Sewage Pollution Promotes the Invasion-Related Traits of Impatiens glandulifera in an Oligotrophic Habitat of the Sharr Mountain (Western Balkans)
Abstract
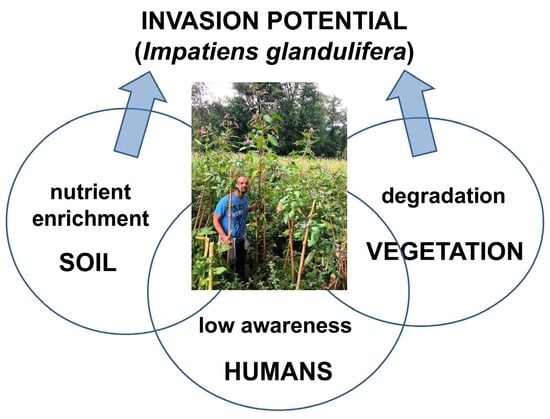
:1. Introduction
2. Results
2.1. The Invader Is Already Well-Established
2.2. Life History Traits Are Correlated with Resident Vegetation and Soils
2.3. Local Practices and Attitudes
3. Discussion
3.1. Sewage Pollution Favors the Invasion in an Oligotrophic Habitat
3.2. Phenotypic Plasticity in Different Habitats
3.3. Local Perception Is Not Yet Negative
3.4. Himalayan Balsam Might Be Underreported in the Western Balkans
3.5. Implicit Risks for the Region
4. Material and Methods
4.1. Study Area
4.2. Vegetation Survey
4.3. Soil Survey and Analyses
4.4. Local Knowledge Survey
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tylianakis, J.M.; Didham, R.K.; Bascompte, J.; Wardle, D.A. Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 2008, 11, 1351–1363. [Google Scholar] [CrossRef] [PubMed]
- Ehrenfeld, J.G. Ecosystem consequences of biological invasions. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 59–80. [Google Scholar] [CrossRef] [Green Version]
- Vilà, M.; Espinar, J.L.; Hejda, M.; Hulme, P.E.; Jarošík, V.; Maron, J.L.; Pergl, J.; Schaffner, U.; Sun, Y.; Pyšek, P. Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 2011, 14, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef]
- Beerling, D.J.; Perrins, J.M. Biological flora of the British Isles: Impatiens glandulifera Royle (Impatiens Roylei Walp.). J. Ecol. 1993, 81, 367–382. [Google Scholar] [CrossRef]
- GBIF Secretariat. GBIF Backbone Taxonomy. Checklist Dataset. 2021. Available online: https://www.gbif.org/species/2891770 (accessed on 13 September 2021).
- Pyšek, P.; Prach, K. Invasion dynamics of Impatiens glandulifera—A century of spreading reconstructed. Biol. Conserv. 1995, 74, 41–48. [Google Scholar] [CrossRef]
- Tanner, R.A.; Gange, A.C. Himalayan balsam, Impatiens glandulifera: Its ecology, invasion and management. Weed Res. 2020, 60, 4–7. [Google Scholar] [CrossRef]
- Helsen, K.; Diekmann, M.; Decocq, G.; De Pauw, K.; Govaert, S.; Jessen Graae, B.J.; Hagenblad, J.; Liira, J.; Orczewska, A.; Sanczuk, P.; et al. Biological flora of Central Europe: Impatiens glandulifera Royle. Perspect. Plant Ecol. Evol. Syst. 2021, 50, 125609. [Google Scholar] [CrossRef]
- European Commission. List of Invasive Alien Species of Union Concern. 2017. Available online: https://ec.europa.eu/environment/nature/invasivealien/list/index_en.htm (accessed on 10 September 2021).
- CABI. Impatiens glandulifera. In Invasive Species Compendium; CAB International: Wallingford, UK, 2020; Available online: https://www.cabi.org/isc/datasheet/28766 (accessed on 3 September 2021).
