moghaddam, M.M.; Kahnamoei, M.B. Evaluation of antimicrobial and cytotoxic effects of Echinacea and Arctium extracts and Zataria essential oil. AMB Express 2022, 12, 75. [Google Scholar] [CrossRef] [PubMed]
- Muhamad, N.; Plengsuriyakarn, T.; Na-Bangchang, K. Application of active targeting nanoparticle delivery system for chemotherapeutic drugs and traditional/herbal medicines in cancer therapy: A systematic review. Int. J. Nanomed. 2018, 13, 3921–3935. [Google Scholar] [CrossRef]
- Hadiman, J.; Susanah, S.; Sugianli, A.K. Prevalence of hematotoxic effect of intravenous chemotherapy among retinoblastoma population in tertiary hospital in Bandung, Indonesia. Int. J. Integr. Health Sci. 2019, 7, 34–38. [Google Scholar]
- Stepanov, I.A.; Shameeva, M.A.; Kruchinin, D.B.; Byvaltsev, V.A.; Shagdurova, I.A. Hematotoxic adverse drug reactions associated with vascular endothelial growth factor inhibitors and cytotoxic drugs in the treatment of glioblastoma: A systematic review. Sib. J. Oncol. 2020, 5, 121–130. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, Q.; Han, Y.; Huang, Z.; Chai, Z.; Liu, J.; Wang, J.; Sun, Z.; Zhao, T.; Wang, G.; et al. VHL expression level in the pathological tissue is significantly associated with hematotoxicity of platinum-based chemotherapy in non-small cell lung cancer patients. Res. Sq. 2021, 2021, 1–14. [Google Scholar]
- Gasmi, S.; Benaicha, B.; Rouabhi, R.; Kebieche, M. Hematotoxicity resulting from chemotherapy in patients with breast cancer in eastern Algeria. Ann. Rom. Soc. Cell Biol. 2021, 25, 20308–20319. [Google Scholar]
- Costa, E.V.; Souza, C.A.S.; Galvão, A.F.C.; Silva, V.R.; Santos, L.S.; Dias, R.B.; Rocha, C.A.G.; Soares, M.B.P.; Silva, F.M.A.; Koolen, H.H.F.; et al. Duguetia pycnastera Sandwith (Annonaceae) leaf essential oil inhibits HepG2 cell growth in vitro and in vivo. Molecules 2022, 27, 5664. [Google Scholar] [CrossRef]
- Khakzad, S.; Rahmani, F.; Hojjati, M.; Tabandeh, M.R. Anti-carcinogenic effects of Satureja khuzistanica and Zataria multiflora essential oils on K562 cell line proliferation. J. Food Process Eng. 2019, 2, 127–132. [Google Scholar]
- Quintans, J.S.S.; Soares, B.M.; Ferraz, R.P.C.; Oliveira, A.C.A.; Silva, T.B.; Menezes, L.R.A.; Sampaio, M.F.C.; Prata, A.P.N.; Moraes, M.O.; Pessoa, C.; et al. Chemical constituents and anticancer effects of the essential oil from leaves of Xylopia laevigata. Planta Med. 2013, 79, 123–130. [Google Scholar] [CrossRef]
- Sahasrabudhe, S.A.; Terluk, M.R.; Kartha, R.V. N-acetylcysteine pharmacology and applications in rare diseases—Repurposing an old antioxidant. Antioxidants 2023, 12, 1316. [Google Scholar] [CrossRef]
- Thalappil, M.A.; Butturini, E.; Prati, A.C.; Bettin, I.; Antonini, L.; Sapienza, F.U.; Garzoli, S.; Ragno, R.; Mariotto, S. Pinus mugo essential oil impairs STAT3 activation through oxidative stress and induces apoptosis in prostate cancer cells. Molecules 2022, 27, 4834. [Google Scholar] [CrossRef] [PubMed]
- Catalani, S.; Palma, F.; Battistelli, S.; Benedetti, S. Oxidative stress and apoptosis induction in human thyroid carcinoma cells exposed to the essential oil from Pistacia lentiscus aerial parts. PLoS ONE 2017, 12, e0172138. [Google Scholar] [CrossRef]
