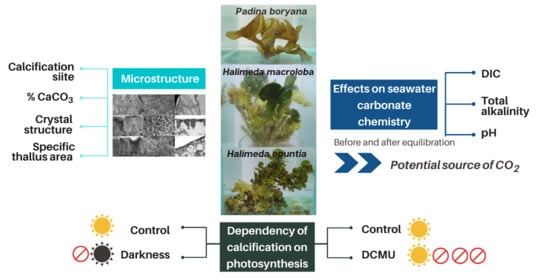
Calcification in Three Common Calcified Algae from Phuket, Thailand: Potential Relevance on Seawater Carbonate Chemistry and Link to Photosynthetic Process
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Sampling and Acclimatization
4.2. Characterization of Calcification and Its Effect on Seawater Chemistry
4.2.1. Experimental Design
4.2.2. Determination of Surface Area, CaCO3 Accumulation and Microstructural Analyses
4.2.3. Determination of Carbonate Chemistry-Related Parameters and Fates of Carbon Related to Biological Processes
4.3. Interrelation between Photosynthesis and Calcification
4.4. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Houghton, J.T.; Ding, Y.; Griggs, D.J.; Noguer, M.; van der Linden, P.J.; Dai, X.; Maskell, K.; Johnson, C.A. Climate Change 2001: The Scientific Basis. Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Meehl, G.A.; Stocker, T.F.; Collins, W.D.; Friedlingstein, P.; Gaye, A.T.; Gregory, J.M.; Kitoh, A.; Knutti, R.; Murphy, J.M.; Noda, A.; et al. Global Climate Projections. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, NY, USA, 2007. [Google Scholar]
- Albright, R.; Benthuysen, J.; Cantin, N.; Caldeira, K.; Anthony, K. Coral reef metabolism and carbon chemistry dynamics of a coral reef flat. Geophys. Res. Lett. 2015, 42. [Google Scholar] [CrossRef]
- Rasmusson, L.M.; Nualla-ong, A.; Wutiruk, T.; Björk, M.; Gullström, M.; Buapet, P. Sensitivity of photosynthesis to warming in two similar species of the aquatic angiosperm Ruppia from tropical and temperate habitats. Sustainability 2021, 13, 9433. [Google Scholar] [CrossRef]
- Yucharoen, M.; Sinutok, S.; Chotikarn, P.; Buapet, P. Experimental assessment of vulnerability to warming in tropical shallow-water marine organisms. Front. Mar. Sci. 2021, 8, 767628. [Google Scholar] [CrossRef]
- Tano, S.; Eggertsen, M.; Wikström, S.A.; Berkström, C.; Buriyo, A.S.; Halling, C. Tropical seaweed beds are important habitats for mobile invertebrate epifauna. Estuar. Coast. Shelf Sci. 2016, 183, 1–12. [Google Scholar] [CrossRef]
- Eggertsen, L.; Ferreira, C.E.L.; Fontoura, L.; Kautsky, N.; Gullström, M.; Berkström, C. Seaweed beds support more juvenile reef fish than seagrass beds in a south-western Atlantic tropical seascape. Estuar. Coast. Shelf Sci. 2017, 196, 97–108. [Google Scholar] [CrossRef] [Green Version]
- Hill, R.; Bellgrove, A.; Macreadie, P.I.; Petrou, K.; Beardall, J.; Steven, A.; Ralph, P.J. Can macroalgae contribute to blue carbon? An Australian perspective. Limnol. Oceanogr. 2015, 60, 1689–1706. [Google Scholar] [CrossRef]
- Krause-Jensen, D.; Lavery, P.; Serrano, O.; Marbà, N.; Masque, P.; Duarte, C.M. Sequestration of macroalgal carbon: The elephant in the Blue Carbon room. Biol. Lett. 2018, 14, 20180236. [Google Scholar] [CrossRef] [Green Version]
- Raven, J. Blue carbon: Past, present and future, with emphasis on macroalgae. Biol. Lett. 2018, 14, 20180336. [Google Scholar] [CrossRef] [Green Version]
- Ortega, C.; Vargas, G.; Rojas, M.; Rutllant, J.A.; Muñoz, P.; Lange, C.B.; Pantoja, S.; Dezileau, L.; Ortlieb, L. Extreme ENSO-driven torrential rainfalls at the southern edge of the Atacama Desert during the Late Holocene and their projection into the 21th century. Glob. Planet. Chang. 2019, 175, 226–237. [Google Scholar] [CrossRef]
