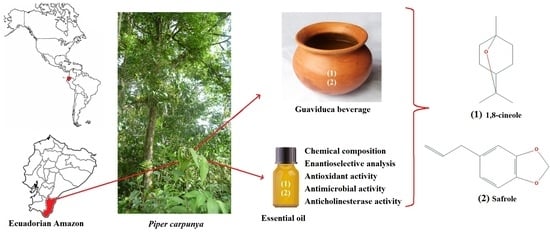
Study of Volatile Secondary Metabolites Present in Piper carpunya Leaves and in the Traditional Ecuadorian Beverage Guaviduca
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition
2.2. Enantioselective Analysis
2.3. Antimicrobial Activity
2.4. Antioxidant Capacity
2.5. Anticholinesterase Activity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Plant Material
4.3. Essential Oil Isolation
4.4. Determination of Physical Properties of Essential Oil
4.5. Elaboration of the Beverage
4.6. Extraction of Compounds from Beverage
4.7. Compounds Identification
4.8. Enantioselective Analysis
4.9. Antimicrobial Activity
4.10. Antioxidant Capacity
4.10.1. DPPH Radical Scavenging Activity
4.10.2. ABTS Radical Cation Scavenging Activity
4.11. Anticholinesterase Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Plant List. Piperaceae. Available online: http://www.theplantlist.org/1.1/browse/A/Piperaceae/ (accessed on 4 July 2020).
- Missouri Botanical Garden. Angiosperm Phylogeny Website. Available online: http://www.mobot.org/MOBOT/research/APweb/ (accessed on 29 April 2020).
- Salehi, B.; Zakaria, Z.A.; Gyawali, R.; Ibrahim, S.A.; Rajkovic, J.; Shinwari, Z.K.; Khan, T.; Sharifi-Rad, J.; Ozleyen, A.; Turkdonmez, E.; et al. Piper Species: A Comprehensive Review on Their Phytochemistry, Biological Activities and Applications. Molecules 2019, 24, 1364. [Google Scholar] [CrossRef] [Green Version]
- Jørgesen, P.M.; León-Yáñez, S. Catalogue of the Vascular Plants of Ecuador. Missouri Botanical Garden Press; Missouri Botanical Garden Press: St. Louis, MI, USA, 1999; pp. 1–1181. [Google Scholar]
- León-Yánez, S.; Valencia, R.; Pitmam, N.; Endara, L.; Ulloa Ulloa, C.; Navarrete, H. Libro Rojo de Plantas Endémicas del Ecuador. Available online: https://bioweb.bio/floraweb/librorojo (accessed on 4 May 2020).
- Rondón, M.E.; Velasco, J.; Cornejo, X.; Fernández, J.; Morocho, V. Chemical composition and antibacterial activity of Piper lenticellosum C.D.C essential oil collected in Ecuador. J. Appl. Pharm. Sci. 2016, 6, 156–159. [Google Scholar] [CrossRef] [Green Version]
- Ballesteros, J.L.; Tacchini, M.; Spagnoletti, A.; Grandini, A.; Paganetto, G.; Neri, L.M.; Marengo, A.; Angiolella, L.; Guerrini, A.; Sacchetti, G. Rediscovering Medicinal Amazonian Aromatic Plants: Piper carpunya (Piperaceae) Essential Oil as Paradigmatic Study. Evid. Based Complement. Altern. Med. 2019, 2019, 6194640. [Google Scholar] [CrossRef]
- Diaz, P.P.; Ramos, B.C.; Matta, G.E. New C6-C3 and C6-C1 compounds from piper lenticellosum. J. Nat. Prod. 1986, 49, 690–691. [Google Scholar] [CrossRef]
- Ortega, T.; Carretero, M.E.; Pascual, E.; Villar, A.M.; Chiriboga, X. Anti-inflammatory activity of ethanolic extracts of plants used in traditional medicine in Ecuador. Phytother. Res. 1996, 10, S121–S122. [Google Scholar]
- De Las Heras, B.; Slowing, K.; Benedí, J.; Carretero, E.; Ortega, T.; Toledo, C.; Bermejo, P.; Iglesias, I.; Abad, M.J.; Gómez-Serranillos, P.; et al. Antiinflammatory and antioxidant activity of plants used in traditional medicine in Ecuador. J. Ethnopharmacol. 1998, 61, 161–166. [Google Scholar] [CrossRef]
- Trabadela, C.; Sánchez-Fidalgo, S.; Miño, P.; Berenguer, B.; Quilez, A.; De La Puerta, R.; Martín, M.J. Gastroprotective effects of piper carpunya against diclofenac-induced gastric lesions in rats. Pharm. Biol. 2008, 46, 829–837. [Google Scholar] [CrossRef]
