So Shiho Tang Reduces Inflammation in Lipopolysaccharide-Induced RAW 264.7 Macrophages and Dextran Sodium Sulfate-Induced Colitis Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Solution Preparation
2.3. Sample Preparation
2.4. HPLC Analysis Conditions
2.5. Validation of HPLC Quantification Method
2.5.1. Specificity
2.5.2. Working Range (Linearity, Detection Limit [DL], and Quantitation Limit [QL])
2.5.3. Accuracy
2.5.4. Precision (Repeatability, Intermediate Precision, and Reproducibility)
2.5.5. Robustness
2.6. Cell Culture
2.7. Cell Viability Assay
2.8. Griess Assay
2.9. Western Blot Analysis
2.10. Animals Experiment
2.11. General Assessment of Colitis
2.12. Statistical Analysis
3. Results
3.1. Chromatography and Quantitation of the Five Compounds in SSHT
3.2. Effects of Saikosaponin A, Saikosaponin B2, Ginsenoside Rb1, Baicalin, and Glycyrrhizic Acid on Nitrite Production in LPS-Stimulated RAW 264.7 Cells
3.3. Effect of SSHT Extract on Nitrite Production and iNOS and COX-2 Expression in LPS-Stimulated RAW 264.7 Cells
3.4. Effect of SSHT on Modulating MAPK Signaling Pathway in LPS-Stimulated RAW 264.7 Cells
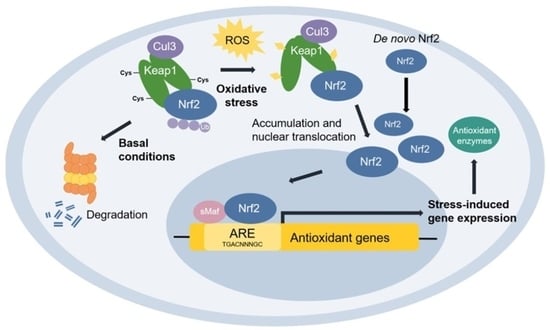
3.5. Effect of SSHT on NRF2 Expression in LPS-Stimulated RAW 264.7 Cells
3.6. Effect of SSHT in DSS-Induced Colitis Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204. [Google Scholar] [CrossRef] [PubMed]
- Colitis-Pathophysiology, U. Inflammatory bowel disease part I: Ulcerative colitis-pathophysiology and conventional and alternative treatment options. Altern. Med. Rev. 2003, 8, 247–283. [Google Scholar]
- Conrad, K.; Roggenbuck, D.; Laass, M.W. Diagnosis and classification of ulcerative colitis. Autoimmun. Rev. 2014, 13, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Porter, R.J.; Kalla, R.; Ho, G.-T. Ulcerative colitis: Recent advances in the understanding of disease pathogenesis. F1000Research 2020, 9, 294. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.; Sturm, A. Current treatment of ulcerative colitis. World J. Gastroenterol. 2011, 17, 3204. [Google Scholar] [PubMed]
- Li, S.; Wu, B.; Fu, W.; Reddivari, L. The anti-inflammatory effects of dietary anthocyanins against ulcerative colitis. Int. J. Mol. Sci. 2019, 20, 2588. [Google Scholar] [CrossRef] [PubMed]
- Bode, J.G.; Ehlting, C.; Häussinger, D. The macrophage response towards LPS and its control through the p38MAPK–STAT3 axis. Cell Signal. 2012, 24, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Yu, Z.; Zheng, Y.; Wang, L.; Qin, X.; Cheng, G.; Ci, X. Isovitexin exerts anti-inflammatory and anti-oxidant activities on lipopolysaccharide-induced acute lung injury by inhibiting MAPK and NF-κB and activating HO-1/Nrf2 pathways. Int. J. Biol. Sci. 2016, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lu, C.; Liu, Z.; Zhang, P.; Guo, H.; Wang, T. Schizandrin B protects LPS-induced sepsis via TLR4/NF-κB/MyD88 signaling pathway. Am. J. Transl. Res. 2018, 10, 1155. [Google Scholar]
- Innamorato, N.G.; Rojo, A.I.; García-Yagüe, A.J.; Yamamoto, M.; De Ceballos, M.L.; Cuadrado, A. The transcription factor Nrf2 is a therapeutic target against brain inflammation. J. Immunol. 2008, 181, 680–689. [Google Scholar] [CrossRef]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef]