- Bergstrom, E.; Silva, J.; Martins, C.; Horta, P. Seagrass can mitigate negative ocean acidification effects on calcifying algae. Sci. Rep. 2019, 9, 1932. [Google Scholar] [CrossRef] [PubMed]
- McNicholl, C.; Koch, M.S.; Swarzenski, P.W.; Oberhaensli, F.R.; Taylor, A.; Batista, M.G.; Metian, M. Ocean acidification effects on calcification and dissolution in tropical reef macroalgae. Coral Reefs 2020, 39, 1635–1647. [Google Scholar] [CrossRef]
- McNicholl, C.; Koch, M.S. Irradiance, photosynthesis and elevated pCO2 effects on net calcification in tropical reef macroalgae. J. Exp. Mar. Bio. Ecol. 2021, 535, 151489. [Google Scholar] [CrossRef]
- Yang, F.; Wei, Z.; Long, L. Transcriptomic and Physiological Responses of the Tropical Reef Calcified Macroalga Amphiroa fragilissima to Elevated Temperature1. J. Phycol. 2021, 57, 1254–1265. [Google Scholar] [CrossRef]
- Johnson, M.D.; Price, N.N.; Smith, J.E. Contrasting effects of ocean acidification on tropical fleshy and calcareous algae. PeerJ 2014, 2, e411. [Google Scholar] [CrossRef] [Green Version]
- Arina, N.; Raynusha, C.; Hidayah, N.; Zainee, N.F.A.; Prathep, A.; Rozaimi, M. Coralline macroalgae contribution to ecological services of carbon storage in a disturbed seagrass meadow. Mar. Environ. Res. 2020, 162, 105156. [Google Scholar] [CrossRef]
- Kleypas, J.A.; Anthony, K.R.N.; Gattuso, J.-P. Coral reefs modify their seawater carbon chemistry—Case study from a barrier reef (Moorea, French Polynesia). Glob. Chang. Biol. 2011, 17, 3667–3678. [Google Scholar] [CrossRef]
- Macreadie, P.I.; Anton, A.; Raven, J.A.; Beaumont, N.; Connolly, R.M.; Friess, D.A.; Kelleway, J.J.; Kennedy, H.; Kuwae, T.; Lavery, P.S.; et al. The future of Blue Carbon science. Nat. Commun. 2019, 10, 3998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalokora, O.J.; Buriyo, A.S.; Asplund, M.E.; Gullström, M.; Mtolera, M.S.P.; Björk, M. An experimental assessment of algal calcification as a potential source of atmospheric CO2. PLoS ONE 2020, 15, e0231971. [Google Scholar] [CrossRef]
- Ware, J.R.; Smith, S.V.; Reaka-Kudla, M.L. Coral reefs: Sources or sinks of atmospheric CO2? Coral Reefs 1992, 11, 127–130. [Google Scholar] [CrossRef]
- Frankignoulle, M.; Canon, C.; Gattuso, J.-P. Marine calcification as a source of carbon dioxide: Positive feedback of increasing atmospheric CO2. Limnol. Oceanogr. 1994, 39, 458–462. [Google Scholar] [CrossRef]
- Barker, S.; Higgins, J.A.; Elderfield, H. The future of the carbon cycle: Review, calcification response, ballast and feedback on atmospheric CO2. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Eng. Sci. 2003, 361, 1977–1999. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Calcification in aquatic plants. Plant Cell Environ. 1984, 7, 457–466. [Google Scholar] [CrossRef]
- McConnaughey, T. Biomineralization Mechanisms. In Origin, Evolution, and Modern Aspects of Biomineralization in Plants and Animals; Springer: Bostan, MA, USA, 1989. [Google Scholar]
- Buapet, P.; Gullström, M.; Björk, M. Photosynthetic activity of seagrasses and macroalgae in temperate shallow waters can alter seawater pH and total inorganic carbon content at the scale of a coastal embayment. Mar. Freshw. Res. 2013, 64, 1040–1048. [Google Scholar] [CrossRef]