- Poma, P.; Labbozzetta, M.; Notarbartolo, M.; Bruno, M.; Maggio, A.; Rosselli, S.; Sajeva, M.; Zito, P. Chemical composition, in vitro antitumor and pro-oxidant activities of Glandora rosmarinifolia (Boraginaceae) essential oil. PLoS ONE 2018, 13, e0196947. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y.; Shi, J. Reactive oxygen species (ROS)-based nanomedicine. Chem. Rev. 2019, 119, 4881–4985. [Google Scholar] [CrossRef]
- Sajadimajd, S.; Khazaei, M. Oxidative stress and cancer: The role of Nrf2. Curr. Cancer Drug Targets 2018, 18, 538–557. [Google Scholar] [CrossRef]
- Huang, Y.-J.; Nan, G.-X. Oxidative stress-induced angiogenesis. J. Clin. Neurosci. 2019, 63, 13–16. [Google Scholar] [CrossRef]
- Kirtonia, A.; Sethi, G.; Garg, M. The multifaceted role of reactive oxygen species in tumorigenesis. Cell. Mol. Life Sci. 2020, 77, 4459–4483. [Google Scholar] [CrossRef] [PubMed]
- Davalli, P.; Marverti, G.; Lauriola, A.; D’arca, D. Targeting oxidatively induced DNA damage response in cancer: Opportunities for novel cancer therapies. Oxid. Med. Cell. Longev. 2018, 2018, 2389523. [Google Scholar] [CrossRef]
- Albasher, G.; Alkahtane, A.A.; Saud Alarifi, D.A.; Alessia, M.S.; Almeer, R.S.; Abdel-Daim, M.M.; Al-Sultan, N.K.; Al-Qahtani, A.A.; Ali, H.; Alkahtani, S. Methotrexate-induced apoptosis in human ovarian adenocarcinoma SKOV-3 cells via ROS-mediated bax/bcl-2-cyt-c release cascading. OncoTargets Ther. 2019, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Chen, Y.; Putluri, N.; Coarfa, C.; Robertson, M.J.; Putluri, V.; Stossi, F.; Dubrulle, J.; Mancini, M.A.; Pang, J.C.; et al. Acquisition of cisplatin resistance shifts head and neck squamous cell carcinoma metabolism toward neutralization of oxidative stress. Cancers 2020, 12, 1670. [Google Scholar] [CrossRef]
- Sinha, B.K.; Tokar, E.J.; Bushel, P.R. Elucidation of mechanisms of topotecan-induced cell death in human breast MCF-7 cancer cells by gene expression analysis. Front. Genet. 2020, 11, 775. [Google Scholar] [CrossRef]
- Vo, A.H.; Van Vleet, T.R.; Gupta, R.R.; Liguori, M.J.; Rao, M.S. An overview of machine learning and big data for drug toxicity evaluation. Chem. Res. Toxicol. 2019, 33, 20–37. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Patton, E.E.; Zon, L.I.; Langenau, D.M. Zebrafish disease models in drug discovery: From preclinical modelling to clinical trials. Nat. Rev. Drug Discov. 2021, 20, 611–628. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.; Maity, S.; Singh, M.; Saraf, S.A.; Saha, S. Phytochemistry, pharmacology and toxicology of Spilanthes acmella: A review. Adv. Pharmacol. Sci. 2013, 2013, 423750. [Google Scholar]
- Nishimura, Y.; Inoue, A.; Sasagawa, S.; Koiwa, J.; Kawaguchi, K.; Kawase, R.; Maruyama, T.; Kim, S.; Tanaka, T. Using zebrafish in systems toxicology for developmental toxicity testing. Congenit. Anom. 2016, 56, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.J.; Li, H.; Wu, D.T.; Zhuang, Q.G.; Li, H.B.; Geng, F.; Gan, R.Y. Recent development in zebrafish model for bioactivity and safety evaluation of natural products. Crit. Rev. Food Sci. Nutr. 2022, 62, 8646–8674. [Google Scholar] [CrossRef] [PubMed]
- Akermi, S.; Smaoui, S.; Elhadef, K.; Fourati, M.; Louhichi, N.; Chaari, M.; Mtibaa, A.C.; Baanannou, A.; Masmoudi, S.; Mellouli, L. Cupressus sempervirens Essential Oil: Exploring the Antibacterial Multitarget Mechanisms, Chemcomputational Toxicity Prediction, and Safety Assessment in Zebrafish Embryos. Molecules 2022, 27, 2630. [Google Scholar] [CrossRef]
