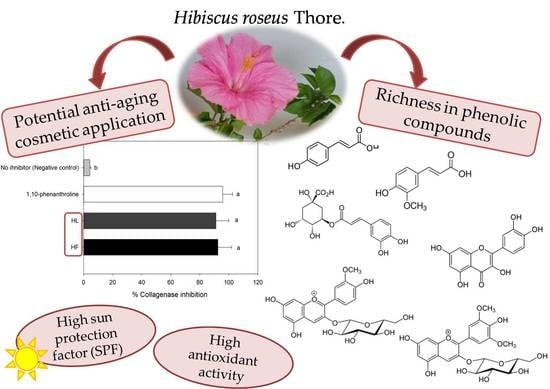
Phenolic Compounds from Leaves and Flowers of Hibiscus roseus: Potential Skin Cosmetic Applications of an Under-Investigated Species
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phenolic Characterization and Quantification
2.2. Antioxidant Activity Assays
2.3. In Vitro Sun Protection Factor (SPF)
2.4. Collagenase Inhibition Activity
3. Materials and Methods
3.1. Plant Material
3.2. Ultrasound-Assisted Extraction
3.3. LC-MS Analysis: Phenolic Profile of the Extracts
3.4. HPLC-DAD Analysis: Quantification of Phenolics
3.5. Antioxidant Activity Assays
3.6. In Vitro Sun Protection Factor (SPF) Assay
3.7. Collagenase Activity Inhibitory Assay
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dorni, A.C.; Amalraj, A.; Gopi, S.; Varma, K.; Anjana, S.N. Novel cosmeceuticals from plants—An industry guided review. J. Appl. Res. Med. Aromat. Plants 2017, 7, 1–26. [Google Scholar] [CrossRef]
- González-Minero, F.J.; Bravo-Díaz, L. The use of plants in skin-care products, cosmetics and fragrances: Past and present. Cosmetics 2018, 5, 50. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S. Exploratory analysis of global cosmetic industry: Major players, technology and market trends. Technovation 2005, 25, 1263–1272. [Google Scholar] [CrossRef]
- Schmidt, B.M. Responsible use of medicinal plants for cosmetics. Hort. Sci. 2012, 47, 985–991. [Google Scholar] [CrossRef]
- Aziz, A.A.; Taher, Z.M.; Muda, R.; Aziz, R. Cosmeceuticals and Natural Cosmetics. In Recent Trends in Research into Malaysian Medicinal Plants; Penerbit UTM Press: Johor, Malaysia, 2017; pp. 126–175. [Google Scholar]
- Przybylska-Balcerek, A.; Stuper-Szablewska, K. Phenolic acids used in the cosmetics industry as natural antioxidants. Eur. J. Med. Technol. 2019, 4, 24–32. [Google Scholar]
- Bijauliya, R.K.; Alok, S.; Kumar, M.; Chanchal, D.K.; Yadav, S. A comprehensive review on herbal cosmetics. Int. J. Pharm. Sci. Res. 2017, 8, 4930–4949. [Google Scholar] [CrossRef]
- Mahesh, S.K.; Fathima, J.; Veena, V.G. Cosmetic potential of natural products: Industrial applications. In Natural Bio-Active Compounds; Swamy, M., Akhtar, M., Eds.; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [Green Version]
- Espinosa-Leal, C.A.; Garcia-Lara, S. Current methods for the discovery of new active ingredients from natural products for cosmeceutical applications. Planta Med. 2019, 85, 535–551. [Google Scholar] [CrossRef] [Green Version]
- Draelos, Z.D. Cosmeceuticals: Undefined, unclassified, and unregulated. Clin. Dermatol. 2009, 27, 431–434. [Google Scholar] [CrossRef]
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as active ingredients for cosmetic products. Int. J. Cosmet Sci. 2015, 37, 455–464. [Google Scholar] [CrossRef]
- de Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Fariña, L.; da Silva de Lucca, R.A. Polyphenols as natural antioxidants in cosmetics applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, A.; Ghasemzadeh, N. Flavonoids and phenolic acids: Role and biochemical activity in plants and human. J. Med. Plants Res. 2011, 5, 6697–6703. [Google Scholar] [CrossRef]
