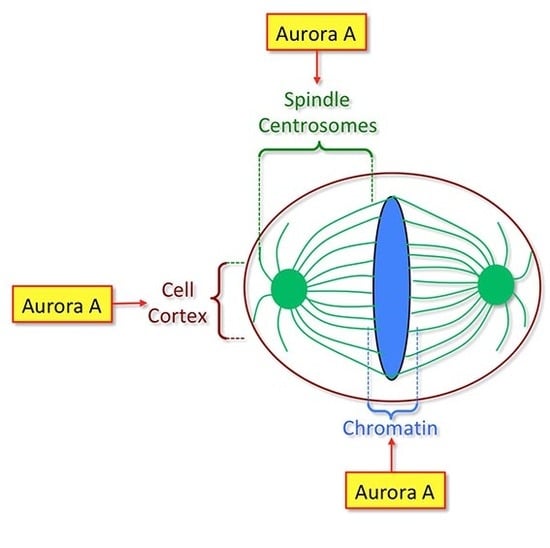
Aurora A Protein Kinase: To the Centrosome and Beyond
Abstract
:1. Introduction
2. Aurora A Regulates Centrosome Maturation, Centrosome Integrity and Centrosomal Microtubule Polymerisation
3. Aurora A Controls Mitotic Spindle Morphogenesis
4. Regulation of Cortical Proteins by Aurora A
5. Regulation of Centromeric Proteins by Aurora A
5.1. Role of Aurora A in Kinetochore-Microtubules (KT-MTs) Attachment
5.2. Aurora A and Chromatin-Associated Transcription Factors during Mitosis
5.3. Role of Aurora A in the Maintenance of Sister Chromatid Cohesion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nigg, E.A. Mitotic kinases as regulators of cell division and its checkpoints. Nat. Rev. Mol. Cell Biol. 2001, 2, 21–32. [Google Scholar] [CrossRef]
- Francisco, L.; Chan, C.S. Regulation of yeast chromosome segregation by Ipl1 protein kinase and type 1 protein phosphatase. Cell. Mol. Biol. Res. 1994, 40, 207–213. [Google Scholar] [PubMed]
- Glover, D.M.; Leibowitz, M.H.; McLean, D.A.; Parry, H. Mutations in aurora prevent centrosome separation leading to the formation of monopolar spindles. Cell 1995, 81, 95–105. [Google Scholar] [CrossRef]
- Abe, Y.; Okumura, E.; Hosoya, T.; Hirota, T.; Kishimoto, T. A single starfish Aurora kinase performs the combined functions of Aurora-A and Aurora-B in human cells. J. Cell Sci. 2010, 123, 3978–3988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, J.; Hagan, I.M. S. Pombe aurora kinase/survivin is required for chromosome condensation and the spindle checkpoint attachment response. Curr. Biol. 2003, 13, 590–597. [Google Scholar] [CrossRef]
- Carmena, M.; Earnshaw, W.C. The cellular geography of aurora kinases. Nat. Rev. Mol. Cell Biol. 2003, 4, 842–854. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, R.D.; Tse, A.; Schwartz, G.K. Aurora kinases: New targets for cancer therapy. Clin. Cancer Res. 2006, 12, 6869–6875. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Gao, K.; Chu, L.; Zhang, R.; Yang, J.; Zheng, J. Aurora kinases: Novel therapy targets in cancers. Oncotarget 2017, 8, 23937–23954. [Google Scholar] [CrossRef] [PubMed]
- Barr, A.R.; Gergely, F. Aurora-A: The maker and breaker of spindle poles. J. Cell Sci. 2007, 120, 2987–2996. [Google Scholar] [CrossRef]
- Giet, R.; McLean, D.; Descamps, S.; Lee, M.J.; Raff, J.W.; Prigent, C.; Glover, D.M. Drosophila aurora A kinase is required to localize D-TACC to centrosomes and to regulate astral microtubules. J. Cell Biol. 2002, 156, 437–451. [Google Scholar] [CrossRef]
- Roghi, C.; Giet, R.; Uzbekov, R.; Morin, N.; Chartrain, I.; Le Guellec, R.; Couturier, A.; Doree, M.; Philippe, M.; Prigent, C. The Xenopus protein kinase pEg2 associates with the centrosome in a cell cycle-dependent manner, binds to the spindle microtubules and is involved in bipolar mitotic spindle assembly. J. Cell Sci. 1998, 111 Pt 5, 557–572. [Google Scholar]
- Eot-Houllier, G.; Magnaghi-Jaulin, L.; Fulcrand, G.; Moyroud, F.X.; Monier, S.; Jaulin, C. Aurora A-dependent CENP-A phosphorylation at inner centromeres protects bioriented chromosomes against cohesion fatigue. Nat. Commun. 2018, 9, 1888. [Google Scholar] [CrossRef] [PubMed]
- Afonso, O.; Figueiredo, A.C.; Maiato, H. Late mitotic functions of aurora kinases. Chromosoma 2017, 126, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Carmena, M.; Wheelock, M.; Funabiki, H.; Earnshaw, W.C. The chromosomal passenger complex (CPC): From easy rider to the godfather of mitosis. Nat. Rev. Mol. Cell Biol. 2012, 13, 789–803. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Stukenberg, P.T. A centromere-signaling network underlies the coordination among mitotic events. Trends Biochem. Sci. 2015, 41, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.T.; Tang, C.J.; Tang, T.K. Possible role of aurora-C in meiosis. Front. Oncol. 2015, 5, 178. [Google Scholar] [CrossRef] [PubMed]
- Balboula, A.Z.; Nguyen, A.L.; Gentilello, A.S.; Quartuccio, S.M.; Drutovic, D.; Solc, P.; Schindler, K. Haspin kinase regulates microtubule-organizing center clustering and stability through aurora kinase C in mouse oocytes. J. Cell Sci. 2016, 129, 3648–3660. [Google Scholar] [CrossRef]
