Chemical and Biological Characterization of the Anticancer Potency of Salvia fruticosa in a Model of Human Malignant Melanoma
Abstract
:1. Introduction
2. Results
2.1. S. fruticosa Extracts Induce Cytotoxicity in A375 Cells
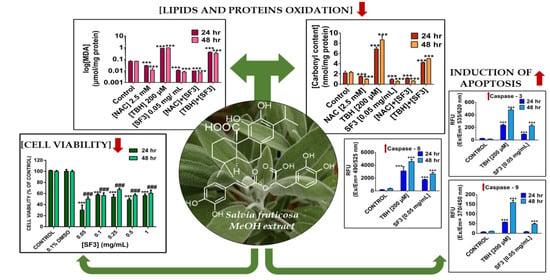
2.2. The Methanolic Extract Fraction (SF3) Exerts Either No Cytotoxicity or a Minimal One in A431 and HaCaT Cells, Respectively
2.3. The Methanolic Extract Fraction (SF3) Is a Rich Source of Phenolic and Flavonoid Compounds
2.4. The Methanolic Extract Fraction (SF3) Exerts a Strong Antioxidant Capacity by Inhibiting Lipid and Protein Oxidation
2.5. The Methanolic Extract Fraction (SF3) Induces Apoptosis in A375 Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material and Extract Preparation
4.3. Cell Lines
4.4. Determination of Cell Viability Levels
4.5. Determination of Total Phenolic Content (TPC)
4.6. Determination of Total Flavonoid Content (TFC)
4.7. Determination of Malondialdehyde and Protein Carbonyl Contents
4.8. Determination of Caspase Activity
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chandra Pal, H.; Marchiony Hunt, K.; Diamond, A.; Elmets, C.A.; Afaq, F. Phytochemicals for the Management of Melanoma. Mini-Reviews Med. Chem. 2016, 16, 953–979. [Google Scholar] [CrossRef]
- Ombra, M.N.; Paliogiannis, P.; Stucci, L.S.; Colombino, M.; Casula, M.; Sini, M.C.; Manca, A.; Palomba, G.; Stanganelli, I.; Mandalà, M.; et al. Dietary compounds and cutaneous malignant melanoma: Recent advances from a biological perspective. Nutr. Metab. 2019, 16, 33. [Google Scholar] [CrossRef]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current state of melanoma diagnosis and treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Sheikh, M.S. Melanoma: Molecular pathogenesis and therapeutic management. Mol. Cell. Pharmacol. 2014, 6, 31–44. [Google Scholar] [CrossRef]
- Domingues, B.; Lopes, J.; Soares, P.; Populo, H. Melanoma treatment in review. ImmunoTargets Ther. 2018, 7, 35–49. [Google Scholar] [CrossRef] [Green Version]
- Ward, W.H.; Farma, J.M. Cutaneous Melanoma: Etiology and Therapy; Ward, W.H., Farma, J.M., Eds.; Codon Publications: Brisbane, Australia, 2017; Volume 6, ISBN 9780994438140. [Google Scholar]
- Ng, C.Y.; Yen, H.; Hsiao, H.Y.; Su, S.C. Phytochemicals in skin cancer prevention and treatment: An updated review. Int. J. Mol. Sci. 2018, 19, 941. [Google Scholar] [CrossRef] [Green Version]
- Mitsiogianni, M.; Kyriakou, S.; Anestopoulos, I.; Trafalis, D.T.; Deligiorgi, M.V.; Franco, R.; Pappa, A.; Panayiotidis, M. An Evaluation of the Anti-Carcinogenic Response of Major Isothiocyanates in Non-Metastatic and Metastatic Melanoma Cells. Antioxidants 2021, 10, 284. [Google Scholar] [CrossRef]
- Kyriakou, S.; Mitsiogianni, M.; Mantso, M.; Cheung, W.; Todryk, S.; Veuger, S.; Pappa, A.; Tetard, D.; Panayiotidis, M.I. Anticancer activity of a novel methylated analogue of L-mimosine against an in vitro model of human malignant melanoma. Invest New Drugs. 2020, 38, 621–633. [Google Scholar] [CrossRef] [Green Version]