- Thitinarongwate, W.; Mektrirat, R.; Nimlamool, W.; Khonsung, P.; Pikulkaew, S.; Okonogi, S.; Kunanusorn, P. Phytochemical and safety evaluations of Zingiber ottensii Valeton essential oil in zebrafish embryos and rats. Toxics 2021, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- He, Y.L.; Shi, J.Y.; Peng, C.; Hu, L.J.; Liu, J.; Zhou, Q.M.; Guo, L.; Xiong, L. Angiogenic effect of motherwort (Leonurus japonicus) alkaloids and toxicity of motherwort essential oil on zebrafish embryos. Fitoterapia 2018, 128, 36–42. [Google Scholar] [CrossRef]
- Asghar, A.; Yousuf, M.; Fareed, G.; Nazir, R.; Hassan, A.; Maalik, A.; Noor, T.; Iqbal, N.; Rasheed, L. Synthesis, acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) activities, and molecular docking studies of a novel compound based on combination of flurbiprofen and isoniazide. RSC Adv. 2020, 10, 19346–19352. [Google Scholar] [CrossRef]
- Haridevamuthu, B.; Guru, A.; Murugan, R.; Sudhakaran, G.; Pachaiappan, R.; Almutairi, M.H.; Juliet, A.; Arockiaraj, J. Neuroprotective effect of biochanin a against bisphenol A-induced prenatal neurotoxicity in zebrafish by modulating oxidative stress and locomotory defects. Neurosci. Lett. 2022, 790, 136889. [Google Scholar] [CrossRef]
- Pullaguri, N.; Nema, S.; Bhargava, Y.; Bhargava, A. Triclosan alters adult zebrafish behavior and targets acetylcholinesterase activity and expression. Environ. Toxicol. Pharmacol. 2019, 75, 103311. [Google Scholar] [CrossRef]
- Loughland, I.; Lau, G.Y.; Jolly, J.; Seebacher, F. Rates of warming impact oxidative stress in zebrafish (Danio rerio). J. Exp. Biol. 2022, 225, jeb243740. [Google Scholar] [CrossRef]
- Félix, L.M.; Luzio, A.; Antunes, L.; Coimbra, A.M.; Valentim, A.M. Malformations and mortality in zebrafish early stages associated with elevated caspase activity after 24 h exposure to MS-222. Toxicol. Appl. Pharmacol. 2021, 412, 115385. [Google Scholar] [CrossRef]
- Félix, L.; Carreira, P.; Peixoto, F. Effects of chronic exposure of naturally weathered microplastics on oxidative stress level, behaviour, and mitochondrial function of adult zebrafish (Danio rerio). Chemosphere 2023, 310, 136895. [Google Scholar] [CrossRef] [PubMed]






| Compound | Area (%) | Rt a (min) | RI b Kovats (Calculated) | RI b Kovats (Literature) |
|---|---|---|---|---|
| α-thujene | 0.03 | 6.320 | 928.792 | 925.0 |
| (-)-α-pinene | 0.66 | 6.458 | 937.300 | 937.0 |
| Sabinene | 0.74 | 7.131 | 978.792 | 972.0 |
| β-pinene | 1.70 | 7.209 | 983.600 | 976.0 |
| Mircene | 0.51 | 7.376 | 993.896 | 993.0 |
| p-cymene | 0.08 | 7.990 | 1032.198 | 1024.0 |
| Limonene | 14.26 | 8.062 | 1036.699 | 1038.0 |
| (E)-β-ocimene | 0.04 | 8.160 | 1042.826 | 1047.0 |
| (Z)-β-ocimene | 1.32 | 8.333 | 1053.642 | 1036.0 |
| Terpinen-4-ol | 0.11 | 10.500 | 1189.122 | 1177.0 |
| (E,E)-2,6-dimethyl-3,5,7-octatriene-2-ol | 0.16 | 10.873 | 1214.039 | 1209.2 |
| Thymol | 0.24 | 12.134 | 1302.998 | 1297.0 |
| Carvacrol | 0.61 | 12.282 | 1302.114 | 1300.0 |
| (E)-caryophyllene | 4.19 | 14.046 | 1442.840 | 1433.0 |
| (E)-α-bergamotene | 0.64 | 14.144 | 1450.658 | 1434.0 |
| (E)-β-farnesene | 0.75 | 14.316 | 1464.380 | 1446.0 |
| (+)-β-funebrene | 0.20 | 14.388 | 1470.124 | 1415.0 |
| α-humulene | 0.41 | 14.495 | 1478.660 | 1459.0 |
| 1-(1,5-dimethyl-4-hexenyl)-4-methylbenzene | 0.38 | 14.730 | 1497.407 | 1484.0 |