- Nguyen, A.L.; Drutovic, D.; Vazquez, B.N.; El Yakoubi, W.; Gentilello, A.S.; Malumbres, M.; Solc, P.; Schindler, K. Genetic interactions between the aurora kinases reveal new requirements for AURKB and AURKC during oocyte meiosis. Curr. Biol. 2018, 28, 3458–3468.e3455. [Google Scholar] [CrossRef]
- Carmena, M.; Ruchaud, S.; Earnshaw, W.C. Making the auroras glow: Regulation of aurora A and B kinase function by interacting proteins. Curr. Opin. Cell Biol. 2009, 21, 796–805. [Google Scholar] [CrossRef]
- Li, S.; Deng, Z.; Fu, J.; Xu, C.; Xin, G.; Wu, Z.; Luo, J.; Wang, G.; Zhang, S.; Zhang, B.; et al. Spatial compartmentalization specializes the function of aurora A and aurora B. J. Biol. Chem. 2015, 290, 17546–17558. [Google Scholar] [CrossRef]
- Ferrari, S.; Marin, O.; Pagano, M.A.; Meggio, F.; Hess, D.; El-Shemerly, M.; Krystyniak, A.; Pinna, L.A. Aurora-A site specificity: A study with synthetic peptide substrates. Biochem. J. 2005, 390, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Kettenbach, A.N.; Schweppe, D.K.; Faherty, B.K.; Pechenick, D.; Pletnev, A.A.; Gerber, S.A. Quantitative phosphoproteomics identifies substrates and functional modules of aurora and Polo-like kinase activities in mitotic cells. Sci. Signal 2011, 4, rs5. [Google Scholar] [CrossRef] [PubMed]
- Giet, R.; Petretti, C.; Prigent, C. Aurora kinases, aneuploidy and cancer, a coincidence or a real link? Trends Cell Biol. 2005, 15, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Berdnik, D.; Knoblich, J.A. Drosophila aurora-a is required for centrosome maturation and actin-dependent asymmetric protein localization during mitosis. Curr. Biol. 2002, 12, 640–647. [Google Scholar] [CrossRef]
- Giet, R.; Prigent, C. The non-catalytic domain of the Xenopus laevis auroraA kinase localises the protein to the centrosome. J. Cell Sci. 2001, 114, 2095–2104. [Google Scholar]
- Hannak, E.; Kirkham, M.; Hyman, A.A.; Oegema, K. Aurora-A kinase is required for centrosome maturation in Caenorhabditis elegans. J. Cell Biol. 2001, 155, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Kufer, T.A.; Sillje, H.H.; Korner, R.; Gruss, O.J.; Meraldi, P.; Nigg, E.A. Human TPX2 is required for targeting aurora-A kinase to the spindle. J. Cell Biol. 2002, 158, 617–623. [Google Scholar] [CrossRef]
- Schumacher, J.M.; Ashcroft, N.; Donovan, P.J.; Golden, A. A highly conserved centrosomal kinase, AIR-1, is required for accurate cell cycle progression and segregation of developmental factors in Caenorhabditis elegans embryos. Development 1998, 125, 4391–4402. [Google Scholar]
- Tillery, M.M.L.; Blake-Hedges, C.; Zheng, Y.; Buchwalter, R.A.; Megraw, T.L. Centrosomal and non-centrosomal microtubule-organizing centers (MTOCs) in Drosophila melanogaster. Cells 2018, 7, 121. [Google Scholar] [CrossRef]
- Muller, H.; Schmidt, D.; Steinbrink, S.; Mirgorodskaya, E.; Lehmann, V.; Habermann, K.; Dreher, F.; Gustavsson, N.; Kessler, T.; Lehrach, H.; et al. Proteomic and functional analysis of the mitotic Drosophila centrosome. EMBO J. 2010, 29, 3344–3357. [Google Scholar] [CrossRef]
- Reboutier, D.; Troadec, M.B.; Cremet, J.Y.; Fukasawa, K.; Prigent, C. Nucleophosmin/B23 activates aurora A at the centrosome through phosphorylation of serine 89. J. Cell Biol. 2012, 197, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Joukov, V.; De Nicolo, A.; Rodriguez, A.; Walter, J.C.; Livingston, D.M. Centrosomal protein of 192 kDa (Cep192) promotes centrosome-driven spindle assembly by engaging in organelle-specific aurora A activation. Proc. Natl. Acad. Sci. USA 2010, 107, 21022–21027. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, L.; Ozlu, N.; Hannak, E.; Cowan, C.; Habermann, B.; Ruer, M.; Muller-Reichert, T.; Hyman, A.A. The Caenorhabditis elegans centrosomal protein SPD-2 is required for both pericentriolar material recruitment and centriole duplication. Curr. Biol. 2004, 14, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Lawo, S.; Bird, A.; Pinchev, D.; Ralph, A.; Richter, C.; Muller-Reichert, T.; Kittler, R.; Hyman, A.A.; Pelletier, L. The mammalian SPD-2 ortholog CEP192 regulates centrosome biogenesis. Curr. Biol. 2008, 18, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Joukov, V.; Walter, J.C.; De Nicolo, A. The CEP192-organized aurora A-Plk1 cascade is essential for centrosome cycle and bipolar spindle assembly. Mol. Cell 2014, 55, 578–591. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Park, J.E.; Kim, T.S.; Lee, E.H.; Park, S.Y.; Zhou, M.; Bang, J.K.; Lee, K.S. Bimodal interaction of mammalian Polo-like kinase 1 and a centrosomal scaffold, CEP192, in the regulation of bipolar spindle formation. Mol. Cell Biol. 2015, 35, 2626–2640. [Google Scholar] [CrossRef] [PubMed]