- Abu-Dahab, R.; Afifi, F.; Kasabri, V.; Majdalawi, L.; Naffa, R. Comparison of the antiproliferative activity of crude ethanol extracts of nine salvia species grown in Jordan against breast cancer cell line models. Pharmacogn. Mag. 2012, 8, 319–324. [Google Scholar] [CrossRef] [Green Version]
- Boukhary, R.; Raafat, K.; Ghoneim, A.I.; Aboul-Ela, M.; El-Lakany, A. Anti-Inflammatory and Antioxidant Activities of Salvia fruticosa (S. fruticosa): An HPLC Determination of Phenolic Contents. Evid. Based Complement. Altern. Med. 2016, 2016, 7178105. [Google Scholar] [CrossRef] [Green Version]
- Xavier, C.P.R.; Lima, C.F.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Salvia fruticosa, Salvia officinalis, and rosmarinic acid induce apoptosis and inhibit proliferation of human colorectal cell lines: The role in MAPK/ERK pathway. Nutr. Cancer. 2009, 61, 564–571. [Google Scholar] [CrossRef] [Green Version]
- Hossan, M.S.; Rahman, S.; Bashar, A.B.M.A.; Jahan, R.; Al-Nahain, A.; Rahmatullah, M. Rosmarinic acid: A review of its anticancer action. World J. Pharm. Pharm. Sci. 2014, 3, 57–70. [Google Scholar]
- Sevindik, N.; Rencuzogullari, E. The genotoxic and antigenotoxic effects of S. fruticosa leaf extract in human blood lymphocytes. Drug Chem. Toxicol. 2014, 37, 295–302. [Google Scholar] [CrossRef]
- Altay, A.; Bozoğlu, F. Salvia fruticosa Modulates mRNA Expressions and Activity Levels of Xenobiotic Metabolizing CYP1A2, CYP2E1, NQO1, GPx, and GST Enzymes in Human Colorectal Adenocarcinoma HT-29 Cells. Nutr. Cancer 2017, 69, 892–903. [Google Scholar] [CrossRef]
- Koutsoulas, A.; Čarnecká, M.; Slanina, J.; Tóth, J.; Slaninová, I. Characterization of Phenolic Compounds and Antiproliferative Effects of Salvia pomifera and S. fruticosa Extracts. Molecules 2019, 24, 2921. [Google Scholar] [CrossRef] [Green Version]
- Arakawa, N.; Okubo, A.; Yasuhira, S.; Takahashi, K.; Amano, H.; Akasaka, T.; Masuda, T.; Shibazaki, M.; Maesawa, C. Carnosic acid, an inducer of NAD(P)H quinone oxidoreductase 1,enhances the cytotoxicity of β-lapachone in melanoma cell lines. Oncol. Lett. 2018, 15, 2393–2400. [Google Scholar] [CrossRef]
- Ververis, A.; Savvidou, G.; Ioannou, K.; Nicolaou, P.; Christodoulou, K.; Plioukas, M. Greek Sage Exhibits Neuroprotective Activity against Amyloid Beta-Induced Toxicity. Evid. Based Complement. Altern. Med. 2020, 2020, 2975284. [Google Scholar] [CrossRef]
- Cummins, D.L.; Cummins, J.M.; Pantle, H.; Silverman, M.A.; Leonard, A.L.; Chanmugam, A. Cutaneous malignant melanoma. Mayo Clin. Proc. 2006, 81, 500–507. [Google Scholar] [CrossRef] [Green Version]
- Rodic, N.; Zampella, J.; Sharma, R.; Burns, K.H.; Taube, J.M. Diagnostic utility of 5-hydroxymethylcytosine immunohistochemistry in melanocytic proliferations. J. Cutan. Pathol. 2015, 42, 807–814. [Google Scholar] [CrossRef] [Green Version]
- Che, G.; Huang, B.; Xie, Z.; Zhao, J.; Yan, Y.; Wu, J.; Sun, H.; Ma, H. Trends in incidence and survival in patients with melanoma, 1974–2013. Am. J. Cancer Res. 2019, 9, 1396–1414. [Google Scholar]
- Miller, R.; Walker, S.; Shui, I.; Brandtmüller, A.; Cadwell, K.; Scherrer, E. Epidemiology and survival outcomes in stages II and III cutaneous melanoma: A systematic review. Melanoma Manag. 2020, 7, 39–53. [Google Scholar] [CrossRef] [Green Version]