- Khor, T.O.; Huang, M.-T.; Kwon, K.H.; Chan, J.Y.; Reddy, B.S.; Kong, A.-N. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium–induced colitis. Cancer Res. 2006, 66, 11580–11584. [Google Scholar] [CrossRef] [PubMed]
- Ke, F.; Yadav, P.K.; Ju, L.Z. Herbal medicine in the treatment of ulcerative colitis. Saudi J. Gastroenterol. 2012, 18, 3. [Google Scholar] [PubMed]
- Chan, K.; Lin, T. Treatments used in complementary and alternative medicine. In Side Effects of Drugs Annual; Elsevier: Amsterdam, The Netherlands, 2009; Volume 31, pp. 745–756. [Google Scholar]
- Guideline, I.H.T. Validation of Analytical Procedures Q2 (R2); ICH: Geneva, Switzerland, 2005. [Google Scholar]
- Pandurangan, A.K.; Mohebali, N.; Esa, N.M.; Looi, C.Y.; Ismail, S.; Saadatdoust, Z. Gallic acid suppresses inflammation in dextran sodium sulfate-induced colitis in mice: Possible mechanisms. Int. Immunopharmacol. 2015, 28, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Salum Mchenga, S.; Wang, D.; Janneh, F.; Feng, Y.; Zhang, P.; Li, Z.; Lu, C. Differential dose effects of recombinant IL-25 on the development of dextran sulfate sodium-induced colitis. Inflamm. Res. 2010, 59, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Jia, M.; Zhao, L.; Zhang, S. Bu-Zhong-Yi-Qi Granule enhances colonic tight junction integrity via TLR4/NF-κB/MLCK signaling pathway in ulcerative colitis rats. Evid. Based Complement. Alternat. Med. 2021, 2021, 6657141. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Han, J.; Hui, L. MAPK signaling in inflammation-associated cancer development. Protein Cell 2010, 1, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Locascio, L.M.; Doré, S. Critical role of Nrf2 in experimental ischemic stroke. Front. Pharmacol. 2019, 10, 153. [Google Scholar] [CrossRef]
- Kahroba, H.; Davatgaran-Taghipour, Y. Exosomal Nrf2: From anti-oxidant and anti-inflammation response to wound healing and tissue regeneration in aged-related diseases. Biochimie 2020, 171, 103–109. [Google Scholar] [CrossRef]
- Li, C.; Jia, W.-w.; Yang, J.-l.; Cheng, C.; Olaleye, O.E. Multi-compound and drug-combination pharmacokinetic research on Chinese herbal medicines. Acta Pharmacol. Sin. 2022, 43, 3080–3095. [Google Scholar] [CrossRef]
- Tran, N.K.S.; Lee, J.H.; Lee, M.J.; Park, J.Y.; Kang, K.S. Multitargeted herbal prescription So Shiho Tang: A scoping review on biomarkers for the evaluation of therapeutic effects. Pharmaceuticals 2023, 16, 1371. [Google Scholar] [CrossRef] [PubMed]
- Kaistha, A.; Levine, J. Inflammatory bowel disease: The classic gastrointestinal autoimmune disease. Curr. Probl. Pediatr. Adolesc. Health Care 2014, 44, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Tatiya-Aphiradee, N.; Chatuphonprasert, W.; Jarukamjorn, K. Immune response and inflammatory pathway of ulcerative colitis. J. Basic Clin. Physiol. Pharmacol. 2018, 30, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Di Sabatino, A.; Lenti, M.V.; Giuffrida, P.; Vanoli, A.; Corazza, G.R. New insights into immune mechanisms underlying autoimmune diseases of the gastrointestinal tract. Autoimmun. Rev. 2015, 14, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Gajendran, M.; Loganathan, P.; Jimenez, G.; Catinella, A.P.; Ng, N.; Umapathy, C.; Ziade, N.; Hashash, J.G. A comprehensive review and update on ulcerative colitis. Dis. Mon. 2019, 65, 100851. [Google Scholar] [CrossRef]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15.25.1–15.25.14. [Google Scholar] [CrossRef] [PubMed]