- Brunetti, C.; Di Ferdinando, M.; Fini, A.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants and developmental regulators: Relative significance in plants and humans. Int. J. Mol. Sci. 2013, 14, 3540–3555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faria-Silva, C.; Ascenso, A.; Costa, A.M.; Marto, J.; Carvalheiro, M.; Ribeiro, H.M.; Simões, S. Feeding the skin: A new trend in food and cosmetics convergence. Trends Food Sci. Technol. 2020, 95, 21–32. [Google Scholar] [CrossRef]
- Maganha, E.G.; da Costa Halmenschlager, R.; Rosa, R.M.; Henriques, J.A.P.; de Paula Ramos, A.L.L.; Saffi, J. Pharmacological evidences for the extracts and secondary metabolites from plants of the genus Hibiscus. Food Chem. 2010, 118, 1–10. [Google Scholar] [CrossRef]
- Khristi, V.; Patel, V.H. Therapeutic potential of Hibiscus rosa-sinensis: A review. Int. J. Nut. Diet. 2016, 4, 105–123. [Google Scholar] [CrossRef]
- Vadivel, V. Distribution of flavonoids among malvaceae family members—A review. Int. J. Green Pharm. 2016, 10. [Google Scholar] [CrossRef]
- ThePlant List. Available online: http://www.theplantlist.org/ (accessed on 15 December 2020).
- Vasudeva, N.; Sharma, S.K. Biologically active compounds from the genus Hibiscus. Pharm. Biol. 2008, 46, 145–153. [Google Scholar] [CrossRef] [Green Version]
- Fiori, A. Nuova Flora Analitica d’Italia; New York Botanical Garden: New York, NY, USA, 1923; Volume 2, p. 164. [Google Scholar] [CrossRef]
- Winters, H.F. Our hardy Hibiscus species as ornamentals. Econ. Bot. 1970, 1, 155–164. [Google Scholar] [CrossRef]
- Pinto, G.; Aurilia, M.; Illiano, A.; Fontanarosa, C.; Sannia, G.; Trifuoggi, M.; Amoresano, A. From untargeted metabolomics to the multiple reaction monitoring-based quantification of polyphenols in chocolates from different geographical areas. J. Mass Spectrom. 2021, 56, e4651. [Google Scholar] [CrossRef]
- Wang, J.; Cao, X.; Jiang, H.; Qi, Y.; Chin, K.L.; Yue, Y. Antioxidant activity of leaf extracts from different Hibiscus sabdariffa accessions and simultaneous determination five major antioxidant compounds by LC-Q-TOF-MS. Molecules 2014, 19, 21226–21238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhen, J.; Villani, T.S.; Guo, Y.; Qi, Y.; Chin, K.; Pan, M.H.; Wu, Q. Phytochemistry, antioxidant capacity, total phenolic content and anti-inflammatory activity of Hibiscus sabdariffa leaves. Food Chem. 2016, 190, 673–680. [Google Scholar] [CrossRef]
- Jabeur, I.; Pereira, E.; Barros, L.; Calhelha, R.C.; Soković, M.; Oliveira, M.B.P.; Ferreira, I.C. Hibiscus sabdariffa L. as a source of nutrients, bioactive compounds and colouring agents. Food Res. Int. 2017, 100, 717–723. [Google Scholar] [CrossRef] [Green Version]