- Macdonald, E.J. Epidemiology of melanoma. Prog. Clin. Cancer. 1975, 6, 139–149. [Google Scholar] [CrossRef]
- Tundis, R.; Iacopetta, D.; Sinicropi, M.S.; Bonesi, M.; Leporini, M.; Passalacqua, N.G.; Ceramella, J.; Menichini, F.; Loizzo, M.R. Assessment of antioxidant, antitumor and pro-apoptotic effects of S. fruticosa Mill. subsp. thomasii (Lacaita) Brullo, Guglielmo, Pavone & Terrasi (Lamiaceae). Food Chem. Toxicol. 2017, 106, 155–164. [Google Scholar] [CrossRef]
- Ramos, A.A.; Pedro, D.; Collins, A.R.; Pereira-Wilson, C. Protection by salvia extracts against oxidative and alkylation damage to DNA in human HCT15 and CO115 cells. In Proceedings of the Journal of Toxicology and Environmental Health—Part A: Current Issues. J. Toxicol. Environ. Health A. 2012, 75, 765–775. [Google Scholar] [CrossRef]
- Kar, S.; Palit, S.; Ball, W.B.; Das, P.K. Carnosic acid modulates Akt/IKK/NF-κB signaling by PP2A and induces intrinsic and extrinsic pathway mediated apoptosis in human prostate carcinoma PC-3 cells. Apoptosis 2012, 17, 735–747. [Google Scholar] [CrossRef]
- Bahri, S.; Jameleddine, S.; Shlyonsky, V. Relevance of carnosic acid to the treatment of several health disorders: Molecular targets and mechanisms. Biomed. Pharmacother. 2016, 84, 569–582. [Google Scholar] [CrossRef]
- Bahri, S.; Mies, F.; Ali, R.B.; Mlika, M.; Jameleddine, S.; Entee, K.M.; Shlyonsky, V. Rosmarinic acid potentiates carnosic acid induced apoptosis in lung fibroblasts. PLoS ONE 2017, 12, e0184368. [Google Scholar] [CrossRef] [Green Version]
- Park, S.Y.; Song, H.; Sung, M.-K.; Kang, Y.-H.; Lee, K.W.; Park, J.H.Y. Carnosic Acid Inhibits the Epithelial-Mesenchymal Transition in B16F10 Melanoma Cells: A Possible Mechanism for the Inhibition of Cell Migration. Int. J. Mol. Sci. 2014, 15, 12698–12713. [Google Scholar] [CrossRef] [Green Version]
- Tosun, M.; Ercisli, S.; Sengul, M.; Ozer, H.; Polat, T.; Ozturk, E. Antioxidant properties and total phenolic content of eight Salvia species from Turkey. Biol. Res. 2009, 42, 175–181. [Google Scholar] [CrossRef]
- Godic, A.; Poljšak, B.; Adamic, M.; Dahmane, R. The Role of Antioxidants in Skin Cancer Prevention and Treatment. Oxidative Med. Cell. Longev. 2014, 2014, 860479. [Google Scholar] [CrossRef]
- Obrador, E.; Liu-Smith, F.; Dellinger, R.W.; Salvador, R.; Meyskens, F.L.; Estrela, J.M. Oxidative stress and antioxidants in the pathophysiology of malignant melanoma. Biol. Chem. 2019, 400, 589–612. [Google Scholar] [CrossRef] [Green Version]
- Hanafi, R.; Anestopoulos, I.; Voulgaridou, G.P.; Franco, R.; Georgakilas, A.G.; Ziech, D.; Malamou-Mitsi, V.; Pappa, A.; Panayiotidis, M. Oxidative stress based-biomarkers in oral carcinogenesis: How far have we gone? Curr. Mol. Med. 2012, 12, 698–703. [Google Scholar] [CrossRef]
- Liou, G.-Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 679–696. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, V.; Singh Tuli, H.; Varol, A.A.; Thakral, F.; Yerer, M.B.; Sak, K.; Varol, M.; Jain, A.; Khan, A.; Sethi, G. biomolecules Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements. Biomolecules 2019, 9, 735. [Google Scholar] [CrossRef] [Green Version]