| Germacrene-D | 0.78 | 14.835 | 1505.784 | 1519.0 |
| (Z)-2-lachnophyllum ester | 57.24 | 15.105 | 1527.323 | 1512.0 |
| β-sesquiphellandrene | 7.04 | 15.265 | 1540.088 | 1525.0 |
| (E)-nerolidol | 0.68 | 15.678 | 1573.036 | 1565.0 |
| Germacrene-B | 0.34 | 15.827 | 1584.922 | 1566.0 |
| Spathulenol | 1.67 | 16.065 | 1604.363 | 1605.0 |
| Caryophyllene oxide | 1.22 | 16.158 | 1612.645 | 1613.0 |
| Isospathulenol | 0.41 | 16.758 | 1666.073 | 1666.0 |
| Cadin-4-en-10-ol | 0.33 | 16.937 | 1682.012 | 1673.0 |
| Neophytadiene | 0.21 | 18.698 | 1839.421 | 1849.0 |
| Total | 96.95% |
| Cell Lines a | IC50 b | SI c | ||
|---|---|---|---|---|
| CBEO (µg/mL) | DXR (µM) | CBEO | DXR | |
| SK-MEL-28 | 18.65 ± 1.16 | 3.55 ± 1.67 | 3.03 | 0.08 |
| HeLa | 30.34 ± 1.08 | 3.80 ± 1.10 | 1.86 | 0.07 |
| HCT-116 | 31.28 ± 1.16 | 2.57 ± 0.001 | 1.81 | 0.11 |
| HL-60 | 32.20 ± 1.10 | 0.22 ± 0.001 | 1.75 | 1.27 |
| K562 | 32.13 ± 1.09 | 0.71 ± 1.13 | 1.76 | 0.39 |
| HaCaT | 56.49 ± 1.03 | 0.28 ± 0.001 | - | - |
| Embryotoxicological Endpoints | NOAEL a | LOAEL b | EC50 c |
|---|---|---|---|
| Eye malformation | n.e. † | n.e. † | n.e. † |
| Otolith malformation | n.e. † | n.e. † | n.e. † |
| Mouth malformation | n.e. † | n.e. † | n.e. † |
| Spine malformation | n.e. † | n.e. † | n.e. † |
| Body pigmentation | n.e. † | n.e. † | n.e. † |
| Hatching delay | 0.5 | n.e. † | 0.99 (0.69–1.42) * |
| Yolk sac edema | 1.0 | 0.75 | n.e. † |
| Pericardial edema | 1.0 | 0.75 | 1.36 (1.10–1.70) * |
| Head edema | n.e. † | n.e. † | n.e. † |
| Blood clotting | 1.0 | 0.75 | n.e. † |
| Undersize | n.e. † | n.e. † | n.e. † |
| Mortality (LC50) d | - | - | 1.20 (1.12–1.3) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, R.C.; do Nascimento, Y.M.; de Araújo Loureiro, P.B.; Martins, R.X.; de Souza Maia, M.E.; Farias, D.F.; Tavares, J.F.; Gonçalves, J.C.R.; da Silva, M.S.; Sobral, M.V. Chemical Composition, In Vitro Antitumor Effect, and Toxicity in Zebrafish of the Essential Oil from Conyza bonariensis (L.) Cronquist (Asteraceae). Biomolecules 2023, 13, 1439. https://doi.org/10.3390/biom13101439
Ferreira RC, do Nascimento YM, de Araújo Loureiro PB, Martins RX, de Souza Maia ME, Farias DF, Tavares JF, Gonçalves JCR, da Silva MS, Sobral MV. Chemical Composition, In Vitro Antitumor Effect, and Toxicity in Zebrafish of the Essential Oil from Conyza bonariensis (L.) Cronquist (Asteraceae). Biomolecules. 2023; 13(10):1439. https://doi.org/10.3390/biom13101439
Chicago/Turabian StyleFerreira, Rafael Carlos, Yuri Mangueira do Nascimento, Paulo Bruno de Araújo Loureiro, Rafael Xavier Martins, Maria Eduarda de Souza Maia, Davi Felipe Farias, Josean Fechine Tavares, Juan Carlos Ramos Gonçalves, Marcelo Sobral da Silva, and Marianna Vieira Sobral. 2023. "Chemical Composition, In Vitro Antitumor Effect, and Toxicity in Zebrafish of the Essential Oil from Conyza bonariensis (L.) Cronquist (Asteraceae)" Biomolecules 13, no. 10: 1439. https://doi.org/10.3390/biom13101439
APA StyleFerreira, R. C., do Nascimento, Y. M., de Araújo Loureiro, P. B., Martins, R. X., de Souza Maia, M. E., Farias, D. F., Tavares, J. F., Gonçalves, J. C. R., da Silva, M. S., & Sobral, M. V. (2023). Chemical Composition, In Vitro Antitumor Effect, and Toxicity in Zebrafish of the Essential Oil from Conyza bonariensis (L.) Cronquist (Asteraceae). Biomolecules, 13(10), 1439. https://doi.org/10.3390/biom13101439