- Pimentel-Moral, S.; Borrás-Linares, I.; Lozano-Sánchez, J.; Arráez-Román, D.; Martínez-Férez, A.; Segura-Carretero, A. Microwave-assisted extraction for Hibiscussabdariffa bioactive compounds. J. Pharmaceut Biomed. 2018, 156, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Piovesana, A.; Rodrigues, E.; Noreña, C.P.Z. Composition analysis of carotenoids and phenolic compounds and antioxidant activity from hibiscus calyces (Hibiscussabdariffa L.) by HPLC-DAD-MS/MS. Phytochem. Anal. 2019, 30, 208–217. [Google Scholar] [CrossRef]
- López-Fernández, O.; Domínguez, R.; Pateiro, M.; Munekata, P.E.; Rocchetti, G.; Lorenzo, J.M. Determination of polyphenols using liquid chromatography–tandem mass spectrometry technique (LC–MS/MS): A review. Antioxidants 2020, 9, 479. [Google Scholar] [CrossRef] [PubMed]
- Olech, M.; Pietrzak, W.; Nowak, R. Characterization of free and bound phenolic acids and flavonoid aglycones in Rosa rugosa Thunb. leaves and achenes using LC–ESI–MS/MS–MRM methods. Molecules 2020, 25, 1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurtado-Gaitán, E.; Sellés-Marchart, S.; Martínez-Márquez, A.; Samper-Herrero, A.; Bru-Martínez, R. A focused multiple reaction monitoring (MRM) quantitative method for bioactive grapevine stilbenes by ultra-high-performance liquid chromatography coupled to triple-quadrupole mass spectrometry (UHPLC-QqQ). Molecules 2017, 22, 418. [Google Scholar] [CrossRef] [Green Version]
- Lambert, M.; Meudec, E.; Verbaere, A.; Mazerolles, G.; Wirth, J.; Masson, G.; Cheynier, V.; Sommerer, N. A high-throughput UHPLC-QqQ-MS method for polyphenol profiling in rosé wines. Molecules 2015, 20, 7890–7914. [Google Scholar] [CrossRef]
- Olech, M.; Nowak, R.; Ivanova, D.; Tashev, A.; Boyadzhieva, S.; Kalotova, G.; Gawlik-Dziki, U. LC-ESI-MS/MS-MRM profiling of polyphenols and antioxidant activity evaluation of junipers of different origin. Appl. Sci. 2020, 10, 8921. [Google Scholar] [CrossRef]
- Da-Costa-Rocha, I.; Bonnlaender, B.; Sievers, H.; Pischel, I.; Heinrich, M. Hibiscus sabdariffa L.—A phytochemical and pharmacological review. Food Chem. 2014, 165, 424–443. [Google Scholar] [CrossRef] [Green Version]
- Lyu, J.I.; Kim, J.M.; Kim, D.G.; Kim, J.B.; Kim, S.H.; Ahn, J.W.; Kwon, S.J. Phenolic compound content of leaf extracts from different Roselle (Hibiscus sabdariffa) accessions. Plant. Breed. Biotech. 2020, 8, 1–10. [Google Scholar] [CrossRef]
- Peng, C.H.; Chyau, C.C.; Chan, K.C.; Chan, T.H.; Wang, C.J.; Huang, C.N. Hibiscus sabdariffa polyphenolic extract inhibits hyperglycemia, hyperlipidemia, and glycation-oxidative stress while improving insulin resistance. J. Agric. Food Chem. 2011, 59, 9901–9909. [Google Scholar] [CrossRef]
- Yang, Y.S.; Huang, C.N.; Wang, C.J.; Lee, Y.J.; Chen, M.L.; Peng, C.H. Polyphenols of Hibiscus sabdariffa improved diabetic nephropathy via regulating the pathogenic markers and kidney functions of type 2 diabetic rats. J. Func. Foods 2013, 5, 810–819. [Google Scholar] [CrossRef]
- Escobar-Ortiz, A.; Castaño-Tostado, E.; Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; Reynoso-Camacho, R. Anthocyanins extraction from Hibiscus sabdariffa and identification of phenolic compounds associated with their stability. J. Sci. Food Agric. 2021, 101, 110–119. [Google Scholar] [CrossRef]