- Reczek, C.R.; Chandel, N.S. The Two Faces of Reactive Oxygen Species in Cancer. Annu. Rev. Cancer Biol. 2016, 1, 79–98. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- de Melo, F.H.M.; Molognoni, F.; Galvonas, M. The Role of Oxidative Stress in Melanoma Development, Progression and Treatment. In Recent Advances in the Biology, Therapy and Management of Melanoma; InTech: London, UK, 2013. [Google Scholar] [CrossRef] [Green Version]
- Wittgen, H.G.M.; Van Kempen, L.C.L.T. Reactive oxygen species in melanoma and its therapeutic implications. Melanoma Res. 2007, 17, 400–409. [Google Scholar] [CrossRef]
- Zupkó, I.; Hohmann, J.; Rédei, D.; Falkay, G.; Janicsák, G.; Máthé, I. Antioxidant activity of leaves of Salvia species in enzyme-dependent and enzyme-independent systems of lipid peroxidation and their phenolic constituents. Planta Med. 2001, 67, 366–368. [Google Scholar] [CrossRef]
- Vergine, M.; Nicolì, F.; Negro, C.; Luvisi, A.; Nutricati, E.; Accogli, R.A.; Sabella, E.; Miceli, A. Phytochemical Profiles and Antioxidant Activity of Salvia species from Southern Italy. Nat. Prod. 2019, 13, 205–215. [Google Scholar] [CrossRef]
- Pereira, D.M.; Valentão, P.; Pereira, J.A.; Andrade, P.B. Phenolics: From chemistry to biology. Molecules 2009, 14, 2202–2211. [Google Scholar] [CrossRef]
- Foti, M.C. Antioxidant properties of phenols. J. Pharm. Pharmacol. 2010, 59, 1673–1685. [Google Scholar] [CrossRef]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef]
- De Niero, E.L.O.; Machado-Santelli, G.M. Cinnamic acid induces apoptotic cell death and cytoskeleton disruption in human melanoma cells. J. Exp. Clin. Cancer Res. 2013, 32, 31. [Google Scholar] [CrossRef] [Green Version]
- Pramanik, K.C.; Kudugunti, S.K.; Fofaria, N.M.; Moridani, M.Y.; Srivastava, S.K. Caffeic acid phenethyl ester suppresses melanoma tumor growth by inhibiting PI3K/AKT/XIAP pathway. Carcinogenesis 2013, 34, 2061–2070. [Google Scholar] [CrossRef] [Green Version]
- Aquilato, A.; Lopez, V.; Doonan, B.; Hsieh, T.C.; Pinto, J.T.; Wu, E.; Wu, J.M. BRAF Mutation in Melanoma and Dietary Polyphenols as Adjunctive Treatment Strategy. Polyphen. Hum. Health Dis. 2014, 2, 1353–1365. [Google Scholar] [CrossRef]
- Anantharaju, P.G.; Gowda, P.C.; Vimalambike, M.G.; Madhunapantula, S.V. An overview on the role of dietary phenolics for the treatment of cancers. Nutr. J. 2016, 15, 99. [Google Scholar] [CrossRef] [Green Version]
- De Melo, M.N.O.; Oliveira, A.P.; Wiecikowski, A.F.; Carvalho, R.S.; de Castro, J.L.; de Oliveira, F.A.G.; Pereira, H.M.G.; da Veiga, V.F.; Capella, M.M.A.; Rocha, L.; et al. Phenolic compounds from Viscum album tinctures enhanced antitumor activity in melanoma murine cancer cells. Saudi Pharm. J. 2018, 26, 311–322. [Google Scholar] [CrossRef]
- Brunetti, C.; Di Ferdinando, M.; Fini, A.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants and developmental regulators: Relative significance in plants and humans. Int. J. Mol. Sci. 2013, 14, 3540–3555. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [Green Version]
