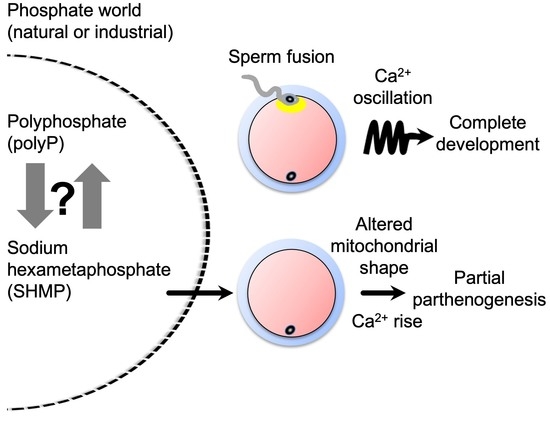
Sodium Hexametaphosphate Serves as an Inducer of Calcium Signaling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Preparation of SHMP Solution
2.2. Animals
2.3. Isolation of Germinal Vesicle (GV) Oocytes and Detection of Phospahate Polymers
2.4. Detection of Phosphate Polymers by Electrophoresis
2.5. Isolation of Metaphase II-Arrested (MII) Oocytes and Incubation in SHMP-Containing Medium
2.6. Observation of Nuclear and Mitochondrial Changes
2.7. Transmission Electron Microscopy (TEM)
2.8. Measurement of Intracellular Ca2+ Concentration
2.9. Statistical Analysis
3. Results
3.1. Presence of Phosphate Polymers in the Outer Region of Mouse Oocytes
3.2. Electrophoretic Analysis of Phosphate Polymers
3.3. Influence of SHMP on Mouse Oocytes before Fertilization
3.4. Partial Parthenogenesis in SHMP-Treated Oocytes
3.5. Mitochondrial Alteration in SHMP-Treated Oocytes
3.6. Ultrastructural Analysis of Mitochondria
3.7. Calcium (Ca2+) Concentration in SHMP-Treated Oocytes
4. Discussion
4.1. Transient Ca2+ Rise and Developmental Ability
4.2. Physiological Functions of PolyP
4.3. Physiological SHMP Activity
4.4. Possible Mechanism of SHMP-Triggered Events
4.5. Phosphate World Inside and Outside Organisms
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuroda, A.; Nomura, K.; Ohtomo, R.; Kato, J.; Ikeda, T.; Takiguchi, N.; Ohtake, H.; Kornberg, A. Role of inorganic polyphosphate in promoting ribosomal protein degradation by the Lon protease in E. coli. Science 2001, 293, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Hurlimann, H.C.; Stadler-Waibel, M.; Werner, T.P.; Freimoser, F.M. Pho91 Is a vacuolar phosphate transporter that regulates phosphate and polyphosphate metabolism in Saccharomyces cerevisiae. Mol. Biol. Cell 2007, 18, 4438–4445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumble, K.D.; Kornberg, A. Inorganic polyphosphate in mammalian cells and tissues. J. Biol. Chem. 1995, 270, 5818–5822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, N.N.; Gomez-Garcia, M.R.; Kornberg, A. Inorganic polyphosphate: Essential for growth and survival. Annu. Rev. Biochem. 2009, 78, 605–647. [Google Scholar] [CrossRef]
- Smith, S.A.; Mutch, N.J.; Baskar, D.; Rohloff, P.; Docampo, R.; Morrissey, J.H. Polyphosphate modulates blood coagulation and fibrinolysis. Proc. Natl. Acad. Sci. USA 2006, 103, 903–908. [Google Scholar] [CrossRef] [Green Version]
- Jacinto, E.; Hall, M.N. Tor signalling in bugs, brain and brawn. Nat. Rev. Mol. Cell Biol. 2003, 4, 117–126. [Google Scholar] [CrossRef]
- Wang, L.; Fraley, C.D.; Faridi, J.; Kornberg, A.; Roth, R.A. Inorganic polyphosphate stimulates mammalian TOR, a kinase involved in the proliferation of mammary cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 11249–11254. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, A.; Kawano, N.; Motomura, K.; Kuroda, A.; Sekiguchi, K.; Miyado, M.; Kang, W.; Miyamoto, Y.; Hanai, M.; Iwai, M.; et al. Degradation of phosphate polymer polyP enhances lactic fermentation in mice. Genes Cells 2018, 23, 904–914. [Google Scholar] [CrossRef] [Green Version]