- Fong, K.W.; Choi, Y.K.; Rattner, J.B.; Qi, R.Z. CDK5RAP2 is a pericentriolar protein that functions in centrosomal attachment of the γ-tubulin ring complex. Mol. Biol. Cell 2008, 19, 115–125. [Google Scholar] [CrossRef]
- Zhang, J.; Megraw, T.L. Proper recruitment of γ-tubulin and D-TACC/Msps to embryonic Drosophila centrosomes requires centrosomin Motif 1. Mol. Biol. Cell 2007, 18, 4037–4049. [Google Scholar] [CrossRef]
- Conduit, P.T.; Feng, Z.; Richens, J.H.; Baumbach, J.; Wainman, A.; Bakshi, S.D.; Dobbelaere, J.; Johnson, S.; Lea, S.M.; Raff, J.W. The centrosome-specific phosphorylation of Cnn by Polo/Plk1 drives Cnn scaffold assembly and centrosome maturation. Dev. Cell 2014, 28, 659–669. [Google Scholar] [CrossRef]
- Conduit, P.T.; Raff, J.W. Different Drosophila cell types exhibit differences in mitotic centrosome assembly dynamics. Curr. Biol. 2015, 25, R650–R651. [Google Scholar] [CrossRef]
- Barros, T.P.; Kinoshita, K.; Hyman, A.A.; Raff, J.W. Aurora A activates D-TACC-Msps complexes exclusively at centrosomes to stabilize centrosomal microtubules. J. Cell Biol. 2005, 170, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, K.; Noetzel, T.L.; Pelletier, L.; Mechtler, K.; Drechsel, D.N.; Schwager, A.; Lee, M.; Raff, J.W.; Hyman, A.A. Aurora A phosphorylation of TACC3/Maskin is required for centrosome-dependent microtubule assembly in mitosis. J. Cell Biol. 2005, 170, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Gergely, F.; Jeffers, K.; Peak-Chew, S.Y.; Raff, J.W. Msps/Xmap215 interacts with the centrosomal protein D-TACC to regulate microtubule behaviour. Nat. Cell Biol. 2001, 3, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Chou, E.J.; Hung, L.Y.; Tang, C.J.; Hsu, W.B.; Wu, H.Y.; Liao, P.C.; Tang, T.K. Phosphorylation of CPAP by aurora-A maintains spindle pole integrity during mitosis. Cell Rep. 2016, 14, 2975–2987. [Google Scholar] [CrossRef] [PubMed]
- De Luca, M.; Brunetto, L.; Asteriti, I.A.; Giubettini, M.; Lavia, P.; Guarguaglini, G. Aurora-A and ch-TOG act in a common pathway in control of spindle pole integrity. Oncogene 2008, 27, 6539. [Google Scholar] [CrossRef]
- Marumoto, T.; Honda, S.; Hara, T.; Nitta, M.; Hirota, T.; Kohmura, E.; Saya, H. Aurora-A kinase maintains the fidelity of early and late mitotic events in Hela cells. J. Biol. Chem. 2003, 278, 51786–51795. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, T.; Boleti, H.; Antony, C.; Karsenti, E.; Vernos, I. Localization of the kinesin-like protein Xklp2 to spindle poles requires a leucine zipper, a microtubule-associated protein, and dynein. J. Cell Biol. 1998, 143, 673–685. [Google Scholar] [CrossRef]
- Wittmann, T.; Wilm, M.; Karsenti, E.; Vernos, I. Tpx2, a novel Xenopus map involved in spindle pole organization. J. Cell Biol. 2000, 149, 1405–1418. [Google Scholar] [CrossRef] [PubMed]
- Carazo-Salas, R.E.; Gruss, O.J.; Mattaj, I.W.; Karsenti, E. Ran-GTP coordinates regulation of microtubule nucleation and dynamics during mitotic-spindle assembly. Nat. Cell Biol. 2001, 3, 228–234. [Google Scholar] [CrossRef]
- Carazo-Salas, R.E.; Guarguaglini, G.; Gruss, O.J.; Segref, A.; Karsenti, E.; Mattaj, I.W. Generation of GTP-bound ran by RCC1 is required for chromatin-induced mitotic spindle formation. Nature 1999, 400, 178–181. [Google Scholar] [CrossRef]
- Bayliss, R.; Sardon, T.; Vernos, I.; Conti, E. Structural basis of aurora-A activation by TPX2 at the mitotic spindle. Mol. Cell 2003, 12, 851–862. [Google Scholar] [CrossRef]
- Eyers, P.A.; Erikson, E.; Chen, L.G.; Maller, J.L. A novel mechanism for activation of the protein kinase aurora A. Curr. Biol. 2003, 13, 691–697. [Google Scholar] [CrossRef]
