Bacillus Calmette–Guérin Vaccine Attenuates Haloperidol-Induced TD-like Behavioral and Neurochemical Alteration in Experimental Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Drug and Chemicals
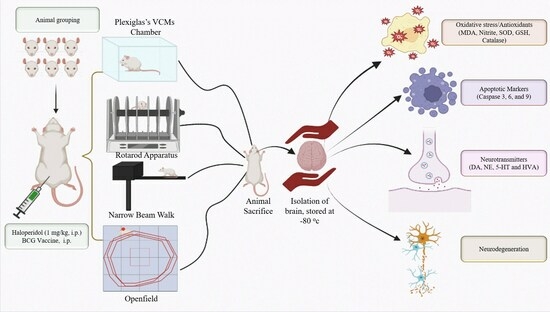
2.3. Protocol Schedule
2.4. Experimental Groups
2.5. Behavioral Assessments
2.5.1. Assessment of Orofacial Movement
2.5.2. Rotarod Activity
2.5.3. Narrow Beam Walk Test (NBW)
2.5.4. Open Field Test (OFT)
2.6. Dissection and Homogenization
2.7. Biochemical Assessment
2.7.1. Estimation of Malondialdehyde (MDA)
2.7.2. Estimation of Nitrite
2.7.3. Estimation of Reduced Glutathione (GSH)
2.7.4. Estimation of Superoxide Dismutase (SOD)
2.7.5. Estimation of Catalase
2.7.6. Protein Estimation
2.7.7. Estimation of IL-6, Cas-3, Cas-6, and Cas-9 Levels
2.8. Neurochemical Analysis
2.9. Statistical Analysis
3. Results
3.1. Effect of BCG Vaccine on VCMs, TPs, and FJs in Haloperidol-Induced TD in Rats
3.2. Effect of BCG Vaccine on Rotarod and NBW Activity in Haloperidol-Induced TD Rats
3.3. Effect of BCG Vaccine on Locomotor Activity in Haloperidol-Induced TD Rats
3.4. Effect of BCG Vaccine on Oxidative Stress Markers in Haloperidol-Induced TD Rats
3.5. Effect of BCG Vaccine on Antioxidants (GSH, SOD, and Catalase) in Haloperidol-Induced TD Rats
3.6. Effect of BCG Vaccine on Apoptotic Markers (Cas-3, Cas-6, and Cas-9) and Inflammatory Cytokines (IL-6) in Haloperidol-Induced TD Rats
3.7. Effect of BCG Vaccine on Haloperidol-Induced TD Alterations in Neurotransmitters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT | Serotonin |
| BCG | Bacillus Calmette–Guérin |
| DA | Dopamine |
| ELISA | Enzyme-linked immune assay |
| GABA | Gama amino butyric acid |
| GSH | Glutathione |
| HVA | Homovanillic acid |
| IL | Interleukin |
| NE | Norepinephrine |
| PD | Parkinson’s disease |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| TD | Tardive dyskinesia |
| TNF | Tumor necrosis factor |
| TPs | Tongue protrusion |
| VCMs | Vacuole chewing movements |
References
- Wang, M.-H.; Yang, C.-C.; Tseng, H.-C.; Fang, C.-H.; Lin, Y.-W.; Soung, H.-S. Naringin ameliorates haloperidol-induced neurotoxicity and orofacial dyskinesia in a rat model of human tardive dyskinesia. Neurotox. Res. 2021, 39, 774–786. [Google Scholar] [CrossRef]
- Sonego, A.B.; Prado, D.d.S.; Guimarães, F.S. PPARγ receptors are involved in the effects of cannabidiol on orofacial dyskinesia and cognitive dysfunction induced by typical antipsychotic in mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 111, 110367. [Google Scholar] [CrossRef]
- Röpke, J.; Busanello, A.; Leal, C.Q.; Reis, E.d.M.; de Freitas, C.M.; Villarinho, J.G.; Figueira, F.H.; Mello, C.F.; Ferreira, J.; Fachinetto, R. Anandamide attenuates haloperidol-induced vacuous chewing movements in rats. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2014, 54, 195–199. [Google Scholar] [CrossRef]
- Kadir, A.; Singh, J.; Rahi, V.; Kumar, P. Berberine ameliorate haloperidol and 3-Nitropropionic acid-induced neurotoxicity in rats. Neurochem. Res. 2022, 47, 3285–3297. [Google Scholar] [CrossRef]
- Jain, R.; Correll, C.U. Tardive Dyskinesia: Recognition, Patient Assessment, and Differential Diagnosis. J. Clin. Psychiatry 2018, 79, 16–23. [Google Scholar] [CrossRef]
- Jackson, R.; Brams, M.N.; Citrome, L.; Hoberg, A.R.; Isaacson, S.H.; Kane, J.M.; Kumar, R. Assessment of the impact of tardive dyskinesia in clinical practice: Consensus panel recommendations. Neuropsychiatr. Dis. Treat. 2021, 17, 1589. [Google Scholar] [CrossRef]