- Pahl, A.T.; Kollmann, J.; Mayer, A.; Haider, S. No evidence for local adaptation in an invasive alien plant: Field and greenhouse experiments tracing a colonization sequence. Ann. Bot. 2013, 112, 1921–1930. [Google Scholar] [CrossRef] [Green Version]
- Petřík, P.; Pergl, J.; Wild, J. Recording effort biases the species richness cited in plant distribution atlases. Perspect. Plant Ecol. Evol. Syst. 2010, 12, 57–65. [Google Scholar] [CrossRef]
- Hejda, M.; Pyšek, P. What is the impact of Impatiens glandulifera on species diversity of invaded riparian vegetation? Biol. Conserv. 2006, 132, 143–152. [Google Scholar] [CrossRef]
- Kiełtyk, P.; Delimat, A. Impact of the alien plant Impatiens glandulifera on species diversity of invaded vegetation in the northern foothills of the Tatra Mountains, Central Europe. Plant Ecol. 2019, 220, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Coakley, S.; Petti, C. Impacts of the invasive Impatiens glandulifera: Lessons learned from one of Europe’s top invasive species. Biology 2021, 10, 619. [Google Scholar] [CrossRef]
- Kueffer, C.; Pyšek, P.; Richardson, D.M. Integrative invasion science: Model systems, multi-site studies, focused meta-analysis and invasion syndromes. New Phytol. 2013, 200, 615–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miles, L.J.; Parker, G.R. DTPA soil extractable and plant heavy metal concentrations with soil-added Cd treatments. Plant Soil 1978, 51, 59–68. [Google Scholar] [CrossRef]
- Tyler, T.; Herbertsson, L.; Olofsson, J.; Olsson, P.A. Ecological indicator and traits values for Swedish vascular plants. Ecol. Indic. 2021, 120, 106923. [Google Scholar] [CrossRef]
- Ellenberg, C.; Leuschner, C. Vegetation Mitteleuropas mit den Alpen: In Ökologischer, Dynamischer und Historischer Sicht, 6th ed.; Ulmer Verlag: Stuttgart, Germany, 2010. [Google Scholar] [CrossRef]
- Topalović, M. Pedološko-bonitetne karakteristike. In Opština Štrpce. Sirinićka Župa—Odlike Prirodne Sredine; Dinić, J., Ed.; SANU: Belgrade, Serbia, 1990; pp. 227–271. (In Serbian) [Google Scholar]
- Hobbs, R.J.; Huenneke, L.F. Disturbance, diversity, and invasion: Implications for conservation. Conserv. Biol. 1992, 6, 324–337. [Google Scholar] [CrossRef] [Green Version]
- Drescher, A.; Prots, B. Warum breitet sich das Drüsen-Springkraut (Impatiens glandulifera Royle) in den Alpen? Wulfenia 2000, 7, 5–26. [Google Scholar]
- Drescher, A.; Prots, B. Distribution patterns of Himalayan Balsam (Impatiens glandulifera Royle) in Austria. Kanitzia 2003, 11, 85–96. [Google Scholar]
- European Environment Agency. Europe’s Ecological Backbone: Recognising the True Value of Our Mountains. EEA Report No 6/2010. 2010. Available online: https://www.eea.europa.eu/publications/europes-ecological-backbone (accessed on 1 November 2011).
- Cisneros, B.J. Safe sanitation in low economic development areas. Treatise Water Sci. 2011, 4, 147–200. [Google Scholar] [CrossRef]
- Menczer, K.; Veselaj, Z.; Hajredini, E. Kosovo Biodiversity Analysis. USAID Publication. 2018. Available online: https://pdf.usaid.gov/pdf_docs/PA00WCZP.pdf (accessed on 17 October 2021).
- Banks, D.; Karnachuk, O.V.; Parnachev, V.P.; Holden, W.B.; Frengstad, B. Groundwater contamination from rural pit latrines: Examples from Siberia and Kosova. Water Environ. J. 2007, 16, 147–152. [Google Scholar] [CrossRef]
- Sweeney, C.; Wingler, A. Effect of environmental factors on size and fecundity of field populations of Impatiens glandulifera. Plant Ecol. Divers. 2020, 13, 413–424. [Google Scholar] [CrossRef]
- Kollmann, J.; Bañuelos, M.J. Latitudinal trends in growth and phenology of the invasive alien plant Impatiens glandulifera (Balsaminaceae). Divers. Distrib. 2004, 10, 377–385. [Google Scholar] [CrossRef]
- Bieberich, J.; Müller, S.; Feldhaar, H.; Lauerer, M. Invasive Impatiens glandulifera: A driver of changes in native vegetation? Ecol. Evol. 2021, 12, 1320–1333. [Google Scholar] [CrossRef]
- Mujuni, N. The Effect of Latitude, Litter and Vegetation Type on the Performance of the Invasive Species Impatiens glandulifera. Master’s Thesis, Norwegian Institute of Science and Technology, Trondheim, Norway, 11 May 2015. Available online: https://ntnuopen.ntnu.no/ntnu-xmlui/handle/11250/2351510 (accessed on 10 November 2021).