- Zorba, A.; Buosi, V.; Kutter, S.; Kern, N.; Pontiggia, F.; Cho, Y.J.; Kern, D. Molecular mechanism of aurora A kinase autophosphorylation and its allosteric activation by TPX2. Elife 2014, 3, e02667. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.Y.; Wiese, C.; Cao, K.; Martin, O.; Donovan, P.; Ruderman, J.; Prigent, C.; Zheng, Y. A Ran signalling pathway mediated by the mitotic kinase aurora A in spindle assembly. Nat. Cell Biol. 2003, 5, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; He, X.; Wang, S.; Jia, J.; Wan, Y.; Wang, Y.; Zeng, R.; Yates, J., 3rd; Zhu, X.; Zheng, Y. A microtubule-associated zinc finger protein, BuGZ, regulates mitotic chromosome alignment by ensuring Bub3 stability and kinetochore targeting. Dev. Cell 2014, 28, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, S.; Huang, Y.; He, X.; Cui, H.; Zhu, X.; Zheng, Y. Phase transition of spindle-associated protein regulate spindle apparatus assembly. Cell 2015, 163, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, T.; Ems-McClung, S.C.; Walczak, C.E.; Prigent, C.; Zhu, X.; Zhang, X.; Zheng, Y. Aurora A activation in mitosis promoted by BuGZ. J. Cell Biol. 2018, 217, 107–116. [Google Scholar] [CrossRef]
- Haren, L.; Remy, M.H.; Bazin, I.; Callebaut, I.; Wright, M.; Merdes, A. Nedd1-dependent recruitment of the γ-tubulin ring complex to the centrosome is necessary for centriole duplication and spindle assembly. J. Cell Biol. 2006, 172, 505–515. [Google Scholar] [CrossRef]
- Luders, J.; Patel, U.K.; Stearns, T. GCP-WD is a γ-tubulin targeting factor required for centrosomal and chromatin-mediated microtubule nucleation. Nat. Cell Biol. 2006, 8, 137–147. [Google Scholar] [CrossRef]
- Pinyol, R.; Scrofani, J.; Vernos, I. The role of NEDD1 phosphorylation by aurora A in chromosomal microtubule nucleation and spindle function. Curr. Biol. 2013, 23, 143–149. [Google Scholar] [CrossRef]
- Goshima, G.; Mayer, M.; Zhang, N.; Stuurman, N.; Vale, R.D. Augmin: A protein complex required for centrosome-independent microtubule generation within the spindle. J. Cell Biol. 2008, 181, 421–429. [Google Scholar] [CrossRef]
- Petry, S.; Groen, A.C.; Ishihara, K.; Mitchison, T.J.; Vale, R.D. Branching microtubule nucleation in Xenopus egg extracts mediated by augmin and TPX2. Cell 2013, 152, 768–777. [Google Scholar] [CrossRef]
- Uehara, R.; Nozawa, R.S.; Tomioka, A.; Petry, S.; Vale, R.D.; Obuse, C.; Goshima, G. The augmin complex plays a critical role in spindle microtubule generation for mitotic progression and cytokinesis in human cells. Proc. Natl. Acad. Sci. USA 2009, 106, 6998–7003. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Lin, Y.T.; Wei, R.; Chen, Y.; Shan, Z.; Lee, W.H. Hice1, a novel microtubule-associated protein required for the maintenance of spindle integrity and chromosomal stability in human cells. Mol. Cell Biol. 2008, 28, 3652–3662. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Ngo, B.; Tapadia, A.; Hsu, P.H.; Wu, G.; Lee, W.H. Aurora-A phosphorylates augmin complex component Hice1 protein at an N-terminal serine/threonine cluster to modulate its microtubule binding activity during spindle assembly. J. Biol. Chem. 2011, 286, 30097–30106. [Google Scholar] [CrossRef]
- McHedlishvili, N.; Matthews, H.K.; Corrigan, A.; Baum, B. Two-step interphase microtubule disassembly aids spindle morphogenesis. BMC Biol. 2018, 16, 14. [Google Scholar] [CrossRef]
- Petrone, A.; Adamo, M.E.; Cheng, C.; Kettenbach, A.N. Identification of candidate cyclin-dependent kinase 1 (Cdk1) substrates in mitosis by quantitative phosphoproteomics. Mol. Cell Proteom. 2016, 15, 2448–2461. [Google Scholar] [CrossRef]
- Verde, F.; Dogterom, M.; Stelzer, E.; Karsenti, E.; Leibler, S. Control of microtubule dynamics and length by cyclin A- and cyclin B-dependent kinases in xenopus egg extracts. J. Cell Biol. 1992, 118, 1097–1108. [Google Scholar] [CrossRef]
- Verde, F.; Labbe, J.C.; Doree, M.; Karsenti, E. Regulation of microtubule dynamics by CDC2 protein kinase in cell-free extracts of Xenopus eggs. Nature 1990, 343, 233–238. [Google Scholar] [CrossRef]
- Rome, P.; Montembault, E.; Franck, N.; Pascal, A.; Glover, D.M.; Giet, R. Aurora A contributes to p150(glued) phosphorylation and function during mitosis. J. Cell Biol. 2010, 189, 651–659. [Google Scholar] [CrossRef]