- Sinutok, S. Interactive Effects of the Ocean Acidification and Warming on Sediment Dwelling Marine Calcifers. Ph.D. Thesis, University of Technology Sydney, Sydney, Australia, 2013. [Google Scholar]
- McNicholl, C.; Koch, M.S.; Hofmann, L.C. Photosynthesis and light-dependent proton pumps increase boundary layer pH in tropical macroalgae: A proposed mechanism to sustain calcification under ocean acidification. J. Exp. Mar. Bio. Ecol. 2019, 521, 151208. [Google Scholar] [CrossRef] [Green Version]
- Van der Heijden, L.H.; Kamenos, N.A. Calculating the global contribution of coralline algae to carbon burial. Biogeosciences 2015, 12, 7845–7877. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, L.C.; Bischof, K.; Baggini, C.; Johnson, A.; Koop-Jakobsen, K.; Teichberg, M. CO2 and inorganic nutrient enrichment affect the performance of a calcifying green alga and its noncalcifying epiphyte. Oecologia 2015, 177, 1157–1169. [Google Scholar] [CrossRef]
- Hofmann, L.C.; Koch, M.; de Beer, D. Biotic control of surface pH and evidence of light-induced H+ pumping and Ca2+-H+ exchange in a tropical crustose coralline alga. PLoS ONE 2016, 11, e0159057. [Google Scholar] [CrossRef]
- Sinutok, S.; Hill, R.; Doblin, M.A.; Kühl, M.; Ralph, P.J. Microenvironmental changes support evidence of photosynthesis and calcification inhibition in Halimeda under ocean acidification and warming. Coral Reefs 2012, 31, 1201–1213. [Google Scholar] [CrossRef]
- Schulz, K.G.; Riebesell, U. Diurnal changes in seawater carbonate chemistry speciation at increasing atmospheric carbon dioxide. Mar. Biol. 2013, 160, 1889–1899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinutok, S.; Pongparadon, S.; Prathep, A. Seasonal variation in density, growth rate and calcium carbonate accumulation of Halimeda macroloba Decaisne at Tangkhen Bay, Phuket Province, Thailand. Malays. J. Sci. 2008, 27, 1–8. [Google Scholar]
- Sinutok, S.; Hill, R.; Doblin, M.A.; Wuhrer, R.; Ralph, P.J. Warmer more acidic conditions cause decreased productivity and calcification in subtropical coral reef sediment-dwelling calcifiers. Limnol. Oceanogr. 2011, 56, 1200–1212. [Google Scholar] [CrossRef]
- Kleypas, J.A.; Buddemeier, R.W.; Archer, D.; Gattuso, J.-P.; Langdon, D.; Opdyke, B.N. Geochemical consequences of increased atmospheric carbon dioxide on coral reefs. Science 1999, 284, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, L.C.; Bischof, K. Ocean acidification effects on calcifying macroalgae. Aquat. Biol. 2014, 22, 261–279. [Google Scholar] [CrossRef] [Green Version]
- Iluz, D.; Fermani, S.; Ramot, M.; Reggi, M.; Caroselli, E.; Prada, F.; Dubinsky, Z.; Goffredo, S.; Falini, G. Calcifying response and recovery potential of the brown alga Padina pavonica under ocean acidification. ACS Earth Sp. Chem. 2017, 1, 316–323. [Google Scholar] [CrossRef]
- Peach, K.E.; Koch, M.S.; Blackwelder, P.L.; Manfrino, C. Calcification and photophysiology responses to elevated pCO2 in six Halimeda species from contrasting irradiance environments on Little Cayman Island reefs. J. Exp. Mar. Bio. Ecol. 2017, 486, 114–126. [Google Scholar] [CrossRef]
- Okazaki, M.; Pentecost, A.; Tanaka, Y.; Miyata, M. A study of calcium carbonate deposition in the genus Padina (Phaeophyceae, Dictyotales). Br. Phycol. J. 1986, 21, 217–224. [Google Scholar] [CrossRef]