- Mandala, V.S.; Loh, D.M.; Shepard, S.M.; Geeson, M.B.; Sergeyev, I.V.; Nocera, D.G.; Cummins, C.C.; Hong, M. Bacterial Phosphate Granules Contain Cyclic Polyphosphates: Evidence from 31P Solid-State NMR. J. Am. Chem. Soc. 2020, 142, 18407–18421. [Google Scholar] [CrossRef]
- Murphy, J.; Beighton, D.; Clark, D.; Bartlett, D. The potential for sodium hexametaphosphate (SHMP) found in common children drinks to limit acid production in the oral biofilm. J. Dent. 2007, 35, 214–217. [Google Scholar] [CrossRef]
- Shirashoji, N.; Jaeggi, J.J.; Lucey, J.A. Effect of sodium hexametaphosphate concentration and cooking time on the physicochemical properties of pasteurized process cheese. J. Dairy Sci. 2010, 93, 2827–2837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Reddy, K.R.; Zhan, H.; Fan, R.; Liu, S.; Xue, Q.; Du, Y. Hydraulic conductivity of soil-bentonite backfill comprised of SHMP-amended Ca-bentonite to Cr(VI)-impacted groundwater. J. Contam. Hydrol. 2021, 242, 103856. [Google Scholar] [CrossRef]
- Terezhalmy, G.; He, T.; Anastasia, M.K.; Eusebio, R. A Randomized Controlled Clinical Trial to Evaluate Extrinsic Stain Removal of a Whitening Dentifrice. J. Clin. Dent. 2016, 27, 114–117. [Google Scholar]
- Shindo, M.; Miyado, K.; Kang, W.; Fukami, M.; Miyado, M. Efficient Superovulation and Egg Collection from Mice. Bio-Protocol 2022, 12, e4439. [Google Scholar] [CrossRef] [PubMed]
- Hasuwa, H.; Muro, Y.; Ikawa, M.; Kato, N.; Tsujimoto, Y.; Okabe, M. Transgenic mouse sperm that have green acrosome and red mitochondria allow visualization of sperm and their acrosome reaction in vivo. Exp. Anim. 2010, 59, 105–107. [Google Scholar] [CrossRef] [Green Version]
- Gomes, F.M.; Ramos, I.B.; Wendt, C.; Girard-Dias, W.; De Souza, W.; Machado, E.A.; Miranda, K. New insights into the in situ microscopic visualization and quantification of inorganic polyphosphate stores by 4’,6-diamidino-2-phenylindole (DAPI)-staining. Eur. J. Histochem. 2013, 57, e34. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, S.; Yuzaki, M.; Nakada, K.; Shirakawa, H.; Nakanishi, S.; Nakade, S.; Mikoshiba, K. Block of Ca2+ wave and Ca2+ oscillation by antibody to the inositol 1,4,5-trisphosphate receptor in fertilized hamster eggs. Science 1992, 257, 251–255. [Google Scholar] [CrossRef]
- Maiolino, M.; O’Neill, N.; Lariccia, V.; Amoroso, S.; Sylantyev, S.; Angelova, P.R.; Abramov, A.Y. Inorganic Polyphosphate Regulates AMPA and NMDA Receptors and Protects Against Glutamate Excitotoxicity via Activation of P2Y Receptors. J. Neurosci. 2019, 39, 6038–6048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mata-Martinez, E.; Sanchez-Cardenas, C.; Chavez, J.C.; Guerrero, A.; Trevino, C.L.; Corkidi, G.; Montoya, F.; Hernandez-Herrera, P.; Buffone, M.G.; Balestrini, P.A.; et al. Role of calcium oscillations in sperm physiology. Biosystems 2021, 209, 104524. [Google Scholar] [CrossRef]