- Venoux, M.; Basbous, J.; Berthenet, C.; Prigent, C.; Fernandez, A.; Lamb, N.J.; Rouquier, S. ASAP is a novel substrate of the oncogenic mitotic kinase aurora-A: Phosphorylation on Ser625 is essential to spindle formation and mitosis. Hum. Mol. Genet. 2008, 17, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Rong, R.; Jiang, L.Y.; Sheikh, M.S.; Huang, Y. Mitotic kinase aurora-A phosphorylates RASSF1A and modulates RASSF1A-mediated microtubule interaction and M-phase cell cycle regulation. Oncogene 2007, 26, 7700–7708. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.; Wong, N.; Pagano, M.; Lun, S.W.; Nakayama, K.I.; Nakayama, K.; Lo, K.W. Regulation of APC/CCdc20 activity by RASSF1A-APC/CCDC20 circuitry. Oncogene 2012, 31, 1975–1987. [Google Scholar] [CrossRef] [PubMed]
- Song, S.J.; Kim, S.J.; Song, M.S.; Lim, D.S. Aurora B-mediated phosphorylation of RASSF1A maintains proper cytokinesis by recruiting syntaxin16 to the midzone and midbody. Cancer Res. 2009, 69, 8540–8544. [Google Scholar] [CrossRef]
- Yu, T.W.; Mochida, G.H.; Tischfield, D.J.; Sgaier, S.K.; Flores-Sarnat, L.; Sergi, C.M.; Topcu, M.; McDonald, M.T.; Barry, B.J.; Felie, J.M.; et al. Mutations in WDR62, encoding a centrosome-associated protein, cause microcephaly with simplified gyri and abnormal cortical architecture. Nat. Genet. 2010, 42, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Lim, N.R.; Yeap, Y.Y.; Zhao, T.T.; Yip, Y.Y.; Wong, S.C.; Xu, D.; Ang, C.S.; Williamson, N.A.; Xu, Z.; Bogoyevitch, M.A.; et al. Opposing roles for JNK and aurora A in regulating the association of wdr62 with spindle microtubules. J. Cell Sci. 2015, 128, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.F.; Zhang, Y.; Wilde, J.; Hansen, K.C.; Lai, F.; Niswander, L. Microcephaly disease gene WDR62 regulates mitotic progression of embryonic neural stem cells and brain size. Nat. Commun. 2014, 5, 3885. [Google Scholar] [CrossRef] [PubMed]
- Wordeman, L.; Mitchison, T.J. Identification and partial characterization of mitotic centromere-associated kinesin, a kinesin-related protein that associates with centromeres during mitosis. J. Cell Biol. 1995, 128, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Andrews, P.D.; Ovechkina, Y.; Morrice, N.; Wagenbach, M.; Duncan, K.; Wordeman, L.; Swedlow, J.R. Aurora B regulates MCAK at the mitotic centromere. Dev. Cell 2004, 6, 253–268. [Google Scholar] [CrossRef]
- Knowlton, A.L.; Lan, W.; Stukenberg, P.T. Aurora B is enriched at merotelic attachment sites, where it regulates MCAK. Curr. Biol. 2006, 16, 1705–1710. [Google Scholar] [CrossRef]
- Lan, W.; Zhang, X.; Kline-Smith, S.L.; Rosasco, S.E.; Barrett-Wilt, G.A.; Shabanowitz, J.; Hunt, D.F.; Walczak, C.E.; Stukenberg, P.T. Aurora B phosphorylates centromeric MCAK and regulates its localization and microtubule depolymerization activity. Curr. Biol. 2004, 14, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ems-McClung, S.C.; Walczak, C.E. Aurora A phosphorylates MCAK to control ran-dependent spindle bipolarity. Mol. Biol. Cell 2008, 19, 2752–2765. [Google Scholar] [CrossRef]
- Tanenbaum, M.E.; Macurek, L.; van der Vaart, B.; Galli, M.; Akhmanova, A.; Medema, R.H. A complex of Kif18b and MCAK promotes microtubule depolymerization and is negatively regulated by aurora kinases. Curr. Biol. 2011, 21, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Reboutier, D.; Troadec, M.B.; Cremet, J.Y.; Chauvin, L.; Guen, V.; Salaun, P.; Prigent, C. Aurora A is involved in central spindle assembly through phosphorylation of Ser19 in p150glued. J. Cell Biol. 2013, 201, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, C. Spindle orientation, asymmetric division and tumour suppression in Drosophila stem cells. Nat. Rev. Genet. 2007, 8, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Knoblich, J.A. Asymmetric cell division: Recent developments and their implications for tumour biology. Nat. Rev. Mol. Cell Biol. 2010, 11, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Siller, K.H.; Doe, C.Q. Spindle orientation during asymmetric cell division. Nat. Cell Biol. 2009, 11, 365–374. [Google Scholar] [CrossRef]
- Wang, H.; Somers, G.W.; Bashirullah, A.; Heberlein, U.; Yu, F.; Chia, W. Aurora-A acts as a tumor suppressor and regulates self-renewal of Drosophila neuroblasts. Genes Dev. 2006, 20, 3453–3463. [Google Scholar] [CrossRef]
- Lee, C.Y.; Andersen, R.O.; Cabernard, C.; Manning, L.; Tran, K.D.; Lanskey, M.J.; Bashirullah, A.; Doe, C.Q. Drosophila aurora-A kinase inhibits neuroblast self-renewal by regulating aPKC/Numb cortical polarity and spindle orientation. Genes Dev. 2006, 20, 3464–3474. [Google Scholar] [CrossRef]