- Eichele, D.D.; Kharbanda, K.K. Dextran sodium sulfate colitis murine model: An indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J. Gastroenterol. 2017, 23, 6016. [Google Scholar] [CrossRef]
- Chen, Y.; Jin, Y.; Stanton, C.; Paul Ross, R.; Zhao, J.; Zhang, H.; Yang, B.; Chen, W. Alleviation effects of Bifidobacterium breve on DSS-induced colitis depends on intestinal tract barrier maintenance and gut microbiota modulation. Eur. J. Nutr. 2021, 60, 369–387. [Google Scholar] [CrossRef]
- Jiang, S.; Xu, H.; Zhao, C.; Zhong, F.; Li, D. Oyster polysaccharides relieve DSS-induced colitis via anti-inflammatory and maintaining the physiological hypoxia. Int. J. Biol. Macromol. 2023, 238, 124150. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. Int. J. Basic Clin. Pharmacol. 2016, 7, 27. [Google Scholar] [CrossRef]
- Sun, C.; Gao, M.; Qiao, M. Research progress of traditional Chinese medicine compound “Xiaochaihu Decoction” in the treatment of depression. Biomed. Pharmacother. 2023, 159, 114249. [Google Scholar] [CrossRef] [PubMed]
- Mowat, A.M.; Bain, C.C. Mucosal macrophages in intestinal homeostasis and inflammation. J. Innate Immun. 2011, 3, 550–564. [Google Scholar] [CrossRef]
- Na, Y.R.; Stakenborg, M.; Seok, S.H.; Matteoli, G. Macrophages in intestinal inflammation and resolution: A potential therapeutic target in IBD. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Lissner, D.; Schumann, M.; Batra, A.; Kredel, L.-I.; Kühl, A.A.; Erben, U.; May, C.; Schulzke, J.-D.; Siegmund, B. Monocyte and M1 macrophage-induced barrier defect contributes to chronic intestinal inflammation in IBD. Inflamm. Bowel Dis. 2015, 21, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Malayil, D.; House, N.C.; Puthenparambil, D.; Job, J.T.; Narayanankutty, A. Borassus flabellifer haustorium extract prevents pro-oxidant mediated cell death and LPS-induced inflammation. Drug Chem. Toxicol. 2022, 45, 1716–1722. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.-H.; Hwang, Y.; Kim, K.-N.; Jun, H.-S. Palmitate induces nitric oxide production and inflammatory cytokine expression in zebrafish. Fish Shellfish Immunol. 2018, 79, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhou, S.; Takeda, R.; Okazaki, K.; Sekita, M.; Sakamoto, K. Anti-inflammatory activities of amber extract in lipopolysaccharide-induced RAW 264.7 macrophages. Biomed. Pharmacother. 2021, 141, 111854. [Google Scholar] [CrossRef] [PubMed]
- Ya Sklyarov, A.; Panasyuk, N.; Fomenko, I. Role of nitric oxide-synthase and cyclooxygenase/lipooxygenase systems in development of experimental ulcerative colitis. J. Physiol. Pharmacol. 2011, 62, 65. [Google Scholar]
- Han, H.; Sun, W.; Feng, L.; Wen, Z.; Yang, M.; Ma, Y.; Fu, J.; Ma, X.; Xu, X.; Wang, Z. Differential relieving effects of shikonin and its derivatives on inflammation and mucosal barrier damage caused by ulcerative colitis. PeerJ 2021, 9, e10675. [Google Scholar] [CrossRef]