- Johnson, V.R.; Russell, B.D.; Fabricius, K.E.; Brownlee, C.; Hall-Spencer, J.M. Temperate and tropical brown macroalgae thrive, despite decalcification, along natural CO2 gradients. Glob. Chang. Biol. 2012, 18, 2792–2803. [Google Scholar] [CrossRef] [Green Version]
- Koch, M.; Bowes, G.; Ross, C.; Zhang, X.-H. Climate change and ocean acidification effects on seagrasses and marine macroalgae. Glob. Chang. Biol. 2013, 19, 103–132. [Google Scholar] [CrossRef]
- Wei, Z.; Long, C.; Zhang, Y.; Huo, Y.; Yang, F.; Long, L. Increased light availability enhances tolerance against ocean acidification stress in Halimeda opuntia. bioRxiv 2020, 1, 1–38. [Google Scholar]
- Mayakun, J.; Bunruk, P.; Kongsaeng, R. Growth rate and calcium carbonate accumulation of Halimeda macroloba Decaisne (Chlorophyta: Halimedaceae) in Thai waters. Songklanakarin J. Sci. Technol. 2014, 36, 419–423. [Google Scholar]
- Wichachucherd, B.; Prathep, A.; Zuccarello, G.C. Phylogeography of Padina boryana (Dictyotales, Phaeophyceae) around the Thai-Malay Peninsula. Eur. J. Phycol. 2014, 49, 313–323. [Google Scholar] [CrossRef]
- Kaewsrikhaw, R.; Prathep, A.; Darakrai, A.; Beer, S. Photosynthesis and calcification in two Halimeda species from Phuket, Thailand. Bot. Mar. 2016, 59, 187–192. [Google Scholar] [CrossRef]
- Prathep, A.; Kaewsrikhaw, R.; Mayakun, J.; Darakrai, A. The effects of light intensity and temperature on the calcification rate of Halimeda macroloba. J. Appl. Phycol. 2018, 30, 3405–3412. [Google Scholar] [CrossRef]
- Diaz-Pulido, G.; Cornwall, C.; Gartrell, P.; Hurd, C.; Tran, D. V Strategies of dissolved inorganic carbon use in macroalgae across a gradient of terrestrial influence: Implications for the Great Barrier Reef in the context of ocean acidification. Coral Reefs 2016, 35, 1327–1341. [Google Scholar] [CrossRef]
- Cornwall, C.E.; Revill, A.T.; Hall-Spencer, J.M.; Milazzo, M.; Raven, J.A.; Hurd, C.L. Inorganic carbon physiology underpins macroalgal responses to elevated CO2. Sci. Rep. 2017, 7, srep46297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borowitzka, M.A.; Larkum, A.W.D. Calcification in the Green Alga Halimeda: III. The sources of inorganic carbon for photosynthesis and calcification and a model of the mechanism of calcification. J. Exp. Bot. 1976, 27, 879–893. [Google Scholar] [CrossRef]
- Böhm, L.; Fütterer, D.; Kaminski, E. Algal calcification in some Codiaceae (Chlorophyta): Ultrastructure and location of skeletal deposits 1, 2. J. Phycol. 1978, 14, 486–493. [Google Scholar] [CrossRef]
- Mauffrey, A.R.L.; Cappelatti, L.; Griffin, J.N. Seaweed functional diversity revisited: Confronting traditional groups with quantitative traits. J. Ecol. 2020, 108, 2390–2405. [Google Scholar] [CrossRef]
- Jokiel, P.L. Ocean acidification and control of reef coral calcification by boundary layer limitation of proton flux. Bull. Mar. Sci. 2011, 87, 639–657. [Google Scholar] [CrossRef]
- Ries, J.B. A physicochemical framework for interpreting the biological calcification response to CO2-induced ocean acidification. Geochim. Cosmochim. Acta 2011, 75, 4053–4064. [Google Scholar] [CrossRef]
- Tyler, C.; Kai, S.; Jokiel, P.L. Predicting the impact of ocean acidification on coral reefs: Evaluating the assumptions involved. ICES J. Mar. Sci. 2016, 73, 550–557. [Google Scholar] [CrossRef] [Green Version]