- Nikolic, N.; Kostic, L.; Djordjevic, A.; Nikolic, M. Phosphorus deficiency is the major limiting factor for wheat on alluvium polluted by the copper mine pyrite tailings: A black box approach. Plant Soil 2011, 339, 485–498. [Google Scholar] [CrossRef]
- Nikolic, N.; Kostic, L.; Nikolic, M. To dam, or not do dam? Abolishment of further flooding impedes the natural revegetation processes after long-term fluvial deposition of copper tailings. Land Degrad. Dev. 2017, 29, 1915–1924. [Google Scholar] [CrossRef]
- Bauer, J.T. Invasive species: “back-seat drivers” of ecosystem change? Biol. Invasions 2012, 14, 1295–1304. [Google Scholar] [CrossRef]
- Čuda, J.; Vıtkova, M.; Albrechtova, M.; Guo, W.Y.; Barney, J.N.; Pyšek, P. Invasive herb Impatiens glandulifera has minimal impact on multiple components of temperate forest ecosystem function. Biol. Invasions 2017, 19, 3051–3066. [Google Scholar] [CrossRef]
- Greenwood, P.; Baumann, P.; Pulley, S.; Kuhn, N.J. The invasive alien plant, Impatiens glandulifera (Himalayan Balsam), and increased soil erosion: Causation or association? Case studies from a river system in Switzerland and the UK. J. Soils Sediments 2018, 18, 3463–3477. [Google Scholar] [CrossRef]
- Maskell, L.C.; Bullock, J.M.; Smart, S.M.; Thompson, K.; Hulme, P.E. The distribution and habitat associations of non-native plant species in urban riparian habitats. J. Veg. Sci. 2006, 17, 449–508. [Google Scholar] [CrossRef]
- Gruntman, M.; Pehl, A.K.; Joshi, S.; Tielbörger, K. Competitive dominance of the invasive plant Impatiens glandulifera: Using competitive effect and response with a vigorous neighbour. Biol. Invasions 2014, 16, 141–151. [Google Scholar] [CrossRef]
- Tvedt, T.; Oestigaard, T. Ideas of Water from Ancient Societies to the Modern World; Series II; Tauris: London, UK, 2010; Volume I. [Google Scholar] [CrossRef]
- Weiss, W. Impatiens glandulifera in Mitteleuropa: Geschichte seiner Verbreitung und Wertschätzung. In Die Rote Pest aus Grüner Sicht: Springkräuter—von Imkern Geschätzt, von Naturschützern Bekämpft; Leopold Stocker: Graz, Austria, 2015; pp. 50–115. [Google Scholar]
- Flügel, H.J. Das Drüsige Springkraut (Impatiens glandulifera)—Bedrohung oder Bereicherung? Nat. Landsch. 2017, 92, 268–273. [Google Scholar] [CrossRef]
- Veselaj, Z.; Behxhet, M.; Hajdari, A.; Krasniqi, Z. 2012. Biodiversity conservation status in the Republic of Kosovo with focus on biodiversity centres. J. Environ. Biol. 2012, 33, 307–310. [Google Scholar]
- Malý, K. Mitteilungen über die Flora von Bosnien-Hercegovina. Glasn. Zem. Muz. BiH 1935, 47, 101–112. [Google Scholar]
- Pavlović, D.; Panjković, B.; Stojšić, V. Impatiens glandulifera Royle—nova adventivna vrsta u flori Srbije. Glas. Prir. Muz. Beogr. 1995, 49–50, 73–76. (In Serbian) [Google Scholar]
- Pacanoski, Z.; Saliji, A. The invasive Impatiens glandulifera Royle (Himalayan balsam) in the Republic of Macedonia: First record and forecast. EPPO Bull. 2014, 44, 87–93. [Google Scholar] [CrossRef]