- Wirtz-Peitz, F.; Nishimura, T.; Knoblich, J.A. Linking cell cycle to asymmetric division: Aurora-A phosphorylates the par complex to regulate numb localization. Cell 2008, 135, 161–173. [Google Scholar] [CrossRef]
- Bell, G.P.; Fletcher, G.C.; Brain, R.; Thompson, B.J. Aurora kinases phosphorylate Lgl to induce mitotic spindle orientation in Drosophila epithelia. Curr. Biol. 2015, 25, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.A.; Moreira, S.; Ventura, G.; Sunkel, C.E.; Morais-de-Sa, E. Aurora a triggers Lgl cortical release during symmetric division to control planar spindle orientation. Curr. Biol. 2015, 25, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Gibson, M.C. Epithelial cell division: Aurora kicks Lgl to the cytoplasmic curb. Curr. Biol. 2015, 25, R43–R45. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.A.; Hirono, K.; Prehoda, K.E.; Doe, C.Q. Identification of an aurora-A/PinsLINKER/Dlg spindle orientation pathway using induced cell polarity in S2 cells. Cell 2009, 138, 1150–1163. [Google Scholar] [CrossRef] [PubMed]
- Merdes, A.; Heald, R.; Samejima, K.; Earnshaw, W.C.; Cleveland, D.W. Formation of spindle poles by dynein/dynactin-dependent transport of NuMA. J. Cell Biol. 2000, 149, 851–862. [Google Scholar] [CrossRef]
- Merdes, A.; Ramyar, K.; Vechio, J.D.; Cleveland, D.W. A complex of NuMA and cytoplasmic dynein is essential for mitotic spindle assembly. Cell 1996, 87, 447–458. [Google Scholar] [CrossRef]
- di Pietro, F.; Echard, A.; Morin, X. Regulation of mitotic spindle orientation: An integrated view. EMBO Rep. 2016, 17, 1106–1130. [Google Scholar] [CrossRef]
- Gallini, S.; Carminati, M.; De Mattia, F.; Pirovano, L.; Martini, E.; Oldani, A.; Asteriti, I.A.; Guarguaglini, G.; Mapelli, M. NuMa phosphorylation by aurora-a orchestrates spindle orientation. Curr. Biol. 2016, 26, 458–469. [Google Scholar] [CrossRef]
- Mangal, S.; Sacher, J.; Kim, T.; Osorio, D.S.; Motegi, F.; Carvalho, A.X.; Oegema, K.; Zanin, E. TPXL-1 activates aurora A to clear contractile ring components from the polar cortex during cytokinesis. J. Cell Biol. 2018, 217, 837–848. [Google Scholar] [CrossRef]
- Bloom, K.; Joglekar, A. Towards building a chromosome segregation machine. Nature 2010, 463, 446–456. [Google Scholar] [CrossRef] [Green Version]
- Fitzgerald-Hayes, M. Yeast centromeres. Yeast 1987, 3, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Verdaasdonk, J.S.; Bloom, K. Centromeres: Unique chromatin structures that drive chromosome segregation. Nat. Rev. Mol. Cell Biol. 2011, 12, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Westhorpe, F.G.; Straight, A.F. The centromere: Epigenetic control of chromosome segregation during mitosis. Cold Spring Harb. Perspect. Biol. 2014, 7, a015818. [Google Scholar] [CrossRef] [PubMed]
- Willard, H.F. Centromeres of mammalian chromosomes. Trends Genet. 1990, 6, 410–416. [Google Scholar] [CrossRef]
- Cleveland, D.W.; Mao, Y.; Sullivan, K.F. Centromeres and kinetochores: From epigenetics to mitotic checkpoint signaling. Cell 2003, 112, 407–421. [Google Scholar] [CrossRef]
- Allshire, R.C.; Karpen, G.H. Epigenetic regulation of centromeric chromatin: Old dogs, new tricks? Nat. Rev. Genet. 2008, 9, 923–937. [Google Scholar] [CrossRef] [PubMed]
- Bernad, R.; Sanchez, P.; Losada, A. Epigenetic specification of centromeres by CENP-A. Exp. Cell Res. 2009, 315, 3233–3241. [Google Scholar] [CrossRef] [PubMed]
- Black, B.E.; Bassett, E.A. The histone variant CENP-A and centromere specification. Curr. Opin. Cell Biol. 2008, 20, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Black, B.E.; Jansen, L.E.; Foltz, D.R.; Cleveland, D.W. Centromere identity, function, and epigenetic propagation across cell divisions. Cold Spring Harb. Symp. Quant. Biol. 2010, 75, 403–418. [Google Scholar] [CrossRef]
- Choo, K.H. Domain organization at the centromere and neocentromere. Dev. Cell 2001, 1, 165–177. [Google Scholar] [CrossRef]
- Henikoff, S.; Dalal, Y. Centromeric chromatin: What makes it unique? Curr. Opin. Genet. Dev. 2005, 15, 177–184. [Google Scholar] [CrossRef] [PubMed]
