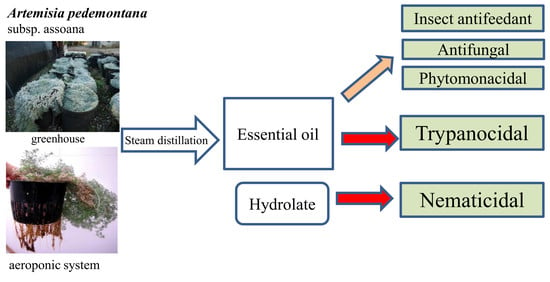
Chemical Composition and Biological Activities of Artemisia pedemontana subsp. assoana Essential Oils and Hydrolate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Cultivation
2.2. Essential Oil Extraction
2.3. Gas Chromatography–Mass Spectrometry Analysis
2.4. Antiparasite Bioassays
2.4.1. Trypanosoma cruzi Susceptibility Assay
2.4.2. Phytomonas davidi Susceptibility Assay
2.4.3. Ferriprotoporphyrin (FP) IX Biocrystallization Inhibition Test (FBIT)
2.5. Insect Bioassays
2.6. Phytotoxic Activity
2.7. Antifungal Bioassays
2.8. Nematicidal Bioassays
2.8.1. In Vitro Effect on Juveniles
2.8.2. In Vitro Effect on Egg Hatching
2.8.3. Effect on Juvenile Infection Capacity
3. Results and Discussion
3.1. Biomass Production and Essential Oil (EO) Composition
3.2. Antiparasitic Effects
3.3. Insect Antifeedant and Biocidal Effects
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Abad, M.J.; Bedoya, L.M.; Apaza, L.; Bermejo, P. The Artemisia L. genus: A review of bioactive essential oils. Molecules 2012, 17, 2542–2566. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Qian, C.; Xu, H.; Huang, Y. Antibacterial activity of Artemisia asiatica essential oil against some common respiratory infection causing bacterial strains and its mechanism of action in Haemophilus influenzae. Microb. Pathog. 2018, 114, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Pandey, B.; Thapa, R.; Upreti, A. Chemical composition, antioxidant and antibacterial activities of essential oil and methanol extract of Artemisia vulgarias and Gaultheria fragantissima collected from Nepal. Asia Pac. J. Trop. Med. 2017, 10, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Han, Z.; Xu, Y.; Yao, L. In vitro and in vivo anti-tobacco mosaic virus activities of essential oils and individual compounds. J. Microbiol. Biotechnol. 2013, 23, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Bailén, M.; Julio, L.F.; Diaz, C.E.; Sanz, J.; Martínez-Díaz, R.A.; Cabrera, R.; Burillo, J.; Gonzalez-Coloma, A. Chemical composition and biological effects of essential oils from Artemisia absinthium L. cultivated under different environmental conditions. Ind. Crop Prod. 2013, 49, 102–107. [Google Scholar] [CrossRef]
- Guardo, N.I.; Sainz, P.; González-Coloma, A.; Burillo, J.; Martínez-Díaz, R.A. Trypanocidal effects of essential oils from selected medicinal plants. Synergy among the main components. Nat. Prod. Commun. 2017, 12, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Díaz, R.A.; Ibáñez-Escribano, A.; Burillo, J.; de las Heras, L.; del Prado, G.; Agulló-Ortuño, M.T.; Julio, L.F.; González-Coloma, A. Trypanocidal, trichomonacidal and cytotoxic components of cultivated Artemisia absinthium Linnaeus (Asteraceae) essential oil. Mem. I Oswaldo Cruz 2015, 110, 693–699. [Google Scholar] [CrossRef]
- Ferreira, J.F.S.; Luthria, D.L.; Sasaki, T.; Heyerick, A. Flavonoids from Artemisia annua L. as antioxidant and their potential synergism with artemisinin against malaria and cancer. Molecules 2010, 15, 3135–3170. [Google Scholar] [CrossRef] [PubMed]
- Ivanescu, B.; Miron, A.; Corciova, A. Sesquiterpene lactones from Artemisia genus: Biological activities and methods of analysis. J. Anal. Methods Chem. 2015, 247685. [Google Scholar] [CrossRef]