- Quílez, A.; Berenguer, B.; Gilardoni, G.; Souccar, C.; de Mendonça, S.; Oliveira, L.F.S.; Martín-Calero, M.J.; Vidari, G. Anti-secretory, anti-inflammatory and anti-Helicobacter pylori activities of several fractions isolated from Piper carpunya Ruiz & Pav. J. Ethnopharmacol. 2010, 128, 583–589. [Google Scholar] [CrossRef]
- Vargas, L.; Velasco-Negueruela, A.; Pérez-Alonso, M.J.; Palá-Paúl, J.; Vallejo, M.C.G. Essential Oil Composition of the Leaves and Spikes of Piper carpunya Ruíz et Pavón (Piperaceae) from Peru. J. Essent. Oil Res. 2004, 16, 122–123. [Google Scholar] [CrossRef]
- Burbott, A.J.; Loomis, W.D. Effects of Light and Temperature on the Monoterpenes of Peppermint. Plant Physiol. 1967, 42, 20–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molares, S.; González, S.B.; Ladio, A.; Agueda Castro, M. Etnobotánica, anatomía y caracterización físico-química del aceite esencial de Baccharis obovata Hook. et Arn. (Asteraceae: Astereae). Acta Bot. Bras. 2009, 23, 578–589. [Google Scholar] [CrossRef]
- Pragadheesh, V.S.; Yadav, A.; Chanotiya, C.S. Role of substituents in cyclodextrin derivatives for enantioselective gas chromatographic separation of chiral terpenoids in the essential oils of Mentha spicata. J. Chromatogr. B 2015, 1002, 30–41. [Google Scholar] [CrossRef]
- Takahisa, E.; Engel, K.-H. 2,3-Di-O-methoxymethyl-6-O-tert-butyldimethylsilyl-β-cyclodextrin, a useful stationary phase for gas chromatographic separation of enantiomers. J. Chromatogr. A 2005, 1076, 148–154. [Google Scholar] [CrossRef]
- König, W.A. Enantioselective capillary gas chromatography in the investigation of stereochemical correlations of terpenoids. Chirality 1998, 10, 499–504. [Google Scholar] [CrossRef]
- Tisserand, R.; Young, R. Essential Oil Safety: A Guide for Health Care Professionals, 2nd ed.; Churchill Livingstone: London, UK, 2014. [Google Scholar]
- Xiang, C.-P.; Han, J.-X.; Li, X.-C.; Li, Y.-H.; Zhang, Y.; Chen, L.; Qu, Y.; Hao, C.-Y.; Li, H.-Z.; Yang, C.-R.; et al. Chemical Composition and Acetylcholinesterase Inhibitory Activity of Essential Oils from Piper Species. J. Agric. Food Chem. 2017, 65, 3702–3710. [Google Scholar] [CrossRef]
- Howes, M.-J.R.; Houghton, P.J. Plants used in Chinese and Indian traditional medicine for improvement of memory and cognitive function. Pharmacol. Biochem. Behav. 2003, 75, 513–527. [Google Scholar] [CrossRef]
- Zarotsky, V.; Sramek, J.J.; Cutler, N.R. Galantamine hydrobromide: An agent for Alzheimer’s disease. Am. J. Health Syst. Pharm. 2003, 60, 446–452. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Luca, V.S.; Trifan, A.; Miron, A. Chapter 7—Antigenotoxic Potential of Some Dietary Non-phenolic Phytochemicals. In Studies in Natural Products Chemistry; Attaur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 60, pp. 223–297. [Google Scholar]
- Santos, F.A.; Silva, R.M.; Campos, A.R.; de Araújo, R.P.; Lima Júnior, R.C.P.; Rao, V.S.N. 1,8-cineole (eucalyptol), a monoterpene oxide attenuates the colonic damage in rats on acute TNBS-colitis. Food Chem. Toxicol. 2004, 42, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Yokota, H.; Hashimoto, H.; Motoya, M.; Yuasa, A. Enhancement of UDP-glucuronyltransferase, UDP-glucose dehydrogenase, and glutathione S-transferase activities in rat liver by dietary administration of eugenol. Biochem. Pharmacol. 1988, 37, 799–802. [Google Scholar] [CrossRef]
