Binding of Sperm to the Zona Pellucida Mediated by Sperm Carbohydrate-Binding Proteins is not Species-Specific in Vitro between Pigs and Cattle
Abstract
:1. Introduction

2. Experimental Section
2.1. Materials
2.2. Assessment of Porcine and Bovine Sperm Binding to Glycolipid Analogs
2.3. Assessment of Sperm Binding After Trypsin Treatment
2.4. Collection of Porcine and Bovine Ovarian Oocytes
2.5. In vitro Maturation of Porcine and Bovine Ovarian Oocytes
2.6. Assessment of Homologous and Heterologous Binding of Porcine and Bovine Sperm to the ZP
2.7. Indirect Immunofluorescent Labeling of Porcine Oocytes
2.8. Lectin Blotting and Western Blotting for Porcine ZPGs
2.9. Preparation of Acrosome-Reacted Sperm and Zona-Free Oocytes
2.10. Binding Assay for A23187-Treated Sperm and Glycolipid Analogs
2.11. Binding Assay for Homologous and Heterologous A23187-Treated Sperm and Zona-Free Oocytes
2.12. Lectin Labeling
2.13. Statistical Analysis
3. Results and Discussion
3.1. Binding of Porcine and Bovine Sperm to Glycolipid Analogs
3.2. Homologous and Heterologous Binding of Porcine and Bovine Sperm to ZP


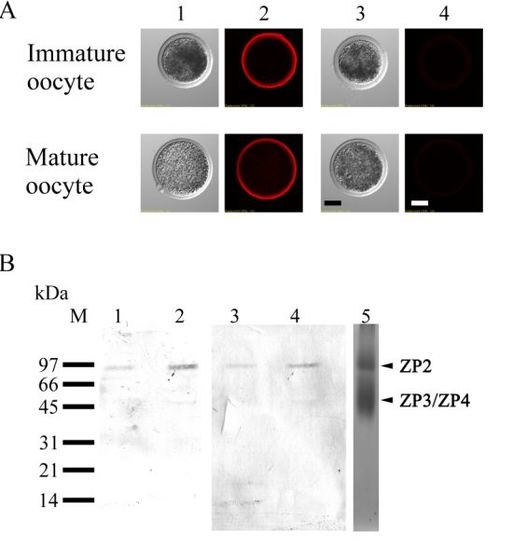
3.3. Lectin Staining in Immature and Mature Oocytes

3.4. Localization of ZP2 in Porcine Oocytes


3.5. Binding of A23187-Treated Porcine and Bovine Sperm to Glycolipid Analogs

3.6. Homologous and Heterologous Binding of A23187-Treated Sperm to Zona-Free Oocytes
3.7. Lectin Labeling in Zona-Free Mature Oocytes

4. Conclusions
Acknowledgments
References
- Yanagimachi, R. Mammalian Fertilization. In The Physiology of Reproduction, 2nd Ed.; Knobil, E., Neill, J.D., Eds.; Raven Press: New York, NY, USA, 1994; pp. 189–317. [Google Scholar]
- Wassarman, P.M.; Jovine, L.; Litscher, E.S. A profile of fertilization in mammals. Nat. Cell Biol. 2001, 3, E59–E64. [Google Scholar] [CrossRef]
- Stetson, I.; Izquierdo-Rico, M.J.; Moros, C.; Chevret, P.; Lorenzo, P.L.; Ballesta, J.; Rebollar, P.G.; Gutierrez-Gallego, R.; Avilés, M. Rabbit zona pellucida composition: A molecular, proteomic and phylogenetic approach. J. Proteomics 2012, 75, 5920–5935. [Google Scholar] [CrossRef]
- Florman, H.M.; Wassarman, P.M. O-linked oligosaccharides of mouse egg ZP3 account for its sperm receptor activity. Cell 1985, 41, 313–324. [Google Scholar] [CrossRef]
- Yurewicz, E.C.; Sacco, A.G.; Gupta, S.K.; Xu, N.; Gage, D.A. Hetero-oligomerization-dependent binding of pig oocyte zona pellucida glycoproteins ZPB and ZPC to boar sperm membrane vesicles. J. Biol. Chem. 1998, 273, 7488–7494. [Google Scholar] [CrossRef]
- Yonezawa, N.; Kudo, K.; Terauchi, H.; Kanai, S.; Yoda, N.; Tanokura, M.; Ito, K.; Miura, K.; Katsumata, T.; Nakano, M. Recombinant porcine zona pellucida glycoproteins expressed in Sf9 cells bind to bovine sperm but not to porcine sperm. J. Biol. Chem. 2005, 280, 20189–20196. [Google Scholar]