- Frankignoulle, M.; Pichon, M.; Gattuso, J.-P. Aquatic Calcification as a Source of Carbon Dioxide. In Carbon Sequestration Biosphere; Springer: Berlin/Heidelberg, Germany, 1995. [Google Scholar]
- Semesi, I.S.; Beer, S.; Björk, M. Seagrass photosynthesis controls rates of calcification and photosynthesis of calcareous macroalgae in a tropical seagrass meadow. Mar. Ecol. Prog. Ser. 2009, 382, 41–47. [Google Scholar] [CrossRef]
- Mayakun, J.; Prathep, A. Calcium carbonate productivity by Halimeda macroloba in the tropical intertidal ecosystem: The significant contributor to global carbonate budgets. Phycol. Res. 2019, 67, 94–101. [Google Scholar] [CrossRef]
- Mayakun, J.; Liao, C.-P.; Liu, S.-L. The standing stock and CaCO3 contribution of Halimeda macroloba in the tropical seagrass-dominated ecosystem in Dongsha Island, the main island of Dongsha Atoll, South China Sea. J. Mar. Biol. Assoc. United Kingd. 2020, 100, 1219–1227. [Google Scholar] [CrossRef]
- Pentecost, A. Calcification Processes in Algae and Cyanobacteria. In Calcareous Algae and Stromatolites; Springer: Berlin/Heidelberg, Germany, 1991. [Google Scholar]
- Egilsdottir, H.; Olafsson, J.; Martin, S. Photosynthesis and calcification in the articulated coralline alga Ellisolandia elongata (Corallinales, Rhodophyta) from intertidal rock pools. Eur. J. Phycol. 2016, 51, 59–70. [Google Scholar] [CrossRef] [Green Version]
- Williamson, C.J.; Perkins, R.; Voller, M.; Yallop, M.L.; Brodie, J. The regulation of coralline algal physiology, an in situ study of Corallina officinalis (Corallinales, Rhodophyta). Biogeosciences 2017, 14, 4485–4498. [Google Scholar] [CrossRef] [Green Version]
- Jensen, P.R.; Gibson, R.A.; Littler, M.M.; Littler, D.S. Photosynthesis and calcification in four deep-water Halimeda species (chlorophyceae, caulerpales). Deep Sea Res. Part A Oceanogr. Res. Pap. 1985, 32, 451–464. [Google Scholar] [CrossRef]
- De Beer, D.; Larkum, A.W.D. Photosynthesis and calcification in the calcifying algae Halimeda discoidea studied with microsensors. Plant. Cell Environ. 2001, 24, 1209–1217. [Google Scholar] [CrossRef] [Green Version]
- McConnaughey, T.A.; Whelan, J.F. Calcification generates protons for nutrient and bicarbonate uptake. Earth-Sci. Rev. 1997, 42, 95–117. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Morphological and Cytological Aspects of Algal Calcification. In International Review of Cytology; Elsevier: Amsterdam, The Netherlands, 1982; pp. 127–162. [Google Scholar]
- Hofmann, L.C.; Schoenrock, K.; de Beer, D. Arctic coralline algae elevate surface pH and carbonate in the dark. Front. Plant Sci. 2018, 9, 1416. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Larkum, A.W.D. Calcification in the green alga Halimeda. I. An Ultrastructure study of thallus development 1. J. Phycol. 1977, 13, 6–16. [Google Scholar] [CrossRef]
- Lee, D.; Carpenter, S.J. Isotopic disequilibrium in marine calcareous algae. Chem. Geol. 2001, 172, 307–329. [Google Scholar] [CrossRef]
- Gil-Díaz, T.; Haroun, R.; Tuya, F.; Betancor, S.; Viera-Rodríguez, M.A. Effects of ocean acidification on the brown alga Padina pavonica: Decalcification due to acute and chronic events. PLoS ONE 2014, 9, e108630. [Google Scholar] [CrossRef] [Green Version]
- Goffredo, S.; Prada, F.; Caroselli, E.; Capaccioni, B.; Zaccanti, F.; Pasquini, L.; Fantazzini, P.; Fermani, S.; Reggi, M.; Levy, O.; et al. Biomineralization control related to population density under ocean acidification. Nat. Clim. Chang. 2014, 4, 593–597. [Google Scholar] [CrossRef] [Green Version]