- Cascone, P.; Iodice, L.; Maffei, M.E.; Bossi, S.; Arimura, G.-I.; Guerrieri, E. Tobacco overexpressing β-ocimene induces direct and indirect responses against aphids in receiver tomato plants. J. Plant Physiol. 2015, 173, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Sayyah, M.; Nadjafnia, L.; Kamalinejad, M. Anticonvulsant activity and chemical composition of Artemisia dracunculus L. essential oil. J. Ethnopharmacol. 2004, 94, 283–287. [Google Scholar] [CrossRef]
- Russo, E.B.; Marcu, J. Chapter Three—Cannabis Pharmacology: The Usual Suspects and a Few Promising Leads. In Advances in Pharmacology; Kendall, D., Alexander, S.P.H., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 80, pp. 67–134. [Google Scholar]
- Bomfim, L.M.; Menezes, L.R.A.; Rodrigues, A.C.B.C.; Dias, R.B.; Gurgel Rocha, C.A.; Soares, M.B.P.; Neto, A.F.S.; Nascimento, M.P.; Campos, A.F.; Silva, L.C.R.C.E.; et al. Antitumour Activity of the Microencapsulation of Annona vepretorum Essential Oil. Basic Clin. Pharmacol. Toxicol. 2016, 118, 208–213. [Google Scholar] [CrossRef]
- Wexler, P. Encyclopedia of Toxicology, 3rd ed.; Academic Press: Bethesda, MD, USA, 2014; p. 5220. [Google Scholar]
- Gates, K. Covalent Modification of DNA by Natural Products. In Comprehensive Natural Products Chemistry; Barton, S.D., Meth-Cohn, O., Nakanishi, K., Eds.; Pergamon Press: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Sue, C. Essential Chemistry for Aromatherapy; Churchill Livingstone: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Siano, F.; Ghizzoni, C.; Gionfriddo, F.; Colombo, E.; Servillo, L.; Castaldo, D. Determination of estragole, safrole and eugenol methyl ether in food products. Food Chem. 2003, 81, 469–475. [Google Scholar] [CrossRef]
- Bogusz, M.J.; Al-Tufail, M. Chapter 18 Toxicological aspects of herbal remedies. In Handbook of Analytical Separations; Bogusz, M.J., Ed.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2008; Volume 6, pp. 589–610. [Google Scholar]
- Van Vuuren, S.; Holl, D. Antimicrobial natural product research: A review from a South African perspective for the years 2009–2016. J. Ethnopharmacol. 2017, 208, 236–252. [Google Scholar] [CrossRef] [PubMed]
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Hendry, E.R.; Worthington, T.; Conway, B.R.; Lambert, P.A. Antimicrobial efficacy of eucalyptus oil and 1,8-cineole alone and in combination with chlorhexidine digluconate against microorganisms grown in planktonic and biofilm cultures. J. Antimicrob. Chemother. 2009, 64, 1219–1225. [Google Scholar] [CrossRef]
- Şimşek, M.; Duman, R. Investigation of Effect of 1,8-cineole on Antimicrobial Activity of Chlorhexidine Gluconate. Pharmacogn. Res. 2017, 9, 234–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dognini, J.; Meneghetti, E.K.; Teske, M.N.; Begnini, I.M.; Rebelo, R.A.; Dalmarco, E.M.; Verdi, M.; de Gasper, A.L. Antibacterial activity of high safrole contain essential oils from Piper xylosteoides (Kunth) Steudel. J. Essent. Oil Res. 2012, 24, 241–244. [Google Scholar] [CrossRef]
- Lobato, A.M.; Ribeiro, A.; Pinheiro, M.D.F.D.S.; Maia, J.G.S. Atividade antimicrobiana de óleos essenciais da Amazônia. Acta Amaz. 1989, 19, 355–363. [Google Scholar] [CrossRef] [Green Version]