- Kanai, S.; Yonezawa, N.; Ishii, Y.; Tanokura, M.; Nakano, M. Recombinant bovine zona pellucidaglycoproteins ZP3 and ZP4 coexpressed in Sf9 cells form a sperm-binding active hetero-complex. FEBS J. 2007, 274, 5390–5405. [Google Scholar] [CrossRef]
- Yonezawa, N.; Kanai-Kitayama, S.; Kitayama, T.; Hamano, A.; Nakano, M. Porcine zona pellucida glycoprotein ZP4 is responsible for the sperm-binding activity of the ZP3/ZP4 complex. Zygote 2012, 20, 389–397. [Google Scholar] [CrossRef]
- Baibakov, B.; Boggs, N.A.; Yauger, B.; Baibakov, G.; Dean, J. Human sperm bind to the N-terminal domain of ZP2 in humanized zonae pellucidae in transgenic mice. J. Cell Biol. 2012, 197, 897–905. [Google Scholar] [CrossRef]
- Hoodbhoy, T.; Dean, J. Insights into the molecular basis of sperm-egg recognition in mammals. Reproduction 2004, 127, 417–422. [Google Scholar] [CrossRef]
- Rankin, T.L.; Coleman, J.S.; Epifano, O.; Hoodbhoy, T.; Turner, S.G.; Castle, P.E.; Lee, E.; Gore-Langton, R.; Dean, J. Fertility and taxon-specific sperm binding persist after replacement of mouse sperm receptors with human homologs. Dev. Cell 2003, 5, 33–43. [Google Scholar] [CrossRef]
- Clark, G.F. The mammalian zona pellucida: a matrix that mediates both gamete binding and immune recognition? Syst. Biol. Reprod. Med. 2010, 56, 349–364. [Google Scholar] [CrossRef]
- Clark, G.F. Molecular models for mouse sperm-oocyte binding. Glycobiology 2011, 21, 3–5. [Google Scholar] [CrossRef]
- Clark, G.F. The molecular basis of mouse sperm-zona pellucida binding: a still unresolved issue in developmental biology. Reproduction 2011, 142, 377–381. [Google Scholar] [CrossRef]
- Tokuhiro, K.; Ikawa, M.; Benham, A.M.; Okabe, M. Protein disulfide isomerase homolog PDILT is required for quality control of sperm membrane protein ADAM3 and male fertility. Proc. Natl. Acad. Sci. USA 2012, 109, 3850–3855. [Google Scholar] [CrossRef] [Green Version]
- Kudo, K.; Yonezawa, N.; Katsumata, T.; Aoki, H.; Nakano, M. Localization of carbohydrate chains of pig sperm ligand in the glycoprotein ZPB of egg zona pellucida. Eur. J. Biochem. 1998, 252, 492–499. [Google Scholar]
- Amari, S.; Yonezawa, N.; Mitsui, S.; Katsumata, T.; Hamano, S.; Kuwayama, M.; Hashimoto, Y.; Suzuki, A.; Takeda, Y.; Nakano, M. Essential role of the nonreducing terminal α-mannosyl residues of the N-linked carbohydrate chain of bovine zona pellucida glycoproteins in sperm-egg binding. Mol. Reprod. Dev. 2001, 59, 221–226. [Google Scholar] [CrossRef]
- Yonezawa, N.; Amari, S.; Takahashi, K.; Ikeda, K.; Imai, F.L.; Kanai, S.; Kikuchi, K.; Nakano, M. Participation of the nonreducing terminal β-galactosyl residues of the neutral N-linked carbohydrate chains of porcine zona pellucida glycoproteins in sperm-egg binding. Mol. Reprod. Dev. 2005, 70, 222–227. [Google Scholar] [CrossRef]
- Yurewicz, E.C.; Pack, B.A.; Sacco, A.G. Isolation, composition, and biological activity of sugar chains of porcine oocyte zona pellucida 55K glycoproteins. Mol. Reprod. Dev. 1991, 30, 126–134. [Google Scholar] [CrossRef]