- Julio, L.F.; González-Coloma, A.; Burillo, J.; Diaz, C.E.; Andrés, M.F. Nematicidal activity of the hydrolate byproduct from the semi industrial vapor pressure extraction of domesticated Artemisia absinthium against Meloidogyne javanica. Crop Prot. 2017, 94, 33–37. [Google Scholar] [CrossRef]
- Julio, L.F.; Burillo, J.; Giménez, C.; Cabrera, R.; Díaz, C.E.; Sanz, J.; González-Coloma, A. Chemical and biocidal characterization of two cultivated Artemisia absinthium populations with different domestication levels. Ind. Crop Prod. 2015, 76, 787–792. [Google Scholar] [CrossRef]
- González-Coloma, A.; Reina, M.; Díaz, C.E.; Fraga, B.M.; Santana-Meridas, O. Natural product-based biopesticides for insect control. In Elsevier Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Reedijk, J., Ed.; Elsevier: Waltham, MA, USA, 2013. [Google Scholar] [CrossRef]
- Sainz, P.; Sanz, J.; Burillo, J.; González-Coloma, A.; Bailén, M.; Martínez-Díaz, R.A. Essential oils for the control of reduviid insects. Phytochem. Rev. 2012, 11, 361–369. [Google Scholar] [CrossRef]
- Sainz, P.; Cruz-Estrada, A.; Díaz, C.E.; González-Coloma, A. The genus Artemisia: Distribution and phytochemistry in the Iberian Peninsula and the Canary and Balearic Islands. Phytochem. Rev. 2017, 16, 1–21. [Google Scholar] [CrossRef]
- Greuter, W. Compositae (pro parte majore). In Compositae. Euro+Med Plantbase the Information Resource for Euro-Mediterranean Plant Diversity; Greuter, W., von Raab-Straube, E., Eds.; Università degli Studi di Palermo: Palermo, Italy, 2005; Available online: http://ww2.bgbm.org/EuroPlusMed (accessed on 15 September 2019).
- Hassler, M. World Plants: Synonymic Checklists of the Vascular Plants of the World. In Species 2000 & ITIS Catalogue of Life; Roskov, Y., Abucay, L., Orrell, T., Nicolson, D., Bailly, N., Kirk, P.M., Bourgoin, T., DeWalt, R.E., Decock, W., De Wever, A., et al., Eds.; Species 2000: Leiden, The Netherlands, 2017; ISSN 2405-8858. Available online: www.catalogueoflife.org/col (accessed on 15 September 2019).
- Perez-Alonso, M.J.; Velasco-Negueruela, A.; Palá-Paúl, J.; Sanz, J. Variations in the essential oil composition of Artemisia pedemontana gathered in Spain: Chemotype camphor-1,8-cineole and chemotype davanone. Byochem. Syst. Ecol. 2003, 31, 77–84. [Google Scholar] [CrossRef]
- Marco, J.A.; Barbera, O.; Martinez, V.; Strack, D.; Meurer, B. Sesquiterpene Lactones from Artemisia assoana. Planta Med. 1988, 54, 460–461. [Google Scholar] [CrossRef] [PubMed]
- Martínez, V.; Barbera, O.; Sánchez-Parareda, J.; Marco, J.A. Phenolic and acetylenic metabolites from Artemisia assoana. Phytochemistry 1987, 26, 2619–2624. [Google Scholar] [CrossRef]
- Jaskowska, E.; Butler, C.; Preston, G.; Kelly, S. Phytomonas: Trypanosomatids Adapted to Plant Environments. PLoS Pathog. 2015, 11, 1–17. [Google Scholar] [CrossRef]
- Bourdy, G.; Oporto, P.; Gimenez, A.; Deharo, E. A search for natural bioactive compounds in Bolivia through a multidisciplinary approach Part VI. Evaluation of the antimalarial activity of plants used by Isoceño-Guaraní Indians. J. Ethnopharmacol. 2004, 93, 269–277. [Google Scholar] [CrossRef]
- Deharo, E.; Garcia, R.; Oporto, P.; Sauvain, M.; Gautret, P.H.; Ginsburg, H. A non-radiolabeled ferriprotoporphyrin IX bomineralization inhibition test (FBIT) for the high throughput screening of antimalarial compounds. Exp. Parasitol. 2002, 100, 252–256. [Google Scholar] [CrossRef]