- Pettit, L.R.; Smart, C.W.; Hart, M.B.; Milazzo, M.; Hall-Spencer, J.M. Seaweed fails to prevent ocean acidification impact on foraminifera along a shallow-water CO2 gradient. Ecol. Evol. 2015, 5, 1784–1793. [Google Scholar] [CrossRef] [Green Version]
- Betancor, S.; Tuya, F.; Gil-Díaz, T.; Figueroa, F.L.; Haroun, R. Effects of a submarine eruption on the performance of two brown seaweeds. J. Sea Res. 2014, 87, 68–78. [Google Scholar] [CrossRef]
- Celis-Plá, P.S.M.; Hall-Spencer, J.M.; Horta, P.A.; Milazzo, M.; Korbee, N.; Cornwall, C.E.; Figueroa, F.L. Macroalgal responses to ocean acidification depend on nutrient and light levels. Front. Mar. Sci. 2015, 2, 26. [Google Scholar] [CrossRef] [Green Version]
- Vogel, N.; Fabricius, K.E.; Strahl, J.; Noonan, S.H.C.; Wild, C.; Uthicke, S. Calcareous green alga Halimeda tolerates ocean acidification conditions at tropical carbon dioxide seeps. Limnol. Oceanogr. 2015, 60, 263–275. [Google Scholar] [CrossRef] [Green Version]
- Price, N.; Hamilton, S.; Tootell, J.; Smith, J. Species-specific consequences of ocean acidification for the calcareous tropical green algae Halimeda. Mar. Ecol. Prog. Ser. 2011, 440, 67–78. [Google Scholar] [CrossRef] [Green Version]
- Schubert, N.; Hofmann, L.C.; Almeida Saá, A.C.; Moreira, A.C.; Arenhart, R.G.; Fernandes, C.P.; de Beer, D.; Horta, P.A.; Silva, J. Calcification in free-living coralline algae is strongly influenced by morphology: Implications for susceptibility to ocean acidification. Sci. Rep. 2021, 11, 11232. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Mechanism in algal calcification. Prog. Phycol. Res. 1982, 1, 137–177. [Google Scholar]
- Ralph, P.J.; Gademann, R. Rapid light curves: A powerful tool to assess photosynthetic activity. Aquat. Bot. 2005, 82, 222–237. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Cappelatti, L.; Mauffrey, A.R.L.; Griffin, J.N. Applying continuous functional traits to large brown macroalgae: Variation across tidal emersion and wave exposure gradients. Mar. Biol. 2019, 166, 145. [Google Scholar] [CrossRef] [Green Version]
- Pierrot, D.; Lewis, E.; Wallace, D.W.R. MS Excel Program Developed for CO2 System Calculations: ORNL/CDIAC-105a. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy, Oak Ridge, Tennessee. 2006. Available online: http://marine.gov.scot/sma/content/ms-excel-program-developed-co2-system-calculations (accessed on 21 October 2021).
- Smith, S.V.; Key, G.S. Carbon dioxide and metabolism in marine environments1. Limnol. Oceanogr. 1975, 20, 493–495. [Google Scholar] [CrossRef]
- Gazeau, F.; Quiblier, C.; Jansen, J.M.; Gattuso, J.-P.; Middelburg, J.J.; Heip, C.H.R. Impact of elevated CO2 on shellfish calcification. Geophys. Res. Lett. 2007, 34, L07603. [Google Scholar] [CrossRef] [Green Version]
- Haynes, D.; Ralph, P.; Prange, J.; Dennison, B. The Impact of the herbicide Diuron on photosynthesis in three species of tropical Seagrass. Mar. Pollut. Bull. 2000, 41, 288–293. [Google Scholar] [CrossRef]
- Laurent, J.; Tambutté, S.; Tambutté, É.; Allemand, D.; Venn, A. The influence of photosynthesis on host intracellular pH in scleractinian corals. J. Exp. Biol. 2012, 216, 1398–1404. [Google Scholar] [CrossRef] [Green Version]