- Velásquez, J.G.; Canovas, S.; Barajas, P.; Marcos, J.; Jiménez-Movilla, M.; Gallego, R.G.; Ballesta, J.; Avilés, M.; Coy, P. Role of sialic acid in bovine sperm-zona pellucida binding. Mol. Reprod. Dev. 2007, 74, 617–628. [Google Scholar] [CrossRef]
- Sutovsky, P. Sperm-egg adhesion and fusion in mammals. Exp. Rev. Mol. Med. 2009, 11, 1–12. [Google Scholar] [CrossRef]
- Evans, J.P. Sperm-Egg Interaction. Annu. Rev. Physiol. 2012, 74, 477–502. [Google Scholar] [CrossRef]
- Boldt, J.; Howe, A.M.; Parkerson, J.B.; Gunter, L.E.; Kuehn, E. Carbohydrate involvement in sperm-egg fusion in mice. Biol. Reprod. 1989, 40, 887–896. [Google Scholar] [CrossRef]
- Ponce, R.H.; Urch, U.A.; Yanagimachi, R. Inhibition of sperm-egg fusion in the hamster and mouse by carbohydrate. Zygote 1994, 2, 253–262. [Google Scholar]
- Gabriele, A.; Andrea, G.; Cordeschi, G.; Properzi, G.; Giammatteo, M.; Stefano, C.D.; Romano, R.; Francavilla, F.; Francavilla, S. Carbohydrate binding activity in human spermatozoa: localization, specificity, and involvement in sperm-egg fusion. Mol. Hum. Reprod. 1998, 4, 543–553. [Google Scholar] [CrossRef]
- Gougoulidis, T.; Trounson, A.; Dowsing, A. Inhibition of bovine sperm-oocyte fusion by the carbohydrate GalNAc. Mol. Reprod. Dev. 1999, 54, 179–185. [Google Scholar] [CrossRef]
- Tanghe, S.; Soom, A.V.; Duchateau, L.; Kruif, A.D. Inhibition of bovine sperm-oocyte fusion by the p-aminophenyl derivative of D-mannose. Mol. Reprod. Dev. 2004, 67, 224–232. [Google Scholar] [CrossRef]
- Yanagimachi, R. Penetration of guinea pig spermatozoa into hamster eggs in vitro. J. Reprod. Fertil. 1972, 28, 477–480. [Google Scholar] [CrossRef]
- Hanada, A.; Chang, M.C. Penetration of zona-free eggs by spermatozoa of different species. Biol. Reprod. 1972, 6, 300–309. [Google Scholar]
- Bedford, J.M. Sperm/egg interaction: the specificity of human spermatozoa. Anat. Rec. 1977, 188, 477–487. [Google Scholar] [CrossRef]
- Lanzendorf, A.E.; Holmgren, W.J.; Johnson, D.E.; Scobey, M.J.; Jeyendran, R.S. Hemizona assay for measuring zona binding in the lowland gorilla. Mol. Reprod. Dev. 1992, 31, 264–267. [Google Scholar] [CrossRef]
- Oehninger, S.; Mahony, M.C.; Swanson, J.R.; Hodgen, G.D. The specificity of human spermatozoa/zona pellucida interaction under hemizona assay conditions. Mol. Reprod. Dev. 1993, 35, 57–61. [Google Scholar] [CrossRef]
- Canovas, S.; Coy, P.; Gomez, E. First steps in the development of a functional assay for human sperm using pig oocytes. J. Androl. 2007, 28, 273–281. [Google Scholar]
- Sinowatz, F.; Wessa, E.; Neumuller, C.; Palma, G. On the species specificity of sperm bindingand sperm penetration of the zona pellucida. Reprod. Dom. Anim. 2003, 38, 141–146. [Google Scholar] [CrossRef]