- Chae, S.C.; Lee, J.-H.; Park, S.U. Recent studies on flavonoids and their antioxidant activities. EXCLI J. 2013, 12, 226–230. [Google Scholar]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Grace, S.C. Phenolics as Antioxidants. In Antioxidants and Reactive Oxygen Species in Plants; Blackwell Publishing Ltd.: Oxford, UK, 2007; pp. 141–168. ISBN 9781405125291. [Google Scholar]
- Božić, D.; Papaefthimiou, D.; Brückner, K.; De Vos, R.C.H.; Tsoleridis, C.A.; Katsarou, D.; Papanikolaou, A.; Pateraki, I.; Chatzopoulou, F.M.; Dimitriadou, E.; et al. Towards elucidating carnosic acid biosynthesis in Lamiaceae: Functional characterization of the three first steps of the pathway in S. fruticosa and Rosmarinus officinalis. PLoS ONE 2015, 10, e0124106. [Google Scholar] [CrossRef] [Green Version]
- Pavic, V.P.; Jakovljevic´, M.; Molnar, M.; Jokic´, S.J. Extraction of Carnosic Acid and Carnosol from Sage (Salvia officinalis L.) Leaves by Supercritical Fluid Extraction and Their Antioxidant and Antibacterial Activity. Plants 2019, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [Green Version]
- O’Flaherty, C.; Matsushita-Fournier, D. Reactive oxygen species and protein modifications in spermatozoa. Biol. Reprod. 2017, 97, 577–585. [Google Scholar] [CrossRef]
- Lopaczynski, W.; Zeisel, S.H. Antioxidants, programmed cell death, and cancer. Nutr. Res. 2001, 21, 295–307. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Barrera, G. Oxidative Stress and Lipid Peroxidation Products in Cancer Progression and Therapy. ISRN Oncol. 2012, 2012, 137289. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, Y.; Umeno, A.; Shichiri, M. Lipid peroxidation biomarkers for evaluating oxidative stress and assessing antioxidant capacity in vivo. J. Clin. Biochem. Nutr. 2013, 52, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, A.A.; Azqueta, A.; Pereira-Wilson, C.; Collins, A.R. Polyphenolic Compounds from Salvia Species Protect Cellular DNA from Oxidation and Stimulate DNA Repair in Cultured Human Cells. J. Agric. Food Chem. 2010, 58, 7465–7471. [Google Scholar] [CrossRef]
- Kominami, K.; Nakabayashi, J.; Nagai, T.; Tsujimura, Y.; Chiba, K.; Kimura, H.; Miyawaki, A.; Sawasaki, T.; Yokota, H.; Manabe, N.; et al. The molecular mechanism of apoptosis upon caspase-8 activation: Quantitative experimental validation of a mathematical model. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 1825–1840. [Google Scholar] [CrossRef] [Green Version]
- Tummers, B.; Green, D.R. Caspase-8: Regulating life and death. Immunol. Rev. 2017, 277, 76–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobrido-Cameán, D.; Barreiro-Iglesias, A. Role of caspase-8 and fas in cell death after spinal cord injury. Front. Mol. Neurosci. 2018, 11, 101. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Browne, G.; Melino, G.; Cohen, G.M. Ordering of caspases in cells undergoing apoptosis by the intrinsic pathway. Cell Death Differ. 2009, 16, 1053–1061. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Zhou, L.; Zhao, T.; Liu, X.; Zhang, P.; Liu, Y.; Zheng, X.; Li, Q. Caspase-9: Structure, mechanisms and clinical application. Oncotarget. 2017, 8, 23996–24008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Zhao, D.; Zhuang, J.; Zhang, F.; Xu, C. Caspase-8 and Caspase-9 functioned differently at different stages of the cyclic stretch-induced apoptosis in human periodontal ligament cells. PLoS ONE. 2016, 11, e0168268. [Google Scholar] [CrossRef]