- Sauter, I.P.; Rossa, G.E.; Lucas, A.M.; Cibulski, S.P.; Roehe, P.M.; da Silva, L.A.A.; Rott, M.B.; Vargas, R.M.F.; Cassel, E.; von Poser, G.L. Chemical composition and amoebicidal activity of Piper hispidinervum (Piperaceae) essential oil. Ind. Crops Prod. 2012, 40, 292–295. [Google Scholar] [CrossRef]
- Azuero, A.; Jaramillo-Jaramillo, C.; San Martinm, D.; D’Armas, H. Analysis of antimicrobial effect of twelve medicinal plants of ancient use in Ecuador. Cienc. Unemi 2016, 9, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Orjala, J.; Erdelmeier, C.A.; Wright, A.D.; Rali, T.; Sticher, O. Five new prenylated p-hydroxybenzoic acid derivatives with antimicrobial and molluscicidal activity from Piper aduncum leaves. Planta Med. 1993, 59, 546–551. [Google Scholar] [CrossRef]
- Wu, B.; Lin, W.H.; Gao, H.Y.; Zheng, L.; Wu, L.J.; Kim, C.S. Four antibacterial monoterpenoid derivatives from the herba of Senecio Cannabifolius less. Indian J. Pharm. Sci. 2006, 68, 332–336. [Google Scholar] [CrossRef] [Green Version]
- Masuoka, C.; Ono, M.; Ito, Y.; Nohara, T. Antioxidative, Antihyaluronidase and Antityrosinase Activities of Some Constituents from the Aerial Part of Piper elongatum VAHL. Food Sci. Technol. Res. 2003, 9, 197–201. [Google Scholar] [CrossRef]
- Mata, A.T.; Proença, C.; Ferreira, A.R.; Serralheiro, M.L.M.; Nogueira, J.M.F.; Araújo, M.E.M. Antioxidant and antiacetylcholinesterase activities of five plants used as Portuguese food spices. Food Chem. 2007, 103, 778–786. [Google Scholar] [CrossRef]
- Andrade, M.A.; das Graças Cardoso, M.; de Andrade, J.; Silva, L.F.; Teixeira, M.L.; Valério Resende, J.M.; da Silva Figueiredo, A.C.; Barroso, J.G. Chemical Composition and Antioxidant Activity of Essential Oils from Cinnamodendron dinisii Schwacke and Siparuna guianensis Aublet. Antioxidants 2013, 2, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, O.; Ozdemir, I.; Tanyildizi, S.; Yildiz, S.; Oguzturk, H. Antioxidative effects of curcumin, β-myrcene and 1,8-cineole against 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced oxidative stress in rats liver. Toxicol. Ind. Health 2011, 27, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Finley, J.W.; Kong, A.N.; Hintze, K.J.; Jeffery, E.H.; Ji, L.L.; Lei, X.G. Antioxidants in foods: State of the science important to the food industry. J. Agric. Food Chem. 2011, 59, 6837–6846. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Valarezo, E.; Merino, G.; Cruz-Erazo, C.; Cartuche, L. Bioactivity evaluation of the native Amazonian species of Ecuador: Piper lineatum Ruiz & Pav. essential oil. Nat. Volatiles Essent. Oils 2020, 7, 14–25. [Google Scholar] [CrossRef]
- Association Française de Normalisation (AFNOR). Huiles Essentielles. Tome 1, Échantillonnage et Méthodes D’analyse; Paris La Défense, AFNOR: Paris, France, 2000. [Google Scholar]
- Valarezo, E.; Ojeda-Riascos, S.; Cartuche, L.; Andrade-González, N.; González-Sánchez, I.; Meneses, M.A. Extraction and Study of the Essential Oil of Copal (Dacryodes peruviana), an Amazonian Fruit with the Highest Yield Worldwide. Plants 2020, 9, 1658. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas. Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- NIST 05. Mass Spectral Library (NIST/EPA/NIH); National Institute of Standards and Technology: Gaithersburg, MD, USA, 2005.