- Poitout, S.; Bues, S. Elevage de plusieursespeces de Lepidopteres Noctuidae sur milleu artificiel simplifié. Ann. Zool. Ecol. Anim. 1970, 2, 79–91. [Google Scholar]
- Santana, O.; Andres, M.F.; Sanz, J.; Errahmani, N.; Abdeslam, L.; Gonzalez-Coloma, A. Valorization of essential oils from Moroccan aromatic plants. Nat. Prod. Commun. 2014, 9, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Julio, L.F.; Barrero, A.F.; Herrador del Pino, M.M.; Arteaga, J.F.; Burrillo, J.; Andrés, M.F.; Díaz, C.E.; González-Coloma, A. Phytotoxic and nematicidal components of Lavandula luisieri. J. Nat. Prod. 2016, 79, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Clausen, C.A.; Yang, V.W. Colorimetric micro-assay for accelerated screening of mould inhibitors. Int. Biodeterior. Biodegrad. 2013, 77, 68–71. [Google Scholar] [CrossRef]
- Luque, F.; Fernández-Ramos, C.; Entrala, E.; Rosales, M.J.; Salas, C.M.; Navarro, J.A.; Sánchez-Moreno, M. Biochemical and ultrastructutral alterations caused by newly synthesized 1,2,4-triazole [1,5a] pyrimidine derivates against Phytomonas staheli (Trypanosomatidae). Toxicol. In Vitro 2000, 14, 487–495. [Google Scholar] [CrossRef]
- Magán, R.; Marín, C.; Salas, J.M.; Barrera-Pérez, M.; Rosales, M.J.; Sánches-Moreno, M. Cytotoxicity of three new triaxolo-pyrimidine derivates against the plant trypanosomatid: Phytomonas sp. Isolated from Euphorbia characias. Mem. I. Oswaldo Cruz 2004, 99, 651–656. [Google Scholar] [CrossRef]
- Salas, J.M.; Quirós, M.; Haj, M.A.; Magán, R.; Marín, C.; Sánchez-Moreno, M.; Faure, R. Activity of Pt(II) and Ru(III) triazolopyrimidine complexes against parasites of the genus Leishmania, Trypanosoma and Phytomonas. Met.-Based Drugs 2001, 8, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Medina, J.M.; Fernandes, J.C.; Moreira, O.C.; Atella, G.; de Souza, W.; Barrabin, H. Mechanisms of growth inhibition of Phytomonas serpens by the alkaloids tomatine and tomatidine. Mem. I Oswaldo Cruz 2015, 110, 48–55. [Google Scholar] [CrossRef]
- Romagni, J.G.; Allen, S.N.; Dayan, F.E. Allelopathic effects of volatile cineoles on two weedy plant species. J. Chem. Ecol. 2000, 26, 303–3013. [Google Scholar] [CrossRef]
- Vokou, D.; Douvli, P.; Blionis, G.; Halley, J. Effects of monoterpenoids, acting alone or in pairs, on seed germination and subsequent seedling growth. J. Chem. Ecol. 2003, 29, 2281–2301. [Google Scholar] [CrossRef]
- De Martino, L.; Mancini, E.; Almeida, L.F.R.; De Feo, V. The antigerminative activity of twenty-seven monoterpenes. Molecules 2010, 15, 6630–6637. [Google Scholar] [CrossRef]
- Okamoto, Y.; Yamaji, K.; Kobayashi, K. Allelopathic activity of camphor released from camphor tree (Cinnamomum camphora). ACS Symp. Ser. 2011, 27, 123–132. [Google Scholar]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef] [PubMed]
- Wajs-Bonikowska, A.; Sienkiewicz, M.; Stobiecka, A.; Maciag, A.; Szoka, L.; Karna, E. Chemical composition and biological activity of Abies alba and A. koreana seed and cone essential oils and characterization of their seed hydrolates. Chem. Biodivers. 2015, 12, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Andrés, M.F.; González-Coloma, A.; Muñoz, R.; De la Peña, F.; Julio, L.F.; Burillo, J. Nematicidal potential of hydrolates from the semi industrial vapor-pressure extraction of Spanish aromatic plants. Environ. Sci. Pollut. Res. Int. 2017. [Google Scholar] [CrossRef] [PubMed]


| Compounds | Rt a | AasG | AasA |