- Salem, M.A.; Zayed, A.; Beshay, M.E.; Mesih, M.M.A.; Khayal, R.F.B.; George, F.A.; Ezzat, S.M. Hibiscus sabdariffa L.: Phyto constituents, nutritive, and pharmacological applications. Adv. Trad. Med. 2021, 1–11. [Google Scholar] [CrossRef]
- Formagio, A.S.; Ramos, D.D.; Vieira, M.C.; Ramalho, S.R.; Silva, M.M.; Zárate, N.A.; Carvalho, J.E. Phenolic compounds of Hibiscus sabdariffa and influence of organic residues on its antioxidant and antitumoral properties. Braz. J. Biol. 2015, 75, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Pruthi, V. Potential applications of ferulic acid from natural sources. Biotech. Rep. 2014, 4, 86–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salem, M.A.; Michel, H.E.; Ezzat, M.I.; Okba, M.M.; El-Desoky, A.M.; Mohamed, S.O.; Ezzat, S.M. Optimization of an extraction solvent for angiotensin-converting enzyme inhibitors from Hibiscus sabdariffa L. based on its UPLC-MS/MS metabolic profiling. Molecules 2020, 25, 2307. [Google Scholar] [CrossRef]
- Takeda, Y.; Okuyama, Y.; Nakano, H.; Yaoita, Y.; Machida, K.; Ogawa, H.; Imai, K. Antiviral activities of Hibiscus sabdariffa L. tea extract against human influenza A virus rely largely on acidic pH but partially on a low-pH-independent mechanism. Food Environ. Virol. 2020, 12, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Saini, P.; Gayen, P.; Nayak, A.; Kumar, D.; Mukherjee, N.; Pal, B.C.; Babu, S.P.S. Effect of ferulic acid from Hibiscus mutabilis on filarial parasite Setaria cervi: Molecular and biochemical approaches. Parasitol. Int. 2012, 61, 520–531. [Google Scholar] [CrossRef]
- Wong, S.; Chan, E.W.; Chan, H. A review on the phytochemistry and pharmacology of two lesser-known Hibiscus species: H. taiwanensis and H. schizopetalus. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 1341–1346. [Google Scholar]
- Song, K.; An, S.M.; Kim, M.; Koh, J.S.; Boo, Y.C. Comparison of the antimelanogenic effects of p-coumaric acid and its methyl ester and their skin permeabilities. J. Dermatol. Sci. 2011, 63, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. p-Coumaric acid as an active ingredient in cosmetics: A review focusing on its antimelanogenic effects. Antioxidants 2019, 8, 275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varela, M.T.; Ferrarini, M.; Mercaldi, V.G.; da Silva Sufi, B.; Padovani, G.; Nazato, L.I.S.; Fernandes, J.P.S. Coumaric acid derivatives as tyrosinase inhibitors: Efficacy studies through in silico, in vitro and ex vivo approaches. Bioorg. Chem. 2020, 103, 104108. [Google Scholar] [CrossRef]
- Jung, S.Y.; Jung, W.S.; Jung, H.K.; Lee, G.H.; Cho, J.H.; Cho, H.W.; Choi, I.Y. The mixture of different parts of Nelumbo nucifera and two bioactive components inhibited tyrosinase activity and melanogenesis. J. Cosm. Sci. 2014, 65, 377–388. [Google Scholar]
- Riaz, A.; Rasul, A.; Hussain, G.; Zahoor, M.K.; Jabeen, F.; Subhani, Z.; Selamoglu, Z. Astragalin: A bioactive phytochemical with potential therapeutic activities. Adv. Pharmacol. Sci. 2018. [Google Scholar] [CrossRef]