- Kang, W.; Harada, Y.; Yamatoya, K.; Kawano, N.; Kanai, S.; Miyamoto, Y.; Nakamura, A.; Miyado, M.; Hayashi, Y.; Kuroki, Y.; et al. Extra-mitochondrial citrate synthase initiates calcium oscillation and suppresses age-dependent sperm dysfunction. Lab. Investig. 2020, 100, 583–595. [Google Scholar] [CrossRef] [Green Version]
- Mizushima, S.; Hiyama, G.; Shiba, K.; Inaba, K.; Dohra, H.; Ono, T.; Shimada, K.; Sasanami, T. The birth of quail chicks after intracytoplasmic sperm injection. Development 2014, 141, 3799–3806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harada, Y.; Matsumoto, T.; Hirahara, S.; Nakashima, A.; Ueno, S.; Oda, S.; Miyazaki, S.; Iwao, Y. Characterization of a sperm factor for egg activation at fertilization of the newt Cynops pyrrhogaster. Dev. Biol. 2007, 306, 797–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwao, Y. Egg activation in physiological polyspermy. Reproduction 2012, 144, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Deguchi, R.; Takeda, N.; Stricker, S.A. Calcium signals and oocyte maturation in marine invertebrates. Int. J. Dev. Biol. 2015, 59, 271–280. [Google Scholar] [CrossRef] [Green Version]
- York-Andersen, A.H.; Hu, Q.; Wood, B.W.; Wolfner, M.F.; Weil, T.T. A calcium-mediated actin redistribution at egg activation in Drosophila. Mol. Reprod. Dev. 2020, 87, 293–304. [Google Scholar] [CrossRef] [Green Version]
- Angelova, P.R.; Baev, A.Y.; Berezhnov, A.V.; Abramov, A.Y. Role of inorganic polyphosphate in mammalian cells: From signal transduction and mitochondrial metabolism to cell death. Biochem. Soc. Trans. 2016, 44, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Stotz, S.C.; Scott, L.O.; Drummond-Main, C.; Avchalumov, Y.; Girotto, F.; Davidsen, J.; Gomez-Garcia, M.R.; Rho, J.M.; Pavlov, E.V.; Colicos, M.A. Inorganic polyphosphate regulates neuronal excitability through modulation of voltage-gated channels. Mol. Brain 2014, 7, 42. [Google Scholar] [CrossRef] [Green Version]
- Gray, M.J.; Wholey, W.Y.; Wagner, N.O.; Cremers, C.M.; Mueller-Schickert, A.; Hock, N.T.; Krieger, A.G.; Smith, E.M.; Bender, R.A.; Bardwell, J.C.; et al. Polyphosphate is a primordial chaperone. Mol. Cell. 2014, 53, 689–699. [Google Scholar] [CrossRef] [Green Version]
- Bae, W.J.; Auh, Q.S.; Kim, G.T.; Moon, J.H.; Kim, E.C. Effects of sodium tri- and hexameta-phosphate in vitro osteoblastic differentiation in Periodontal Ligament and Osteoblasts, and in vivo bone regeneration. Differentiation 2016, 92, 257–269. [Google Scholar] [CrossRef]
- Simsek-Duran, F.; Li, F.; Ford, W.; Swanson, R.J.; Jones, H.W., Jr.; Castora, F.J. Age-associated metabolic and morphologic changes in mitochondria of individual mouse and hamster oocytes. PLoS ONE 2013, 8, e64955. [Google Scholar] [CrossRef] [Green Version]