- NIST. Libro del Web de Química del NIST, SRD 69. in Base de Datos de Referencia Estándar del NIST Número 69. Available online: http://webbook.nist.gov (accessed on 19 May 2020).
- Valarezo, E.; Benítez, L.; Palacio, C.; Aguilar, S.; Armijos, C.; Calva, J.; Ramírez, J. Volatile and non-volatile metabolite study of endemic ecuadorian specie Piper lanceifolium Kunth. J. Essent. Oil Res. 2020. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Rhee, I.K.; van de Meent, M.; Ingkaninan, K.; Verpoorte, R. Screening for acetylcholinesterase inhibitors from Amaryllidaceae using silica gel thin-layer chromatography in combination with bioactivity staining. J. Chromatogr. A 2001, 915, 217–223. [Google Scholar] [CrossRef]



| Peak | Compound | RI | RIref | PCW | PCS | Beverage | Type | CF | MM | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % a | SD | % a | SD | mg/L | SD | (Da) | ||||||
| 1 | α-Thujene | 926 | 924 | - | - | 0.16 | 0.05 | 0.21 | 0.01 | ALM | C10H16 | 136.13 |
| 2 | α-Pinene | 932 | 932 | 2.65 | 0.34 | 4.71 | 0.44 | 0.79 | 0.04 | ALM | C10H16 | 136.13 |
| 3 | Sabinene | 969 | 969 | - | - | 1.55 | 0.04 | 0.61 | 0.03 | ALM | C10H16 | 136.13 |
| 4 | β-Pinene | 973 | 974 | 2.39 | 0.32 | 3.63 | 0.01 | 0.55 | 0.03 | ALM | C10H16 | 136.13 |
| 5 | Myrcene | 986 | 988 | 0.70 | 0.09 | 1.62 | 0.16 | 0.61 | 0.03 | ALM | C10H16 | 136.13 |
| 6 | δ-2-Carene | 1004 | 1001 | 0.17 | 0.04 | 0.14 | 0.01 | - | - | ALM | C10H16 | 136.13 |
| 7 | α-Terpinene | 1013 | 1014 | 5.90 | 0.44 | 8.93 | 1.29 | 1.83 | 0.09 | ALM | C10H16 | 136.13 |
| 8 | ρ-Cymene | 1021 | 1020 | - | - | 4.72 | 0.41 | 1.23 | 0.06 | ARM | C10H14 | 134.10 |
| 9 | β-Phellandrene | 1026 | 1025 | - | - | 4.63 | 1.20 | - | - | ALM | C10H16 | 136.13 |
| 10 | 1.8-Cineole | 1029 | 1026 | 25.20 | 1.31 | 17.45 | 2.33 | 14.41 | 0.72 | OXM | C10H18O | 154.14 |
| 11 | (Z)-β-Ocimene | 1034 | 1032 | 3.40 | 0.32 | 9.45 | 1.74 | 1.77 | 0.09 | ALM | C10H16 | 136.13 |
| 12 | (E)-β-Ocimene | 1045 | 1044 | 5.66 | 0.53 | 15.50 | 2.53 | 2.67 | 0.13 | ALM | C10H16 | 136.13 |
| 13 | γ-Terpinene | 1055 | 1054 | 2.43 | 0.20 | 5.18 | 1.27 | 0.65 | 0.03 | ALM | C10H16 | 136.13 |
| 14 | cis-Sabinene hydrate | 1063 | 1065 | 0.34 | 0.02 | - | - | - | - | OXM | C10H18O | 154.14 |
| 15 | Terpinolene | 1082 | 1086 | 0.14 | 0.02 | 0.09 | 0.01 | - | - | ALM | C10H16 | 136.13 |
| 16 | Linalool | 1099 | 1095 | 1.06 | 0.05 | 0.44 | 0.05 | - | - | OXM | C10H18O | 154.14 |
| 17 | Chrysanthenone | 1121 | 1124 | 1.35 | 0.05 | - | - | - | - | OXM | C10H14O | 150.10 |
| 18 | Camphor | 1140 | 1141 | 0.15 | 0.01 | - | - | - | - | OXM | C10H16O | 152.12 |