- McKinley, K.L.; Cheeseman, I.M. The molecular basis for centromere identity and function. Nat. Rev. Mol. Cell Biol. 2016, 17, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, B.A.; Karpen, G.H. Centromeric chromatin exhibits a histone modification pattern that is distinct from both euchromatin and heterochromatin. Nat. Struct. Mol. Biol. 2004, 11, 1076–1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasmyth, K.; Haering, C.H. Cohesin: Its roles and mechanisms. Annu. Rev. Genet. 2009, 43, 525–558. [Google Scholar] [CrossRef] [PubMed]
- Kunitoku, N.; Sasayama, T.; Marumoto, T.; Zhang, D.; Honda, S.; Kobayashi, O.; Hatakeyama, K.; Ushio, Y.; Saya, H.; Hirota, T. CENP-A phosphorylation by aurora-A in prophase is required for enrichment of aurora-B at inner centromeres and for kinetochore function. Dev. Cell 2003, 5, 853–864. [Google Scholar] [CrossRef]
- Wike, C.L.; Graves, H.K.; Hawkins, R.; Gibson, M.D.; Ferdinand, M.B.; Zhang, T.; Chen, Z.; Hudson, D.F.; Ottesen, J.J.; Poirier, M.G.; et al. Aurora-A mediated histone H3 phosphorylation of threonine 118 controls condensin I and cohesin occupancy in mitosis. Elife 2016, 5, e11402. [Google Scholar] [CrossRef] [PubMed]
- Godek, K.M.; Kabeche, L.; Compton, D.A. Regulation of kinetochore-microtubule attachments through homeostatic control during mitosis. Nat. Rev. Mol. Cell Biol. 2015, 16, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Barisic, M.; Aguiar, P.; Geley, S.; Maiato, H. Kinetochore motors drive congression of peripheral polar chromosomes by overcoming random arm-ejection forces. Nat. Cell Biol. 2014, 16, 1249–1256. [Google Scholar] [CrossRef]
- Chmatal, L.; Yang, K.; Schultz, R.M.; Lampson, M.A. Spatial regulation of kinetochore microtubule attachments by destabilization at spindle poles in meiosis i. Curr. Biol. 2015, 25, 1835–1841. [Google Scholar] [CrossRef]
- Ye, A.A.; Deretic, J.; Hoel, C.M.; Hinman, A.W.; Cimini, D.; Welburn, J.P.; Maresca, T.J. Aurora A kinase contributes to a pole-based error correction pathway. Curr. Biol. 2015, 25, 1842–1851. [Google Scholar] [CrossRef]
- Kim, Y.; Holland, A.J.; Lan, W.; Cleveland, D.W. Aurora kinases and protein phosphatase 1 mediate chromosome congression through regulation of CENP-E. Cell 2010, 142, 444–455. [Google Scholar] [CrossRef]
- Barisic, M.; Silva e Sousa, R.; Tripathy, S.K.; Magiera, M.M.; Zaytsev, A.V.; Pereira, A.L.; Janke, C.; Grishchuk, E.L.; Maiato, H. Microtubule detyrosination guides chromosomes during mitosis. Science 2015, 348, 799–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lampson, M.A.; Cheeseman, I.M. Sensing centromere tension: Aurora B and the regulation of kinetochore function. Trends Cell Biol. 2011, 21, 133–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lampson, M.A.; Renduchitala, K.; Khodjakov, A.; Kapoor, T.M. Correcting improper chromosome-spindle attachments during cell division. Nat. Cell Biol. 2004, 6, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Katayama, H.; Sasai, K.; Kloc, M.; Brinkley, B.R.; Sen, S. Aurora kinase-A regulates kinetochore/chromatin associated microtubule assembly in human cells. Cell Cycle 2008, 7, 2691–2704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeLuca, K.F.; Meppelink, A.; Broad, A.J.; Mick, J.E.; Peersen, O.B.; Pektas, S.; Lens, S.M.A.; DeLuca, J.G. Aurora A kinase phosphorylates Hec1 to regulate metaphase kinetochore-microtubule dynamics. J. Cell Biol. 2018, 217, 163–177. [Google Scholar] [CrossRef]
- Deng, Z.; Cao, P.; Wan, M.M.; Sui, G. Yin yang 1: A multifaceted protein beyond a transcription factor. Transcription 2010, 1, 81–84. [Google Scholar] [CrossRef]
- Gordon, S.; Akopyan, G.; Garban, H.; Bonavida, B. Transcription factor YY1: Structure, function, and therapeutic implications in cancer biology. Oncogene 2006, 25, 1125–1142. [Google Scholar] [CrossRef]
- Alexander, K.E.; Rizkallah, R. Aurora A phosphorylation of YY1 during mitosis inactivates its DNA binding activity. Sci. Rep. 2017, 7, 10084. [Google Scholar] [CrossRef]
- Chuang, L.S.; Khor, J.M.; Lai, S.K.; Garg, S.; Krishnan, V.; Koh, C.G.; Lee, S.H.; Ito, Y. Aurora kinase-induced phosphorylation excludes transcription factor RUNX from the chromatin to facilitate proper mitotic progression. Proc. Natl. Acad. Sci. USA 2016, 113, 6490–6495. [Google Scholar] [CrossRef]