| Padina boryana | Halimeda opuntia | Halimeda macroloba | |
|---|---|---|---|
| Specific thallus area (cm2 g−1 fw) | 37.09 ± 1.83 a | 6.41 ± 0.28 b | 6.31 ± 0.40 b |
| Specific thallus area (cm2 g−1 dw) | 163.88 ± 13.37 a | 14.84 ± 1.32 b | 23.97 ± 1.50 b |
| % Dry weight of calcium carbonate | 46.89 ± 3.57 a | 81.44 ± 1.71 b | 77.35 ± 1.44 b |
| Calcium carbonate polymorph | Aragonite | Aragonite | Aragonite |
| Site of calcium carbonate | Cell surface | Intercellular spaces | Intercellular spaces |
| Calcium carbonate crystal shape | Needle or rod shape | Needle or rod shape | Needle or rod shape |
| Crystal width (µm) | 0.13 ± 0.02 a | 0.22 ± 0.02 b | 0.12 ± 0.02 a |
| Crystal length (µm) | 1.36 ± 0.05 a | 1.80 ± 0.09 b | 1.24 ± 0.05 a |
| Crystal density (needles µm−2) | 5.26 ± 0.41 a | 3.47 ± 0.12 b | 5.65 ± 0.34 c |
| Surface diameter of primary utricle (µm) | - | 23.67 ± 0.69 a | 28.31 ± 0.40 b |
| Diffusion pathway length (µm) | - | 5.95 ± 0.25 a | 22.11 ± 0.51 b |
| DIC | Padina boryana | ||
|---|---|---|---|
| Initial | After 3 h Incubation | After Equilibration | |
| CO2 (μM) | 8.97 ± 0.22 a | 0.93 ± 0.26 b | 9.64 ± 0.51 a |
| HCO3− (μM) | 1491.83 ± 6.64 a | 628.83 ± 93.61 b | 1438.83 ± 28.28 a |
| CO32− (μM) | 199.57 ± 3.80 a | 436.15 ± 20.38 b | 174.50 ± 6.66 c |
| ΩCa | 4.97 ± 0.09 a | 10.88 ± 0.50 b | 4.35 ± 0.16 a |
| ΩAr | 3.31 ± 0.06 a | 7.24 ± 0.33 b | 2.89 ± 0.11 c |
| DIC | Halimeda opuntia | ||
| Initial | After 3 h Incubation | After Equilibration | |
| CO2 (μM) | 8.86 ± 0.10 a | 12.12 ± 1.41 c | 9.85 ± 0.56 a |
| HCO3− (μM) | 1489.30 ± 6.19 a | 1281.85 ± 73.01 c | 1217.81 ± 71.85 c |
| CO32− (μM) | 200.56 ± 2.16 a | 114.93 ± 9.95 d | 125.61 ± 14.61 d |
| ΩCa | 5.00 ± 0.05 a | 2.86 ± 0.24 d | 3.13 ± 0.36 d |
| ΩAr | 3.33 ± 0.03 a | 1.90 ± 0.16 d | 2.08 ± 0.24 d |
| DIC | Halimeda macroloba | ||
| Initial | After 3 h Incubation | After Equilibration | |
| CO2 (μM) | 8.71 ± 0.40 ad | 7.29 ± 0.81 d | 9.50 ± 0.25 a |
| HCO3− (μM) | 1483.06 ± 4.73 a | 1210.38 ± 75.68 c | 1276.53 ± 53.11 c |
| CO32− (μM) | 203.04 ± 3.97 a | 168.60 ± 11.24 ac | 132.38 ± 10.27 d |
| ΩCa | 5.06 ± 0.09 a | 4.20 ± 0.28 c | 3.30 ± 0.25 d |
| ΩAr | 3.37 ± 0.06 a | 2.80 ± 0.18 c | 2.19 ± 0.17 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buapet, P.; Sinutok, S. Calcification in Three Common Calcified Algae from Phuket, Thailand: Potential Relevance on Seawater Carbonate Chemistry and Link to Photosynthetic Process. Plants 2021, 10, 2537. https://doi.org/10.3390/plants10112537
Buapet P, Sinutok S. Calcification in Three Common Calcified Algae from Phuket, Thailand: Potential Relevance on Seawater Carbonate Chemistry and Link to Photosynthetic Process. Plants. 2021; 10(11):2537. https://doi.org/10.3390/plants10112537
Chicago/Turabian StyleBuapet, Pimchanok, and Sutinee Sinutok. 2021. "Calcification in Three Common Calcified Algae from Phuket, Thailand: Potential Relevance on Seawater Carbonate Chemistry and Link to Photosynthetic Process" Plants 10, no. 11: 2537. https://doi.org/10.3390/plants10112537
APA StyleBuapet, P., & Sinutok, S. (2021). Calcification in Three Common Calcified Algae from Phuket, Thailand: Potential Relevance on Seawater Carbonate Chemistry and Link to Photosynthetic Process. Plants, 10(11), 2537. https://doi.org/10.3390/plants10112537