- Datta, S.; Jamwal, S.; Deshmukh, R.; Kumar, P. Beneficial effects of lycopene against haloperidol induced orofacial dyskinesia in rats: Possible neurotransmitters and neuroinflammation modulation. Eur. J. Pharmacol. 2016, 771, 229–235. [Google Scholar] [CrossRef]
- Bishnoi, M.; Boparai, R.K. An animal model to study the molecular basis of tardive dyskinesia. In Psychiatric Disorders; Springer: Berlin/Heidelberg, Germany, 2012; pp. 193–201. [Google Scholar]
- Sonego, A.B.; Prado, D.S.; Vale, G.T.; Sepulveda-Diaz, J.E.; Cunha, T.M.; Tirapelli, C.R.; Del Bel, E.A.; Raisman-Vozari, R.; Guimarães, F.S. Cannabidiol prevents haloperidol-induced vacuos chewing movements and inflammatory changes in mice via PPARγ receptors. Brain Behav. Immun. 2018, 74, 241–251. [Google Scholar] [CrossRef]
- Takeuchi, H.; Mori, Y.; Tsutsumi, Y. Pathophysiology, prognosis and treatment of tardive dyskinesia. Ther. Adv. Psychopharmacol. 2022, 12, 20451253221117313. [Google Scholar] [CrossRef]
- Leung, J.G.; Breden, E.L. Tetrabenazine for the treatment of tardive dyskinesia. Ann. Pharmacother. 2011, 45, 525–531. [Google Scholar] [CrossRef]
- Caroff, S.N.; Aggarwal, S.; Yonan, C. Treatment of tardive dyskinesia with tetrabenazine or valbenazine: A systematic review. J. Comp. Eff. Res. 2018, 7, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Nemes, E.; Geldenhuys, H.; Rozot, V.; Rutkowski, K.T.; Ratangee, F.; Bilek, N.; Mabwe, S.; Makhethe, L.; Erasmus, M.; Toefy, A.; et al. Prevention of M. tuberculosis infection with H4: IC31 vaccine or BCG revaccination. N. Engl. J. Med. 2018, 379, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Eisenhut, M.; Harris, R.J.; Rodrigues, L.C.; Sridhar, S.; Habermann, S.; Snell, L.; Mangtani, P.; Adetifa, I.; Lalvani, A.; et al. Effect of BCG vaccination against Mycobacterium tuberculosis infection in children: Systematic review and meta-analysis. BMJ 2014, 349, g4643. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Shi, C.; Liu, L.; Han, W. MUC1-based recombinant Bacillus Calmette–Guerin vaccines as candidates for breast cancer immunotherapy. Expert Opin. Biol. Ther. 2010, 10, 1037–1048. [Google Scholar] [CrossRef]
- Kühtreiber, W.M.; Tran, L.; Kim, T.; Dybala, M.; Nguyen, B.; Plager, S.; Huang, D.; Janes, S.; Defusco, A.; Baum, D.; et al. Long-term reduction in hyperglycemia in advanced type 1 diabetes: The value of induced aerobic glycolysis with BCG vaccinations. Npj Vaccines 2018, 3, 23. [Google Scholar] [CrossRef]
- Senousy, M.A.; Hanafy, M.E.; Shehata, N.; Rizk, S.M. Erythropoietin and Bacillus Calmette-Guérin Vaccination Mitigate 3-Nitropropionic Acid-Induced Huntington-like Disease in Rats by Modulating the PI3K/Akt/mTOR/P70S6K Pathway and Enhancing the Autophagy. ACS Chem. Neurosci. 2022, 13, 721–732. [Google Scholar] [CrossRef]
- Yedke, N.G.; Kumar, P. The Neuroprotective Role of BCG Vaccine in Movement Disorders: A Review. In CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders); Bentham Science Publishers: Sharjah, United Arab Emirates, 2023. [Google Scholar]
- Moalem, G.; Gdalyahu, A.; Shani, Y.; Otten, U.; Lazarovici, P.; Cohen, I.R.; Schwartz, M. Production of neurotrophins by activated T cells: Implications for neuroprotective autoimmunity. J. Autoimmun. 2000, 15, 331–345. [Google Scholar] [CrossRef]
- Schwartz, M.; Butovsky, O.; Brück, W.; Hanisch, U.K. Microglial phenotype: Is the commitment reversible? Trends Neurosci. 2006, 29, 68–74. [Google Scholar] [CrossRef]
- Cossu, D.; Yokoyama, K.; Sakanishi, T.; Sechi, L.A.; Hattori, N. Bacillus Calmette-Guérin Tokyo-172 vaccine provides age-related neuroprotection in actively induced and spontaneous experimental autoimmune encephalomyelitis models. Clin. Exp. Immunol. 2023, 212, 70–80. [Google Scholar] [CrossRef]