- Winter, E.; Chiaradia, L.D.; Silva, A.H.; Nunes, R.J.; Yunes, R.A.; Creczynski-Pasa, T.B. Involvement of extrinsic and intrinsic apoptotic pathways together with endoplasmic reticulum stress in cell death induced by naphthylchalcones in a leukemic cell line: Advantages of multi-target action. Toxicol. Vitr. 2014, 28, 769–777. [Google Scholar] [CrossRef] [Green Version]




| Fraction | Extraction Solvent | EC50 (mg/mL) (48 h) |
|---|---|---|
| SF1 | Petroleum ether | 0.82 |
| SF3 | Methanol | 0.048 |
| SF4 | Water | 0.22 |
| SF5 | Diethyl ether | 0.57 |
| SF6 | Ethyl acetate | 0.17 |
| SF7 | n-Butanol | 0.56 |
| SF8 | Water | 0.88 |
| SF1 | SF2 | SF3 | SF4 | SF5 | SF6 | SF7 | SF8 | |
|---|---|---|---|---|---|---|---|---|
| TPC (μg GAE/ g of dry extract) | 136.26 ± 2.8 | 91.5 ± 5.9 | 319.19 ± 10.0 | 198.99 ± 28.7 | 110.01 ± 4.9 | 148.38 ± 3.8 | 82.13 ± 4.4 | 46.38 ± 5.7 |
| TFC (μg RE/ g of dry extract) | 788.33 ± 64.7 | 164.44 ± 6.0 | 1054.66 ± 58.2 | 685.66 ± 10.9 | 189.45 ± 3.2 | 187.97 ± 10.3 | 201.91 ± 1.2 | 583.59 ± 4.1 |
| TFC (μg CE/ g of dry extract) | 583.76 ± 12.7 | 104.08 ± 5.5 | 664.57 ± 15.2 | 235.32 ± 9.2 | 102.53 ± 5.9 | 175.9 ± 4.7 | 100.79 ± 7.9 | 226.88 ± 4.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyriakou, S.; Tragkola, V.; Plioukas, M.; Anestopoulos, I.; Chatzopoulou, P.S.; Sarrou, E.; Trafalis, D.T.; Deligiorgi, M.V.; Franco, R.; Pappa, A.; et al. Chemical and Biological Characterization of the Anticancer Potency of Salvia fruticosa in a Model of Human Malignant Melanoma. Plants 2021, 10, 2472. https://doi.org/10.3390/plants10112472
Kyriakou S, Tragkola V, Plioukas M, Anestopoulos I, Chatzopoulou PS, Sarrou E, Trafalis DT, Deligiorgi MV, Franco R, Pappa A, et al. Chemical and Biological Characterization of the Anticancer Potency of Salvia fruticosa in a Model of Human Malignant Melanoma. Plants. 2021; 10(11):2472. https://doi.org/10.3390/plants10112472
Chicago/Turabian StyleKyriakou, Sotiris, Venetia Tragkola, Michael Plioukas, Ioannis Anestopoulos, Paschalina S. Chatzopoulou, Eirini Sarrou, Dimitrios T. Trafalis, Maria V. Deligiorgi, Rodrigo Franco, Aglaia Pappa, and et al. 2021. "Chemical and Biological Characterization of the Anticancer Potency of Salvia fruticosa in a Model of Human Malignant Melanoma" Plants 10, no. 11: 2472. https://doi.org/10.3390/plants10112472
APA StyleKyriakou, S., Tragkola, V., Plioukas, M., Anestopoulos, I., Chatzopoulou, P. S., Sarrou, E., Trafalis, D. T., Deligiorgi, M. V., Franco, R., Pappa, A., & Panayiotidis, M. I. (2021). Chemical and Biological Characterization of the Anticancer Potency of Salvia fruticosa in a Model of Human Malignant Melanoma. Plants, 10(11), 2472. https://doi.org/10.3390/plants10112472