- Mugnier, S.; Dell'Aquila, M.E.; Pelaez, J.; Douet, C.; Ambruosi, B.; Santis, T.D.; Lacalandra, G.M.; Lebos, C.; Sizaret, P.-Y.; Delaleu, B.; et al. New Insights into the Mechanisms of Fertilization: Comparison of the Fertilization Steps, Composition, and Structure of the Zona Pellucida Between Horses and Pigs. Biol. Reprod. 2009, 81, 856–870. [Google Scholar] [CrossRef]
- Nakano, M.; Yonezawa, N. Localization of sperm ligand carbohydrate chains in pig zona pellucida glycoproteins. Cells Tissues Organs 2001, 168, 65–75. [Google Scholar] [CrossRef]
- Peterson, R.N.; Russel, L.D.; Bundman, D.; Conway, M.; Freund, M. The interaction of living boar sperm and plasma membrane vesicles with the porcine zona pellucida. Dev. Biol. 1981, 84, 144–156. [Google Scholar] [CrossRef]
- Ozawa, M.; Nagai, T.; Fahrudin, M.; Karja, N.W.K.; Kaneko, H.; Noguchi, J.; Ohnuma, K.; Kikuchi, K. Addition of glutathione or thioredoxin to culture medium reduced intracellular redox status of porcine IVM/IVF embryos, resulting in improved development to the blastocyst stage. Mol. Reprod. Dev. 2006, 73, 998–1007. [Google Scholar] [CrossRef]
- Matsushita, S.; Tani, T.; Kato, Y.; Tsunoda, Y. Effect of low-temperature bovine ovary storage on the maturation rate and developmental potential of follicular oocytes after in vitro fertilization, parthenogenetic activation, or somatic cell nucleus transfer. Anim. Reprod. Sci. 2004, 84, 293–301. [Google Scholar] [CrossRef]
- Matsukawa, K.; Akagi, S.; Adachi, N.; Kubo, M.; Hirako, M.; Watanabe, S.; Takahashi, S. Effect of ovary storage on development of bovine oocytes after intracytoplasmic sperm injection, parthenogenetic activation, or somatic cell nuclear transfer. J. Mamm. Ova Res. 2007, 24, 114–119. [Google Scholar] [CrossRef]
- Kikuchi, K.; Onishi, A.; Kashiwazaki, N.; Iwamoto, M.; Noguchi, J.; Kaneko, H.; Akita, T.; Nagai, T. Successful piglet production after transfer of blastocysts produced by a modified in vitro system. Biol. Reprod. 2002, 66, 1033–1041. [Google Scholar] [CrossRef]
- Petters, R.M.; Wells, K.D. Culture of pig embryos. J. Reprod. Fertil. Suppl. 1993, 48, 61–73. [Google Scholar]
- Geshi, M.; Yonai, M.; Sakaguchi, M.; Nagai, T. Improvement of in vitro co-culture systems for bovine embryos using a low concentration of carbon dioxide and medium supplemented with b-mercaptoethanol. Theriogenology 1999, 51, 551–558. [Google Scholar] [CrossRef]
- Yonezawa, N.; Hatanaka, Y.; Takeyama, H.; Nakano, M. Binding of pig sperm receptor in the zona pellucida to the boar sperm acrosome. J. Reprod. Fertil. 1995, 103, 1–8. [Google Scholar] [CrossRef]
- Larson, J.L.; Miller, D.J. Simple histochemical stain for acrosomes on sperm from several species. Mol. Reprod. Dev. 1999, 52, 445–449. [Google Scholar] [CrossRef]
- Noguchi, S.; Yonezawa, N.; Katsumata, T.; Hashizume, K.; Kuwayama, M.; Hamano, S.; Watanabe, S.; Nakano, M. Characterization of the zona pellucida glycoproteins from bovine ovarian and fertilized eggs. Biochim. Biophys. Acta 1994, 1201, 7–14. [Google Scholar] [CrossRef]