|---|---|---|---|
| α-pinene | 3.94 | 0.54 b | 0.89 b |
| Camphene | 4.16 | 1.77 | 5.34 |
| β-pinene | 4.56 | 0.22 | 0.71 |
| α-Terpinene | 5.15 | - | 0.99 |
| p-Cymene | 5.29 | 1.75 | 7.4 |
| 1,8-Cineole | 5.43 | 22.88 | 25.78 |
| γ-Terpinene | 5.92 | - | 1.73 |
| Sabinene | 6.07 | - | 2.53 |
| Linalool | 6.66 | 1.56 | - |
| Sabinene Isomer | 6.68 | - | 1.45 |
| Camphor | 7.69 | 44.03 | 32.4 |
| Borneol | 8.12 | 4.79 | 2.77 |
| Terpinen-4-ol | 8.36 | 8.86 | 5.77 |
| 1-α-Terpineol | 8.64 | 3.6 | 1.6 |
| Bornyl acetate | 10.77 | 0.98 | - |
| Methyl eugenol | 13.34 | 1.27 | 1.82 |
| Nerolidol | 16.78 | 0.82 | - |
| Spathulenol | 17.19 | 1.03 | 0.59 |
| Viridiflorol | 17.51 | 1.69 | 1.59 |
| EO/Compound | Concentration (µg/mL) | T. cruzia | P. davidia |
|---|---|---|---|
| AasG EO | 800 | 100a | 100 |
| 400 | 100a | 0.0 | |
| 200 | 20.3 ± 4.6b | 0.0 | |
| AasA EO | 800 | 99.4 ± 0.6a | 100a |
| 400 | 100a | 72.7 ± 12.9b | |
| 200 | 72.1 ± 3.2b | 0.0 | |
| 100 | 14.6 ± 2.9c | - | |
| 1,8-Cineole | 100 | 2.0 ± 2.5 | 0.0 |
| 10 | 0.0 | 0.0 | |
| Camphor | 100 | 0.0 | 0.0 |
| Cineole:Camphor 1:1 | 100 | 0.0 | 0.0 |
| EO/Compound | Concentration (µg/cm2) | S. littoralis | M. persicae | R. padi |
|---|---|---|---|---|
| %FI a | %SI b | %SI b | ||
| AasG EO | 100 | 85.6 ± 7.9 * | 46.1 ± 8.9 | 56.9 ± 8.1 * |
| 50 | 43.3 ± 19.2 | - | - | |
| AasA EO | 100 | 53.0 ± 8.9 | 76.1 ± 7.2 * | 60.3 ± 7.2 * |
| 50 | - | 29.7 ± 7.6 | - | |
| 1,8-Cineole | 50 | 12.3 ± 7.6 | 42.1 ± 8.8 | 35.8 ± 7.4 |
| Camphor | 50 | 59.9 ± 12.6 | 32.3 ± 7.5 | 33.2 ± 7.8 |
| Cineole:Camphor 1:1 | 50 | 62.7 ± 11.2 | 55.6 ± 8.4 | 40.4 ± 7.8 |
| EO/Compound | Concentration (µg/mL) | % I a |
|---|---|---|
| AasG EO | 800 | 83.7 ± 2.3a |
| 400 | 39.06 ± 6.3b | |
| 200 | 33.9 ± 11.6b | |
| AasA EO | 800 | 67.8 ± 6.3a |
| 400 | 38.6 ± 13.4b | |
| 200 | 16.1 ± 4.3c | |
| 1,8-Cineole | 100 | 0.0 |
| Camphor | 100 | 24.2 ± 9.8 |
| Cineole:Camphor 1:1 | 100 | 26.7 ± 8.0 |
| EO/H | J2 mortality (%) a | LC50 b |
|---|---|---|
| AasG EO | 3.72 ± 0.23a | - |
| AasA EO | 3.06 ± 0.35a | - |
| AasA H | 96.4 ± 1.1b | 74 (70–80) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sainz, P.; Andrés, M.F.; Martínez-Díaz, R.A.; Bailén, M.; Navarro-Rocha, J.; Díaz, C.E.; González-Coloma, A. Chemical Composition and Biological Activities of Artemisia pedemontana subsp. assoana Essential Oils and Hydrolate. Biomolecules 2019, 9, 558. https://doi.org/10.3390/biom9100558
Sainz P, Andrés MF, Martínez-Díaz RA, Bailén M, Navarro-Rocha J, Díaz CE, González-Coloma A. Chemical Composition and Biological Activities of Artemisia pedemontana subsp. assoana Essential Oils and Hydrolate. Biomolecules. 2019; 9(10):558. https://doi.org/10.3390/biom9100558
Chicago/Turabian StyleSainz, Paula, María Fe Andrés, Rafael A. Martínez-Díaz, María Bailén, Juliana Navarro-Rocha, Carmen E. Díaz, and Azucena González-Coloma. 2019. "Chemical Composition and Biological Activities of Artemisia pedemontana subsp. assoana Essential Oils and Hydrolate" Biomolecules 9, no. 10: 558. https://doi.org/10.3390/biom9100558
APA StyleSainz, P., Andrés, M. F., Martínez-Díaz, R. A., Bailén, M., Navarro-Rocha, J., Díaz, C. E., & González-Coloma, A. (2019). Chemical Composition and Biological Activities of Artemisia pedemontana subsp. assoana Essential Oils and Hydrolate. Biomolecules, 9(10), 558. https://doi.org/10.3390/biom9100558