| 19 | Terpinen-4-ol | 1170 | 1174 | 1.60 | 0.22 | 0.17 | 0.01 | 0.86 | 0.04 | OXM | C10H18O | 154.14 |
| 20 | α-Terpineol | 1195 | 1186 | 3.15 | 0.16 | 1.36 | 0.41 | 0.15 | 0.01 | OXM | C10H18O | 154.13 |
| 21 | Ascaridole | 1239 | 1234 | 2.35 | 0.14 | 1.04 | 0.32 | 0.88 | 0.04 | OXM | C10H16O2 | 168.12 |
| 22 | Cumin aldehyde | 1242 | 1238 | - | - | 0.18 | 0.06 | - | - | OXM | C10H12O | 148.09 |
| 23 | Carvone | 1244 | 1239 | - | - | 0.43 | 0.07 | >0.1 | - | OXM | C10H14O | 150.10 |
| 24 | Piperitone | 1248 | 1249 | 0.26 | 0.03 | - | - | - | - | OXM | C10H16O | 152.12 |
| 25 | α-Terpinen-7-al | 1280 | 1283 | 0.09 | 0.03 | - | - | - | - | OXM | C10H14O | 150.10 |
| 26 | Safrole | 1291 | 1285 | 21.91 | 2.79 | 13.18 | 1.72 | 2.43 | 0.12 | OXM | C10H10O2 | 162.07 |
| 27 | Thymol | 1294 | 1289 | - | - | 0.47 | 0.06 | - | - | OXM | C10H14O | 150.10 |
| 28 | Carvacrol | 1301 | 1298 | - | - | 0.26 | 0.04 | 0.79 | 0.04 | OXM | C10H14O | 150.10 |
| 29 | (Z)-Methyl cinnamate | 1304 | 1299 | 2.48 | 0.02 | 0.48 | 0.34 | 0.59 | 0.03 | OXM | C10H10O2 | 162.07 |
| 30 | Thymol acetate | 1346 | 1349 | 0.28 | 0.14 | tr | - | - | - | OTC | C12H16O2 | 192.12 |
| 31 | trans-Caryophyllene | 1405 | 1417 | 0.62 | 0.27 | - | - | - | - | ALS | C15H24 | 204.19 |
| 32 | ρ-Cymen-7-ol acetate | 1421 | 1421 | 1.45 | 0.13 | 0.57 | 0.04 | 0.14 | 0.01 | OTC | C12H16O2 | 192.12 |
| 33 | α-Humulene | 1451 | 1452 | 0.25 | 0.10 | - | - | - | - | ALS | C15H24 | 204.19 |
| 34 | (E)-β-Farnesene | 1459 | 1454 | 0.22 | 0.21 | 0.16 | 0.01 | >0.1 | - | ALS | C15H24 | 204.19 |
| 35 | Germacrene D | 1476 | 1480 | 6.67 | 0.44 | 0.92 | 0.23 | 0.21 | 0.01 | ALS | C15H24 | 204.19 |
| 36 | Bicyclogermacrene | 1495 | 1500 | 3.58 | 0.23 | 0.85 | 0.41 | >0.1 | - | ALS | C15H24 | 204.19 |
| 37 | (E. E)-α-Farnesene | 1503 | 1505 | 0.38 | 0.03 | - | - | - | - | ALS | C15H24 | 204.19 |
| 38 | β-Sesquiphellandrene | 1519 | 1521 | 0.12 | 0.01 | - | - | - | - | ALS | C15H24 | 204.19 |
| 39 | δ-Cadinene | 1521 | 1522 | 0.27 | 0.02 | - | - | - | - | ALS | C15H24 | 204.19 |
| 40 | (E)-Nerolidol | 1557 | 1561 | tr | - | - | - | - | - | OXS | C15H26O | 222.20 |
| 41 | Spathulenol | 1573 | 1577 | 1.18 | 0.10 | tr | - | >0.1 | - | OXS | C15H24O | 220.18 |
| 42 | Carotol | 1590 | 1594 | 0.48 | 0.19 | - | - | - | - | OXS | C15H26O | 222.20 |
| Aliphatic monoterpene hydrocarbons (ALM) | 23.45 | 55.58 | ||||||||||
| Aromatic monoterpene hydrocarbons (ARM) | - | 4.72 | ||||||||||
| Oxygenated monoterpenes (OXM) | 59.94 | 35.45 | ||||||||||
| Aliphatic sesquiterpene hydrocarbons (ALS) | 12.09 | 1.93 | ||||||||||