- Laćan, G.; Dang, H.; Middleton, B.; Horwitz, M.A.; Tian, J.; Melega, W.P.; Kaufman, D.L. Bacillus Calmette-Guerin vaccine-mediated neuroprotection is associated with regulatory T-cell induction in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine mouse model of Parkinson’s disease. J. Neurosci. Res. 2013, 91, 1292–1302. [Google Scholar] [CrossRef]
- Dow, C.T.; Greenblatt, C.L.; Chan, E.D.; Dow, J.F. Evaluation of BCG Vaccination and Plasma Amyloid: A Prospective, Pilot Study with Implications for Alzheimer’s Disease. Microorganisms 2022, 10, 424. [Google Scholar] [CrossRef]
- Thakur, K.S.; Prakash, A.; Bisht, R.; Bansal, P.K. Beneficial effect of candesartan and lisinopril against haloperidol-induced tardive dyskinesia in rat. J. Renin-Angiotensin-Aldosterone Syst. 2015, 16, 917–929. [Google Scholar] [CrossRef]
- Yang, J.; Qi, F.; Yao, Z. Neonatal Bacillus Calmette-Guérin vaccination alleviates lipopolysaccharide-induced neurobehavioral impairments and neuroinflammation in adult mice. Mol. Med. Rep. 2016, 14, 1574–1586. [Google Scholar] [CrossRef]
- Jamwal, S.; Singh, S.; Kaur, N.; Kumar, P. Protective Effect of Spermidine Against Excitotoxic Neuronal Death Induced by Quinolinic Acid in Rats: Possible Neurotransmitters and Neuroinflammatory Mechanism. Neurotox. Res. 2015, 28, 171–184. [Google Scholar] [CrossRef]
- Bishnoi, M.; Chopra, K.; Kulkarni, S.K. Possible anti-oxidant and neuroprotective mechanisms of zolpidem in attenuating typical anti-psychotic-induced orofacial dyskinesia: A biochemical and neurochemical study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2007, 31, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Wills, E. Mechanisms of lipid peroxide formation in animal tissues. Biochem. J. 1966, 99, 667. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G. L Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Kono, Y. Generation of superoxide radicals during auto-oxidation of hydroxyl-amine hydrochloride an assay for SOD. Arch. Biochem. Biophys. 1978, 186, 189–195. [Google Scholar] [CrossRef]
- Lück, H. Catalase. In Methods of Enzymatic Analysis; Elsevier: Amsterdam, The Netherlands, 1965; pp. 885–894. [Google Scholar]
- Rahi, V.; Ram, P.; Kumar, P. Filgrastim, a Recombinant Form of Granulocyte Colony-stimulating Factor, Ameliorates 3-nitropropionic Acid and Haloperidol-induced Striatal Neurotoxicity in Rats. Neurotox. Res. 2022, 40, 2089–2102. [Google Scholar] [CrossRef]
- Montemagni, C.; Del Favero, E.; Cocuzza, E.; Vischia, F.; Rocca, P. Effect of long-acting injectable antipsychotics on hospitalizations and global functioning in schizophrenia: A naturalistic mirror-image study. Ther. Adv. Psychopharmacol. 2022, 12, 20451253221122526. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, O.A.; Jørgensen, H.A. Neurotoxicity associated with neuroleptic-induced oral dyskinesias in rats. Implications for tardive dyskinesia? Prog. Neurobiol. 2000, 61, 525–541. [Google Scholar] [CrossRef] [PubMed]
- Bishnoi, M.; Chopra, K.; Kulkarni, S.K. Protective effect of Curcumin, the active principle of turmeric (Curcuma longa) in haloperidol-induced orofacial dyskinesia and associated behavioural, biochemical and neurochemical changes in rat brain. Pharmacol. Biochem. Behav. 2008, 88, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Mezzomo, N.F.; Schmitz, I.d.S.; de Lima, V.B.; Dorneles, G.P.; Schaffer, L.F.; Boeck, C.R.; Romao, P.R.T.; Peroza, L.R. Reversal of haloperidol-induced orofacial dyskinesia and neuroinflammation by isoflavones. Mol. Biol. Rep. 2022, 49, 1917–1923. [Google Scholar] [CrossRef]