- Lazarević, R.; Kirbus, B. Erozija zemljišta. In Opština Štrpce. Sirinićka Župa—Odlike Prirodne Sredine; Dinić, J., Ed.; SANU: Belgrade, Serbia, 1990; pp. 153–182. (In Serbian) [Google Scholar]
- Ocokoljić, M.; Redžić, R.; Jovanović, V. Hidrografske i hidrološke karakteristike. In Opština Štrpce. Sirinićka Župa—Odlike Prirodne Sredine; Dinić, J., Ed.; SANU: Belgrade, Serbia, 1990; pp. 207–226. (In Serbian) [Google Scholar]
- Krasniqi, F.D. Šumska Vegetacija Brdskog Regiona Kosova i Metohije; Zajednica Naučnih Ustanova Kosova: Priština, SFRY, 1972; Knjiga 27. (In Serbian) [Google Scholar]
- Jovanović, B. Alluvial forests. In Vegetation of Serbia; Sarić, M., Ed.; SANU: Belgrade, Serbia, 1997; Volume II/1, pp. 107–158. (In Serbian) [Google Scholar]
- Bieberich, J.; Feldhaar, H.; Lauerer, M. Micro-habitat and season dependent impact of the invasive Impatiens glandulifera on native vegetation. Neobiota 2020, 57, 109–131. [Google Scholar] [CrossRef]
- Smartt, P. Sampling for vegetation survey: A flexible systematic model for sample location. J. Biogeogr. 1978, 5, 43–56. [Google Scholar] [CrossRef]
- Chambers, R. The origins and practice of Participatory Rural Appraisal. World Dev. 1994, 22, 953–969. [Google Scholar] [CrossRef] [Green Version]




| Invader Physiognomy | Typical Invaded Sites | Code |
|---|---|---|
| Prominently lush, coarse, average height >2.5 m | Highly disturbed riparian but also moist non-riparian areas, visible deposition of organic waste (garbage, manure), sparse tree canopy | A |
| Intermediate | Riparian area with denser forest cover and no obvious garbage deposition (lower anthropogenic influence) | B |
| Slender, average height <1.5 m | Open ruderal habitats, roadsides, deposits of sand and other construction waste, often also household waste; vicinity of waterways, but not flooded | C |
| Soil Parameter | Site Code | ||
|---|---|---|---|
| A | B | C | |
| pH | 7.6 ± 0.3 a | 7.2 ± 0.4 a | 8.1 ± 0.2 b |
| CEC (cmolc kg−1) | 22 ± 5 b | 15 ± 3 a | 24 ± 4 b |
| CaCO3 (%) | 4 ± 2 b | 2.0 ± 1 a | 8 ± 2 c |
| Corg (%) | 3.4 ± 0.9 b | 3.6 ±1.2 b | 2.8 ± 0.6 a |
| Ntot (%) | 0.41 ± 0.05 b | 0.46 ± 0.04 b | 0.31 ± 0.05 a |
| Stot (%) | 0.07 ± 0.03 b | 0.02 ± 0.02 a | 0.02 ± 0.01 a |
| Available P (mg kg−1) | 146 ± 49 c | 28 ± 7 b | 18 ± 5 a |
| Available K (mg kg−1) | 863 ± 229 b | 421 ± 155 a | 418 ± 174 a |
| Available Ca (mg kg−1) | 3363 ± 1181 a | 2379 ± 635 a | 4372 ± 709 a |
| Available Mg (mg kg−1) | 270 ± 82 a | 227 ± 72 a | 145 ± 40 a |
| Available B (mg kg−1) | 0.16 ± 0.08 a | 0.06 ± 0.02 a | 0.11 ± 0.04 a |
| Available Fe (mg kg−1) | 53 ±18 b | 64 ± 9 c | 34 ± 9 a |
| Available Mn (mg kg−1) | 13 ± 3 a | 20 ± 1 b | 11.1 ± 0.7 a |
| Available Cu (mg kg−1) | 4 ± 1 b | 2.7 ± 0.5 a | 2.0 ± 0.4 a |
| Available Zn (mg kg−1) | 7 ± 2 b | 1.9 ± 0.5 a | 3.6 ± 0.8 a |