- Mattioli, M.; Barboni, B.; Lucidi, P.; Seren, E. Identification of capacitation in boar spermatozoa by chlortetracycline staining. Theriogenology 1996, 45, 373–381. [Google Scholar] [CrossRef]
- Watson, P.F. Recent developments and concepts in the cryopreservation of spermatozoa and assessment of their post-thawing function. Reprod. Fertil. Dev. 1995, 7, 871–891. [Google Scholar] [CrossRef]
- Watson, P.F. Cooling of spermatozoa and fertilizing capacity. Reprod. Dom. Anim. 1996, 31, 135–140. [Google Scholar] [CrossRef]
- Ikeda, H.; Kikuchi, K.; Noguchi, J.; Takeda, H.; Shimada, A.; Mizokami, T.; Kaneko, H. Effect of preincubation of cryopreserved porcine epididymal sperm. Theriogenology. 2002, 57, 1309–1318. [Google Scholar] [CrossRef]
- Clark, G.F.; Zimmerman, S.; Lafrenz, D.E.; Yi, Y.-J.; Sutovsky, P. Carbohydrate-Mediated Binding and Induction of Acrosomal Exocytosis in a Boar Sperm-Somatic Cell Adhesion Model. Biol. Reprod. 2010, 83, 623–634. [Google Scholar] [CrossRef]
- Lee, R.T.; Ichikawa, Y.; Fay, M.; Drickamer, K.; Shao, M.C.; Lee, Y.C. Ligand-binding characteristics of rat serum-type mannose-binding protein (MBP-A): homology of binding site architecture with mammalian and chicken hepatic lectins. J. Biol. Chem. 1991, 266, 4810–4815. [Google Scholar]
- Weis, W.I.; Drickamer, K.; Hendrickson, W.A. Structure of a C-type mannose-binding protein complexed with an oligosaccharide. Nature 1992, 360, 127–134. [Google Scholar]
- Hokke, C.H.; Damm, J.B.; Kamerling, J.P.; Vliegenthart, J.F. Structure of three acidic O-linked carbohydrate chains of porcine zona pellucida glycoproteins. FEBS Lett. 1993, 329, 29–34. [Google Scholar] [CrossRef]
- Hokke, C.H.; Damm, J.B.; Penninkhof, B.; Aitken, R.J.; Kamerling, J.P.; Vliegenthart, J.F. Structure of the O-linked carbohydrate chains of porcine zona pellucida glycoproteins. Eur. J. Biochem. 1994, 221, 491–512. [Google Scholar] [CrossRef]
- Noguchi, S.; Hatanaka, Y.; Tobita, T.; Nakano, M. Structural analysis of the N-linked carbohydrate chains of the 55-kDa glycoprotein family (PZP3) from porcine zona pellucida. Eur. J. Biochem. 1992, 204, 1089–1100. [Google Scholar]
- Noguchi, S.; Nakano, M. Structure of the acidic N-linked carbohydrate chains of the 55-kDaglycoprotein family (PZP3) from porcine zona pellucida. Eur. J. Biochem. 1992, 209, 883–894. [Google Scholar] [CrossRef]
- Katsumata, T.; Noguchi, S.; Yonezawa, N.; Tanokura, M.; Nakano, M. Structural characterizationof the N-linked carbohydrate chains of the zona pellucida glycoproteins from bovine ovarian and fertilized eggs. Eur. J. Biochem. 1996, 240, 448–453. [Google Scholar]
- Witzendorff, D.; Ekhlasi-Hunderieser, M.; Dostalova, Z.; Resch, M.; Rath, D.; Michelmann, H.W.; Töpfer-Petersen, E. Analysis of N-linked glycans of porcine zona pellucida glycoprotein ZPA by MALDI-TOF MS: a contribution to understanding zona pellucida structure. Glycobiology. 2005, 15, 475–488. [Google Scholar]