- Holmstrom, K.M.; Marina, N.; Baev, A.Y.; Wood, N.W.; Gourine, A.V.; Abramov, A.Y. Signalling properties of inorganic polyphosphate in the mammalian brain. Nat. Commun. 2013, 4, 1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joo, Y.N.; Jin, H.; Eun, S.Y.; Park, S.W.; Chang, K.C.; Kim, H.J. P2Y2R activation by nucleotides released from the highly metastatic breast cancer cell MDA-MB-231 contributes to pre-metastatic niche formation by mediating lysyl oxidase secretion, collagen crosslinking, and monocyte recruitment. Oncotarget 2014, 5, 9322–9334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorodeski, G.I. Purinergic Signalling in the Reproductive System. Auton. Neurosci. 2015, 191, 82–101. [Google Scholar] [CrossRef]
- Sophocleous, R.A.; Miles, N.A.; Ooi, L.; Sluyter, R. P2Y2 and P2X4 Receptors Mediate Ca2+ Mobilization in DH82 Canine Macrophage Cells. Int. J. Mol. Sci. 2020, 21, 8572. [Google Scholar] [CrossRef]
- Wang, M.J.; Yang, B.R.; Jing, X.Y.; Wang, Y.Z.; Kang, L.; Ren, K.; Kang, L. P2Y1R and P2Y2R: Potential molecular triggers in muscle regeneration. Purinergic. Signal. 2023, 19, 305–313. [Google Scholar] [CrossRef]
- Bartolak-Suki, E.; Imsirovic, J.; Nishibori, Y.; Krishnan, R.; Suki, B. Regulation of Mitochondrial Structure and Dynamics by the Cytoskeleton and Mechanical Factors. Int. J. Mol. Sci. 2017, 18, 1812. [Google Scholar] [CrossRef]
- Fernandez, P.; Pullarkat, P.A. The role of the cytoskeleton in volume regulation and beading transitions in PC12 neurites. Biophys. J. 2010, 99, 3571–3579. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.Y.; Chen, D.Y.; Li, J.S.; Lian, L.; Lei, L.; Han, Z.M.; Sun, Q.Y. Rotation of meiotic spindle is controlled by microfilaments in mouse oocytes. Biol. Reprod. 2003, 68, 943–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Straub, J.S.; Nowotarski, M.S.; Lu, J.; Sheth, T.; Jiao, S.; Fisher, M.P.A.; Shell, M.S.; Helgeson, M.E.; Jerschow, A.; Han, S. Phosphates form spectroscopically dark state assemblies in common aqueous solutions. Proc. Natl. Acad. Sci. USA 2023, 120, e2206765120. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katano, D.; Kang, W.; Harada, Y.; Kawano, N.; Miyado, M.; Saito, T.; Fukuoka, M.; Yamada, M.; Miyado, K. Sodium Hexametaphosphate Serves as an Inducer of Calcium Signaling. Biomolecules 2023, 13, 577. https://doi.org/10.3390/biom13040577
Katano D, Kang W, Harada Y, Kawano N, Miyado M, Saito T, Fukuoka M, Yamada M, Miyado K. Sodium Hexametaphosphate Serves as an Inducer of Calcium Signaling. Biomolecules. 2023; 13(4):577. https://doi.org/10.3390/biom13040577
Chicago/Turabian StyleKatano, Daiki, Woojin Kang, Yuichirou Harada, Natsuko Kawano, Mami Miyado, Takako Saito, Mio Fukuoka, Mitsutoshi Yamada, and Kenji Miyado. 2023. "Sodium Hexametaphosphate Serves as an Inducer of Calcium Signaling" Biomolecules 13, no. 4: 577. https://doi.org/10.3390/biom13040577
APA StyleKatano, D., Kang, W., Harada, Y., Kawano, N., Miyado, M., Saito, T., Fukuoka, M., Yamada, M., & Miyado, K. (2023). Sodium Hexametaphosphate Serves as an Inducer of Calcium Signaling. Biomolecules, 13(4), 577. https://doi.org/10.3390/biom13040577