| Available Mo (mg kg−1) | 0.026 ± 0.002 a | 0.02 ± 0.05 a | 0.0289 ± 0.0008 a |
| Available Ni (mg kg−1) | 1.2 ± 0.6 a | 4 ± 1 b | 0.7 ± 0.2 a |
| Available Cr (mg kg−1) | 0.03 ± 0.01 a | 0.04 ± 0.01 a | 0.03 ± 0.01 a |
| Available Cd (mg kg−1) | 0.03 ± 0.01 a | 0.04 ± 0.01 a | 0.03 ± 0.01 a |
| Available Pb (mg kg−1) | 3.6 ± 0.9 a | 2.7 ± 0.7 a | 3.8 ± 0.9 a |
| Available As (mg kg−1) | 0.02 ± 0.02 a | 0.05 ± 0.03 a | 0.04 ± 0.01 a |
| Resident Vegetation | Site Code | ||
|---|---|---|---|
| A | B | C | |
| Average species number per sample | 7.5 ± 1.5 a | 14.3 ± 3.2 b | 7.0 ± 1.8 a |
| Total number of species a | 39 | 53 | 46 |
| Weighted mean distance (relative Sørensen) | 0.66 | 0.76 | 0.82 |
| Ellenberg indicator value for light | 7.0 ± 0.5 b | 5.7 ± 0.2 a | 7.9 ± 0.6 c |
| Ellenberg indicator value for moisture | 6.8 ± 0.5 b | 7.9 ± 0.3 c | 5.4 ± 0.4 a |
| Ellenberg indicator value for N | 8.1 ± 0.5 c | 6.7 ± 0.4 b | 5.5 ± 0.4 a |
| Comparison of the Invaded Resident Vegetation | Test Statistics | ||
|---|---|---|---|
| T | A | p | |
| “A” vs. “C” | −16.1 | 0.12 | 0.00000000 |
| “B” vs. “C” | −13.6 | 0.11 | 0.00000000 |
| “A” vs. “B” | −7.6 | 0.05 | 0.00000024 |
| Local Attitudes | Respondent Age Group | |
|---|---|---|
| Young (16–30 y) | Elder (30–60 y) | |
| % of Responses | ||
| Awareness | ||
| Know it is present | 44 | 86 |
| Know it is introduced/non-native | 0 | 6 |
| Familiar with its vernacular name | 0 | 15 |
| Perception | ||
| Indifferent to its presence | 97 | 74 |
| Amenity of riparian landscape | 18 | 5 |
| Nuisance weed in gardens | 3 | 23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanojevic, M.; Trailovic, M.; Dubljanin, T.; Krivošej, Z.; Nikolic, M.; Nikolic, N. Sewage Pollution Promotes the Invasion-Related Traits of Impatiens glandulifera in an Oligotrophic Habitat of the Sharr Mountain (Western Balkans). Plants 2021, 10, 2814. https://doi.org/10.3390/plants10122814
Stanojevic M, Trailovic M, Dubljanin T, Krivošej Z, Nikolic M, Nikolic N. Sewage Pollution Promotes the Invasion-Related Traits of Impatiens glandulifera in an Oligotrophic Habitat of the Sharr Mountain (Western Balkans). Plants. 2021; 10(12):2814. https://doi.org/10.3390/plants10122814
Chicago/Turabian StyleStanojevic, Milos, Maja Trailovic, Tijana Dubljanin, Zoran Krivošej, Miroslav Nikolic, and Nina Nikolic. 2021. "Sewage Pollution Promotes the Invasion-Related Traits of Impatiens glandulifera in an Oligotrophic Habitat of the Sharr Mountain (Western Balkans)" Plants 10, no. 12: 2814. https://doi.org/10.3390/plants10122814
APA StyleStanojevic, M., Trailovic, M., Dubljanin, T., Krivošej, Z., Nikolic, M., & Nikolic, N. (2021). Sewage Pollution Promotes the Invasion-Related Traits of Impatiens glandulifera in an Oligotrophic Habitat of the Sharr Mountain (Western Balkans). Plants, 10(12), 2814. https://doi.org/10.3390/plants10122814