- Ferrer, M.D.; Busquets-Cortés, C.; Capó, X.; Tejada, S.; Tur, J.A.; Pons, A.; Sureda, A. Cyclooxygenase-2 inhibitors as a therapeutic target in inflammatory diseases. Curr. Med. Chem. 2019, 26, 3225–3241. [Google Scholar] [CrossRef]
- Rao, K.M.K. MAP kinase activation in macrophages. J. Leukoc. Biol. 2001, 69, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Liou, C.-J.; Len, W.-B.; Wu, S.-J.; Lin, C.-F.; Wu, X.-L.; Huang, W.-C. Casticin inhibits COX-2 and iNOS expression via suppression of NF-κB and MAPK signaling in lipopolysaccharide-stimulated mouse macrophages. J. Ethnopharmacol. 2014, 158, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Yang, L.; He, J. Plantanone C attenuates LPS-stimulated inflammation by inhibiting NF-κB/iNOS/COX-2/MAPKs/Akt pathways in RAW 264.7 macrophages. Biomed. Pharmacother. 2021, 143, 112104. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy—From molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta Proteins Proteom. 2005, 1754, 253–262. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Ahn, C.-B.; Je, J.-Y. Anti-inflammatory action of high molecular weight Mytilus edulis hydrolysates fraction in LPS-induced RAW264. 7 macrophage via NF-κB and MAPK pathways. Food Chem. 2016, 202, 9–14. [Google Scholar] [CrossRef]
- de la Vega, M.R.; Dodson, M.; Gross, C.; Mansour, H.M.; Lantz, R.C.; Chapman, E.; Wang, T.; Black, S.M.; Garcia, J.G.; Zhang, D.D. Role of Nrf2 and autophagy in acute lung injury. Curr. Pharmacol. Rep. 2016, 2, 91–101. [Google Scholar] [CrossRef]
- Notarte, K.I.; Quimque, M.T.; Macaranas, I.T.; Khan, A.; Pastrana, A.M.; Villaflores, O.B.; Arturo, H.C.; Pilapil IV, D.Y.; Tan, S.M.; Wei, D.Q.; et al. Attenuation of lipopolysaccharide-induced inflammatory responses through inhibition of the NF-κB pathway and the increased Nrf2 level by a flavonol-enriched n-butanol fraction from Uvaria alba. ACS Omega 2023, 8, 5377–5392. [Google Scholar] [CrossRef]
- Ci, X.; Zhou, J.; Lv, H.; Yu, Q.; Peng, L.; Hua, S. Betulin exhibits anti-inflammatory activity in LPS-stimulated macrophages and endotoxin-shocked mice through an AMPK/AKT/Nrf2-dependent mechanism. Cell Death Dis. 2017, 8, e2798. [Google Scholar] [CrossRef] [PubMed]
- De Souza, L.F.; Barreto, F.; da Silva, E.G.; Andrades, M.E.; Guimaraes, E.L.; Behr, G.A.; Moreira, J.C.; Bernard, E.A. Regulation of LPS stimulated ROS production in peritoneal macrophages from alloxan-induced diabetic rats: Involvement of high glucose and PPARgamma. Life Sci. 2007, 81, 153–159. [Google Scholar] [CrossRef]
- Kim, Y.J. Rhamnazin inhibits LPS-induced inflammation and ROS/RNS in raw macrophages. J. Nutr. Health 2016, 49, 288–294. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Yang, S.; Yan, S. Mechanism of Nrf2 in the treatment of ulcerative colitis via regulating macrophage polarization. J. Cent. South Univ. Med. Sci. 2023, 48, 1746–1752. [Google Scholar]