- Töpfer-Petersen, E.; Ekhlasi-Hunderieser, M.; Tsolova, M. Glycobiology of fertilization in the pig. Int. J. Dev. Biol. 2008, 52, 717–736. [Google Scholar] [CrossRef]
- Witzendorff, D.; Maass, K.; Pich, A.; Ebeling, S.; Kölle, S.; Kochel, C.; Ekhlasi-Hunderieser, M.; Geyer, H.; Geyer, R.; Töpfer-Petersen, E. Characterization of the acidic N-linked glycans of the zona pellucida of prepuberal pigs by a mass spectrometric approach. Carbohydrate Res. 2009, 344, 1541–1549. [Google Scholar] [CrossRef]
- Hedrick, J.L.; Wardrip, N.J. On the macromolecular composition of the zona pellucida from porcine oocytes. Dev. Biol. 1987, 121, 478–488. [Google Scholar] [CrossRef]
- Rath, D.; Töpfer-Petersen, E.; Michelmann, H.W.; Schwartz, P.; Ebeling, S. Zona pellucida characteristics and sperm-binding patterns of in vivo and in vitro produced porcine oocytes inseminated with differently prepared spermatozoa. Theriogenology. 2005, 63, 352–362. [Google Scholar] [CrossRef]
- Michelmann, H.W.; Rath, D.; Töpfer-Petersen, E.; Schwartz, P. Structural and functional events on the porcine zona pellucida during maturation, fertilization and embryonic development: a scanning electron microscopy analysis. Reprod. Domest. Anim. 2007, 42, 594–602. [Google Scholar] [CrossRef]
- Töpfer-Petersen, E. Carbohydrate-based interactions on the route of a spermatozoon to fertilization. Hum. Reprod. Update 1999, 5, 314–329. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Takahashi, K.; Kikuchi, K.; Uchida, Y.; Kanai-Kitayama, S.; Suzuki, R.; Sato, R.; Toma, K.; Geshi, M.; Akagi, S.; Nakano, M.; et al. Binding of Sperm to the Zona Pellucida Mediated by Sperm Carbohydrate-Binding Proteins is not Species-Specific in Vitro between Pigs and Cattle. Biomolecules 2013, 3, 85-107. https://doi.org/10.3390/biom3010085
Takahashi K, Kikuchi K, Uchida Y, Kanai-Kitayama S, Suzuki R, Sato R, Toma K, Geshi M, Akagi S, Nakano M, et al. Binding of Sperm to the Zona Pellucida Mediated by Sperm Carbohydrate-Binding Proteins is not Species-Specific in Vitro between Pigs and Cattle. Biomolecules. 2013; 3(1):85-107. https://doi.org/10.3390/biom3010085
Chicago/Turabian StyleTakahashi, Kazuya, Kazuhiro Kikuchi, Yasuomi Uchida, Saeko Kanai-Kitayama, Reiichiro Suzuki, Reiko Sato, Kazunori Toma, Masaya Geshi, Satoshi Akagi, Minoru Nakano, and et al. 2013. "Binding of Sperm to the Zona Pellucida Mediated by Sperm Carbohydrate-Binding Proteins is not Species-Specific in Vitro between Pigs and Cattle" Biomolecules 3, no. 1: 85-107. https://doi.org/10.3390/biom3010085
APA StyleTakahashi, K., Kikuchi, K., Uchida, Y., Kanai-Kitayama, S., Suzuki, R., Sato, R., Toma, K., Geshi, M., Akagi, S., Nakano, M., & Yonezawa, N. (2013). Binding of Sperm to the Zona Pellucida Mediated by Sperm Carbohydrate-Binding Proteins is not Species-Specific in Vitro between Pigs and Cattle. Biomolecules, 3(1), 85-107. https://doi.org/10.3390/biom3010085