| Oxygenated sesquiterpene (OXS) | 1.66 | tr | ||||||||||
| Other compounds (OTC) | 1.72 | 0.57 | ||||||||||
| Total identified | 98.86 | 98.26 | ||||||||||
| Enantiomers | RT | RI | Enantiomeric Distribution | e.e. |
|---|---|---|---|---|
| min | min | % | % | |
| (1R,5R)-(+)-Sabinene | 7.6 | 968 | 93.3 | 86.5 |
| (1S,5S)-(-)-Sabinene | 7.83 | 973 | 6.7 | |
| (R)-(−)-α-Phellandrene | 17.09 | 1019 | 78.3 | 56.6 |
| (S)-(+)-α-Phellandrene | 18.64 | 1026 | 21.7 | |
| (S)-(+)-linalool | 24.54 | 1107 | 76.6 | 53.1 |
| (R)-(-)-Linalool | 24.68 | 1109 | 23.4 | |
| (R)-(+)-α-Terpineol | 30.56 | 1187 | 70.0 | 40.0 |
| (S)-(-)-α-Terpineol | 30.74 | 1189 | 30.0 |
| Microorganism | P. carpunya | Positive Control |
|---|---|---|
| MIC (μg/mL) a | ||
| Gram-negative bacteria | ||
| Pseudomonas aeruginosa (ATCC 27853) | >1000 | 15.6 |
| Klebsiella pneumoniae (ATCC 9997) | 500 | 1.95 |
| Proteus vulgaris (ATCC 8427) | >1000 | 3.91 |
| Escherichia coli (ATCC 25922) | >1000 | 1.95 |
| Salmonella typhimurium (LT2) | >1000 | 1.95 |
| Gram-positive bacteria | ||
| Enterococcus faecalis (ATCC 29212) | >1000 | 7.81 |
| Staphylococcus aureus (ATCC 25923) | >1000 | 7.81 |
| Sample | DPPH | ABTS |
|---|---|---|
| SC50 * (μg/mL) | ||
| Essential oil | >1000 | >1000 |
| BHT | 440 ± 30 | 840 ± 30 |
| Trolox | 450 ± 50 | 650 ± 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valarezo, E.; Rivera, J.X.; Coronel, E.; Barzallo, M.A.; Calva, J.; Cartuche, L.; Meneses, M.A. Study of Volatile Secondary Metabolites Present in Piper carpunya Leaves and in the Traditional Ecuadorian Beverage Guaviduca. Plants 2021, 10, 338. https://doi.org/10.3390/plants10020338
Valarezo E, Rivera JX, Coronel E, Barzallo MA, Calva J, Cartuche L, Meneses MA. Study of Volatile Secondary Metabolites Present in Piper carpunya Leaves and in the Traditional Ecuadorian Beverage Guaviduca. Plants. 2021; 10(2):338. https://doi.org/10.3390/plants10020338
Chicago/Turabian StyleValarezo, Eduardo, Jonathan Xavier Rivera, Edgar Coronel, Miguel Andrés Barzallo, James Calva, Luis Cartuche, and Miguel Angel Meneses. 2021. "Study of Volatile Secondary Metabolites Present in Piper carpunya Leaves and in the Traditional Ecuadorian Beverage Guaviduca" Plants 10, no. 2: 338. https://doi.org/10.3390/plants10020338
APA StyleValarezo, E., Rivera, J. X., Coronel, E., Barzallo, M. A., Calva, J., Cartuche, L., & Meneses, M. A. (2021). Study of Volatile Secondary Metabolites Present in Piper carpunya Leaves and in the Traditional Ecuadorian Beverage Guaviduca. Plants, 10(2), 338. https://doi.org/10.3390/plants10020338