- Yang, H.; Lv, H.; Li, H.; Ci, X.; Peng, L. Oridonin protects LPS-induced acute lung injury by modulating Nrf2-mediated oxidative stress and Nrf2-independent NLRP3 and NF-κB pathways. Cell Commun. Signal. 2019, 17, 62. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Luo, C.; Wang, P.; He, Q.; Zhou, J.; Peng, H. Saikosaponin A mediates the inflammatory response by inhibiting the MAPK and NF-Κb pathways in LPS-stimulated RAW 264.7 cells. Exp. Ther. Med. 2013, 5, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-S.; Im, H.-T.; Lee, K.-T. Saikosaponin B2 suppresses inflammatory responses through IKK/IκBα/NF-κB signaling inactivation in LPS-induced RAW 264.7 macrophages. Inflammation 2019, 42, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Uto, T.; Morinaga, O.; Tanaka, H.; Shoyama, Y. Analysis of the synergistic effect of glycyrrhizin and other constituents in licorice extract on lipopolysaccharide-induced nitric oxide production using knock-out extract. Biochem. Biophys. Res. Commun. 2012, 417, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Park, E.K.; Shin, Y.W.; Lee, H.U.; Kim, S.S.; Lee, Y.C.; Lee, B.Y.; Kim, D.H. Inhibitory effect of ginsenoside Rb1 and compound K on NO and prostaglandin E2 biosyntheses of RAW264. 7 cells induced by lipopolysaccharide. Biol. Pharm. Bull. 2005, 28, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.W.; Su, W.L.; Chou, T.C. Baicalin improves the survival in endotoxic mice and inhibits the inflammatory responses in LPS-treated RAW 264.7 macrophages. Eur. J. Inflamm. 2020, 18, 2058739220967767. [Google Scholar] [CrossRef]
- Yarnell, E. Synergy in herbal medicines. J. Restor. Med. 2015, 4, 60. [Google Scholar] [CrossRef]
- Dinda, B.; Dinda, S.; DasSharma, S.; Banik, R.; Chakraborty, A.; Dinda, M. Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. Eur. J. Med. Chem. 2017, 131, 68–80. [Google Scholar] [CrossRef]
- Huang, T.; Liu, Y.; Zhang, C. Pharmacokinetics and bioavailability enhancement of baicalin: A review. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 159–168. [Google Scholar] [CrossRef]
- Wang, X.; Xie, L.; Long, J.; Liu, K.; Lu, J.; Liang, Y.; Cao, Y.; Dai, X.; Li, X. Therapeutic effect of baicalin on inflammatory bowel disease: A review. J. Ethnopharmacol. 2022, 283, 114749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-L.; Zhang, S.; He, W.-X.; Lu, J.-L.; Xu, Y.-J.; Yang, J.-Y.; Liu, D. Baicalin may alleviate inflammatory infiltration in dextran sodium sulfate-induced chronic ulcerative colitis via inhibiting IL-33 expression. Life Sci. 2017, 186, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Feng, L.; Zhang, Z.H.; Jia, X.B. The anti-inflammation effect of baicalin on experimental colitis through inhibiting TLR4/NF-κB pathway activation. Int. Immunopharmacol. 2014, 23, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Liang, W.; Wang, T.; Sui, J.; Wang, J.; Deng, Z.; Chen, D. Saponins regulate intestinal inflammation in colon cancer and IBD. Pharmacol. Res. 2019, 144, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Graebin, C.S. The pharmacological activities of glycyrrhizinic acid (“glycyrrhizin”) and glycyrrhetinic acid. In Sweeteners; Springer: Cham, Switzerland, 2018; p. 245. [Google Scholar] [CrossRef]