- Kabeche, L.; Nguyen, H.D.; Buisson, R.; Zou, L. A mitosis-specific and R loop-driven ATR pathway promotes faithful chromosome segregation. Science 2018, 359, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Castellano-Pozo, M.; Santos-Pereira, J.M.; Rondon, A.G.; Barroso, S.; Andujar, E.; Perez-Alegre, M.; Garcia-Muse, T.; Aguilera, A. R loops are linked to histone H3 S10 phosphorylation and chromatin condensation. Mol. Cell 2013, 52, 583–590. [Google Scholar] [CrossRef]
- Holt, S.V.; Vergnolle, M.A.; Hussein, D.; Wozniak, M.J.; Allan, V.J.; Taylor, S.S. Silencing CENP-F weakens centromeric cohesion, prevents chromosome alignment and activates the spindle checkpoint. J. Cell Sci. 2005, 118, 4889–4900. [Google Scholar] [CrossRef] [PubMed]
- Marston, A.L. Shugoshins: Tension-sensitive pericentromeric adaptors safeguarding chromosome segregation. Mol. Cell Biol. 2015, 35, 634–648. [Google Scholar] [CrossRef] [PubMed]
- Daum, J.R.; Potapova, T.A.; Sivakumar, S.; Daniel, J.J.; Flynn, J.N.; Rankin, S.; Gorbsky, G.J. Cohesion fatigue induces chromatid separation in cells delayed at metaphase. Curr. Biol. 2011, 21, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.; Gassmann, R.; Oegema, K.; Desai, A. Uncoordinated loss of chromatid cohesion is a common outcome of extended metaphase arrest. PLoS ONE 2011, 6, e22969. [Google Scholar] [CrossRef] [PubMed]
- Caous, R.; Pascal, A.; Rome, P.; Richard-Parpaillon, L.; Karess, R.; Giet, R. Spindle assembly checkpoint inactivation fails to suppress neuroblast tumour formation in aura mutant Drosophila. Nat. Commun. 2015, 6, 8879. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Jiang, Y.; Lu, L.; Cao, M.; Qiao, Y.; Liu, X.; Liu, D.; Van Dyke, T.; Wang, F.; Yao, X.; et al. Aurora-A promotes the establishment of spindle assembly checkpoint by priming the Haspin-aurora-B feedback loop in late G2 phase. Cell Discov. 2017, 3, 16049. [Google Scholar] [CrossRef]
- Zhou, L.; Liang, C.; Chen, Q.; Zhang, Z.; Zhang, B.; Yan, H.; Qi, F.; Zhang, M.; Yi, Q.; Guan, Y.; et al. The N-terminal non-kinase-domain-mediated binding of Haspin to Pds5b protects centromeric cohesion in mitosis. Curr. Biol. 2017, 27, 992–1004. [Google Scholar] [CrossRef]
- Thein, K.H.; Kleylein-Sohn, J.; Nigg, E.A.; Gruneberg, U. Astrin is required for the maintenance of sister chromatid cohesion and centrosome integrity. J. Cell Biol. 2007, 178, 345–354. [Google Scholar] [CrossRef] [Green Version]
- Chiu, S.C.; Chen, J.M.; Wei, T.Y.; Cheng, T.S.; Wang, Y.H.; Ku, C.F.; Lian, C.H.; Liu, C.C.; Kuo, Y.C.; Yu, C.T. The mitosis-regulating and protein-protein interaction activities of Astrin are controlled by aurora-A-induced phosphorylation. Am. J. Physiol. Cell Physiol. 2014, 307, C466–C478. [Google Scholar] [CrossRef] [PubMed]
- Mersfelder, E.L.; Parthun, M.R. The tale beyond the tail: Histone core domain modifications and the regulation of chromatin structure. Nucleic Acids Res. 2006, 34, 2653–2662. [Google Scholar] [CrossRef] [PubMed]
- Nerusheva, O.O.; Galander, S.; Fernius, J.; Kelly, D.; Marston, A.L. Tension-dependent removal of pericentromeric shugoshin is an indicator of sister chromosome biorientation. Genes Dev. 2014, 28, 1291–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Indjeian, V.B.; Stern, B.M.; Murray, A.W. The centromeric protein Sgo1 is required to sense lack of tension on mitotic chromosomes. Science 2005, 307, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jia, L.; Yu, H. Phospho-H2a and cohesin specify distinct tension-regulated Sgo1 pools at kinetochores and inner centromeres. Curr. Biol. 2013, 23, 1927–1933. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magnaghi-Jaulin, L.; Eot-Houllier, G.; Gallaud, E.; Giet, R. Aurora A Protein Kinase: To the Centrosome and Beyond. Biomolecules 2019, 9, 28. https://doi.org/10.3390/biom9010028
Magnaghi-Jaulin L, Eot-Houllier G, Gallaud E, Giet R. Aurora A Protein Kinase: To the Centrosome and Beyond. Biomolecules. 2019; 9(1):28. https://doi.org/10.3390/biom9010028
Chicago/Turabian StyleMagnaghi-Jaulin, Laura, Grégory Eot-Houllier, Emmanuel Gallaud, and Régis Giet. 2019. "Aurora A Protein Kinase: To the Centrosome and Beyond" Biomolecules 9, no. 1: 28. https://doi.org/10.3390/biom9010028
APA StyleMagnaghi-Jaulin, L., Eot-Houllier, G., Gallaud, E., & Giet, R. (2019). Aurora A Protein Kinase: To the Centrosome and Beyond. Biomolecules, 9(1), 28. https://doi.org/10.3390/biom9010028