- Guzen, F.P.; Cavalcanti, J.R.L.d.P.; Cavalcanti, D.M.L.d.P.; de Sales, L.G.P.; da Silva, M.S.M.; da Silva, A.N.A.; Pinheiro, F.I.; de Araújo, D.P. Haloperidol-Induced Preclinical Tardive Dyskinesia Model in Rats. Curr. Protoc. Neurosci. 2019, 88, e68. [Google Scholar] [CrossRef] [PubMed]
- Yedke, N.G.; Arthur, R.; Kumar, P. Bacillus calmette gaurine vaccine ameliorates the neurotoxicity of quinolinic acid in rats via the modulation of antioxidant, inflammatory and apoptotic markers. J. Chem. Neuroanat. 2023, 131, 102287. [Google Scholar] [CrossRef] [PubMed]
- Balijepalli, S.; Kenchappa, R.S.; Boyd, M.R.; Ravindranath, V. Protein thiol oxidation by haloperidol results in inhibition of mitochondrial complex I in brain regions: Comparison with atypical antipsychotics. Neurochem. Int. 2001, 38, 425–435. [Google Scholar] [CrossRef]
- Patil, R.; Dhawale, K.; Gound, H.; Gadakh, R. Protective Effect of Leaves of Murraya koenigii on Reserpine-Induced Orofacial Dyskinesia. Iran J. Pharm. Res. 2012, 11, 635–641. [Google Scholar]
- Kulkarni, S.; Mukherjee, S.; Pandey, A.; Dahake, R.; Padmanabhan, U.; Chowdhary, A.S. Bacillus Calmette-Guérin Confers Neuroprotection in a Murine Model of Japanese Encephalitis. Neuroimmunomodulation 2016, 23, 278–286. [Google Scholar] [CrossRef]
- Wu, Q.; Yuan, F.; Zhang, S.; Liu, W.; Miao, Q.; Zheng, X.; Lu, S.; Hou, K. Correlation of Blood Biochemical Markers with Tardive Dyskinesia in Schizophrenic Patients. Dis. Markers 2022, 2022, 1–6. [Google Scholar] [CrossRef]
- Wu, Z.W.; Yu, H.H.; Wang, X.; Guan, H.Y.; Xiu, M.H.; Zhang, X.Y. Interrelationships between oxidative stress, cytokines, and psychotic symptoms and executive functions in patients with chronic schizophrenia. Psychosom. Med. 2021, 83, 485–491. [Google Scholar] [CrossRef]
- Gasnier, M.; Ellul, P.; Plaze, M.; Ahad, P.A. A new look on an old issue: Comprehensive review of neurotransmitter studies in cerebrospinal fluid of patients with schizophrenia and antipsychotic effect on monoamine’s metabolism. Clin. Psychopharmacol. Neurosci. 2021, 19, 395. [Google Scholar] [CrossRef] [PubMed]
- Gofrit, O.N.; Klein, B.Y.; Cohen, I.R.; Ben-Hur, T.; Greenblatt, C.L.; Bercovier, H. Bacillus Calmette-Guérin (BCG) therapy lowers the incidence of Alzheimer’s disease in bladder cancer patients. PLoS ONE 2019, 14, e0224433. [Google Scholar] [CrossRef] [PubMed]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yedke, N.G.; Upadhayay, S.; Singh, R.; Jamwal, S.; Ahmad, S.F.; Kumar, P. Bacillus Calmette–Guérin Vaccine Attenuates Haloperidol-Induced TD-like Behavioral and Neurochemical Alteration in Experimental Rats. Biomolecules 2023, 13, 1667. https://doi.org/10.3390/biom13111667
Yedke NG, Upadhayay S, Singh R, Jamwal S, Ahmad SF, Kumar P. Bacillus Calmette–Guérin Vaccine Attenuates Haloperidol-Induced TD-like Behavioral and Neurochemical Alteration in Experimental Rats. Biomolecules. 2023; 13(11):1667. https://doi.org/10.3390/biom13111667
Chicago/Turabian StyleYedke, Narhari Gangaram, Shubham Upadhayay, Randhir Singh, Sumit Jamwal, Sheikh F. Ahmad, and Puneet Kumar. 2023. "Bacillus Calmette–Guérin Vaccine Attenuates Haloperidol-Induced TD-like Behavioral and Neurochemical Alteration in Experimental Rats" Biomolecules 13, no. 11: 1667. https://doi.org/10.3390/biom13111667
APA StyleYedke, N. G., Upadhayay, S., Singh, R., Jamwal, S., Ahmad, S. F., & Kumar, P. (2023). Bacillus Calmette–Guérin Vaccine Attenuates Haloperidol-Induced TD-like Behavioral and Neurochemical Alteration in Experimental Rats. Biomolecules, 13(11), 1667. https://doi.org/10.3390/biom13111667