- Su, X.; Wu, L.; Hu, M.; Dong, W.; Xu, M.; Zhang, P. Glycyrrhizic acid: A promising carrier material for anticancer therapy. Biomed. Pharmacother. 2017, 95, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Selyutina, O.Y.; Polyakov, N. Glycyrrhizic acid as a multifunctional drug carrier—From physicochemical properties to biomedical applications: A modern insight on the ancient drug. Int. J. Pharm. 2019, 559, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Yuan, B.-C.; Ma, Y.-S.; Zhou, S.; Liu, Y. The anti-inflammatory activity of licorice, a widely used Chinese herb. Pharm. Biol. 2017, 55, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Luo, M.; Fu, Y.; Wang, S.; Efferth, T.; Zu, Y. Glycyrrhizic acid nanoparticles inhibit LPS-induced inflammatory mediators in 264.7 mouse macrophages compared with unprocessed glycyrrhizic acid. Int. J. Nanomed. 2013, 8, 1377–1383. [Google Scholar]
- Wang, Y.M.; Du, G.Q. Glycyrrhizic acid prevents enteritis through reduction of NF-κB p65 and p38MAPK expression in rat. Mol. Med. Rep. 2016, 13, 3639–3646. [Google Scholar] [CrossRef]
- Yuan, B.; Yang, R.; Ma, Y.; Zhou, S.; Zhang, X.; Liu, Y. A systematic review of the active saikosaponins and extracts isolated from Radix Bupleuri and their applications. Pharm. Biol. 2017, 55, 620–635. [Google Scholar] [CrossRef]
- Hu, H.; Zheng, K.; Xu, X.; Li, B.; Yin, Q.; Zeng, H.; Jiang, Y.; Zhang, Z.; Ma, S.-S.; Chen, T. Saikosaponin B1/D alleviate dextran sulfate sodium-induced colitis via regulating the NRF2/HO-1 pathway to inhibit the ferroptosis in zebrafish. Res. Sq. 2023; preprint. [Google Scholar] [CrossRef]
- Shin, B.-K.; Kwon, S.W.; Park, J.H. Chemical diversity of ginseng saponins from Panax ginseng. J. Ginseng Res. 2015, 39, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, Y.; Xiong, Z.; Yu, S.; Zhou, B.; Ling, Y.; Zheng, Z.; Shi, G.; Wu, Y.; Qian, X. Ginsenoside Rb1 inhibits free fatty acids-induced oxidative stress and inflammation in 3T3-L1 adipocytes. Mol. Med. Rep. 2017, 16, 9165–9172. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Gao, S.; Wang, T.; Shen, Y.; Yang, W.; Li, Y.; Hu, H. Ginsenoside Rb1 improves learning and memory ability through its anti-inflammatory effect in Aβ1-40 induced Alzheimer’s disease of rats. Am. J. Transl. Res. 2019, 11, 2955. [Google Scholar]
- Guo, Y.; Xie, J.; Zhang, L.; Yang, L.; Ma, J.; Bai, Y.; Ma, W.; Wang, L.; Yu, H.; Zeng, Y. Ginsenoside Rb1 exerts antidepressant-like effects via suppression inflammation and activation of AKT pathway. Neurosci. Lett. 2021, 744, 135561. [Google Scholar] [CrossRef]








| Glycyrrhizic Acid | Ginsenoside Rb1 | Baicalin | Saikosaponin A and B2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HPLC Instrument | Waters 2695 Separation Module | Waters e2695 Separation Module | Waters e2695 Separation Module | Waters e2695 Separation Module | ||||||||
| Detector | Waters 2996 PDA | Waters 2998 PDA | Waters 2998 PDA | Waters 2998 PDA | ||||||||
| Column | Phenomenex Gemini® C18 110 Å column (5 μm, 4.6 × 250 mm) | YMC-Pack Pro C18 LC column (5 μm, 4.6 × 150 mm) | Luna® C18(2) 100 Å column (5 μm, 4.6 × 250 mm) | Phenomenex Gemini® C18 110 Å column (5 μm, 4.6 × 250 mm) | ||||||||
| Column Temp. | 25 °C | 25 °C | 25 °C | 25 °C | ||||||||
| Sample Temp. | 20 °C | 25 °C | 20 °C | 25 °C | ||||||||
| Detection | 254 nm | 195 nm | 277 nm | 203 nm (saikosaponin A) 253 nm (saikosaponin B2) | ||||||||
| Flow Rate | 1.0 mL/min | 1.0 mL/min | 1.0 mL/min | 0.8 mL/min | ||||||||
| Injection | 10 μL | 10 μL | 10 μL | 10 μL | ||||||||
| Mobile Phase | A: Methanol (0.5% Formic acid) B: Water (0.5% Formic acid) | A: Acetonitrile (0.1% Formic acid) B: Water (0.1% Formic acid) | A: Acetonitrile (1.0% Acetic acid) B: Water (1.0% Acetic acid) | A: Acetonitrile B: Water | ||||||||
| Gradient Condition | Time (min) | A (%) | B (%) | Time (min | A (%) | B (%) | Time (min) | A (%) | B (%) | Time (min) | A (%) | B (%) |
| 0 | 70 | 30 | 0 | 20 | 80 | 0 | 22 | 78 | 0 | 30 | 70 | |
| 30 | 70 | 30 | 40 | 50 | 50 | 35 | 22 | 78 | 30 | 36 | 64 | |
| 31 | 100 | 0 | 45 | 100 | 0 | 36 | 100 | 0 | 50 | 39 | 61 | |
| 40 | 100 | 0 | 50 | 100 | 0 | 45 | 100 | 0 | 55 | 100 | 0 | |
| 41 | 70 | 30 | 51 | 20 | 80 | 46 | 22 | 78 | 60 | 100 | 0 | |
| 50 | 70 | 30 | 60 | 20 | 80 | 60 | 22 | 78 | 61 | 30 | 70 | |
| 70 | 30 | 70 | ||||||||||
| Analyte | Detection Wavelength (nm) | Working Range (µg/mL) | Regression Equation | r2 | DL (µg/mL) | QL (µg/mL) |
|---|---|---|---|---|---|---|
| Glycyrrhizic acid | 254 | 15.625–500 | 0.9990 | 5.24 | 15.89 | |
| Ginsenoside Rb1 | 195 | 50–800 | 0.9996 | 4.40 | 13.33 | |
| Baicalin | 277 | 15.625–1000 | 0.9995 | 3.08 | 9.34 | |
| Saikosaponin A | 203 | 7.8125–250 | 0.9994 | 0.73 | 2.21 | |
| Saikosaponin B2 | 253 | 15.625–1000 | 0.9990 | 3.46 | 10.49 |
| Compound | Content in SSHT Dry Extract (mg/g) | Criteria in KHP (mg/g) |
|---|---|---|
| Glycyrrhizic acid | 5.333 ± 0.040 | 1.6 |
| Ginsenoside Rb1 | 1.301 ± 0.037 | N/A * |
| Baicalin | 55.148 ± 0.138 | 11.9 |
| Saikosaponin A | 0.216 ± 0.013 | N/A |
| Saikosaponin B2 | 0.750 ± 0.007 | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, M.T.; Park, G.; Park, D.H.; Choi, M.; Ku, S.; Go, S.H.; Lee, Y.G.; Song, S.J.; Ahn, C.-W.; Jang, Y.P.; et al. So Shiho Tang Reduces Inflammation in Lipopolysaccharide-Induced RAW 264.7 Macrophages and Dextran Sodium Sulfate-Induced Colitis Mice. Biomolecules 2024, 14, 451. https://doi.org/10.3390/biom14040451
He MT, Park G, Park DH, Choi M, Ku S, Go SH, Lee YG, Song SJ, Ahn C-W, Jang YP, et al. So Shiho Tang Reduces Inflammation in Lipopolysaccharide-Induced RAW 264.7 Macrophages and Dextran Sodium Sulfate-Induced Colitis Mice. Biomolecules. 2024; 14(4):451. https://doi.org/10.3390/biom14040451
Chicago/Turabian StyleHe, Mei Tong, Geonha Park, Do Hwi Park, Minsik Choi, Sejin Ku, Seung Hyeon Go, Yun Gyo Lee, Seok Jun Song, Chang-Wook Ahn, Young Pyo Jang, and et al. 2024. "So Shiho Tang Reduces Inflammation in Lipopolysaccharide-Induced RAW 264.7 Macrophages and Dextran Sodium Sulfate-Induced Colitis Mice" Biomolecules 14, no. 4: 451. https://doi.org/10.3390/biom14040451
APA StyleHe, M. T., Park, G., Park, D. H., Choi, M., Ku, S., Go, S. H., Lee, Y. G., Song, S. J., Ahn, C. -W., Jang, Y. P., & Kang, K. S. (2024). So Shiho Tang Reduces Inflammation in Lipopolysaccharide-Induced RAW 264.7 Macrophages and Dextran Sodium Sulfate-Induced Colitis Mice. Biomolecules, 14(4), 451. https://doi.org/10.3390/biom14040451