- Imran, M.; Rauf, A.; Shah, Z.A.; Saeed, F.; Imran, A.; Arshad, M.U.; Mubarak, M.S. Chemo-preventive and therapeutic effect of the dietary flavonoid kaempferol: A comprehensive review. Phytother. Res. 2019, 33, 263–275. [Google Scholar] [CrossRef]
- Diaconeasa, Z.; Știrbu, I.; Xiao, J.; Leopold, N.; Ayvaz, Z.; Danciu, C.; Socaciu, C. Anthocyanins, vibrant color pigments, and their role in skin cancer prevention. Biomedicines 2020, 8, 336. [Google Scholar] [CrossRef]
- Zhai, Y.; Dang, Y.; Gao, W.; Zhang, Y.; Xu, P.; Gu, J.; Ye, X. P38 and JNK signal pathways are involved in the regulation of phlorizin against UVB-induced skin damage. Exp. Dermatol. 2015, 24, 275–279. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Lu, Y.R.; Lin, I.F.; Kang, W.; Chen, H.B.; Lu, H.F.; Wang, H.M.D. Reversing UVB-induced photoaging with Hibiscus sabdariffa calyx aqueous extract. J. Sci. Food Agric. 2020, 100, 672–681. [Google Scholar] [CrossRef]
- Dueñas, M.; Surco-Laos, F.; González-Manzano, S.; González-Paramás, A.M.; Santos-Buelga, C. Antioxidant properties of major metabolites of quercetin. Eur. Food Res. Technol. 2011, 232, 103–111. [Google Scholar] [CrossRef]
- Li, X.; Chen, B.; Xie, H.; He, Y.; Zhong, D.; Chen, D. Antioxidant structure–activity relationship analysis of five dihydrochalcones. Molecules 2018, 23, 1162. [Google Scholar] [CrossRef] [Green Version]
- Tseng, T.H.; Wang, C.J.; Kao, E.S.; Chu, H.Y. Hibiscus anthocyanins are potent antioxidants in human cells. Chem. Biol. Interact. 1996, 101, 137–145. [Google Scholar] [CrossRef]
- Wang, C.J.; Wang, J.M.; Lin, W.L.; Chu, C.Y.; Chou, F.P.; Tseng, T.H. Protective effect of Hibiscus anthocyanins against tert-butyl hydroperoxide-induced hepatic toxicity in rats. Food Chem. Toxicol. 2000, 38, 411–416. [Google Scholar] [CrossRef]
- Shui, G.; Peng, L.L. An improved method for the analysis of major antioxidants of Hibiscus esculentus Linn. J. Chromatogr. 2004, 1048, 17–24. [Google Scholar] [CrossRef]
- Lyu, J.I.; Ryu, J.; Jin, C.H.; Kim, D.G.; Kim, J.M.; Seo, K.S.; Kwon, S.J. Phenolic compounds in extracts of Hibiscus acetosella (Cranberry Hibiscus) and their antioxidant and antibacterial properties. Molecules 2020, 25, 4190. [Google Scholar] [CrossRef] [PubMed]
- Mohd-Esa, N.; Hern, F.S.; Ismail, A.; Yee, C.L. Antioxidant activity in different parts of roselle (Hibiscus sabdariffa L.) extracts and potential exploitation of the seeds. Food Chem. 2010, 122, 1055–1060. [Google Scholar] [CrossRef]
- Chen, J.H.; Wang, C.J.; Wang, C.P.; Sheu, J.Y.; Lin, C.L.; Lin, H.H. Hibiscus sabdariffa leaf polyphenolic extract inhibits LDL oxidation and foam cell formation involving up-regulation of LXRα/ABCA1 pathway. Food Chem. 2013, 141, 397–406. [Google Scholar] [CrossRef]
- Widowati, W.; Rani, A.P.; Hamzah, R.A.; Arumwardana, S.; Afifah, E.; Kusuma, H.S.W.; Amalia, A. Antioxidant and antiaging assays of Hibiscussabdariffa extract and its compounds. Nat. Prod. Sci. 2017, 23, 192–200. [Google Scholar] [CrossRef] [Green Version]
- McCullough, J.L.; Kelly, K.M. Prevention and treatment of skin aging. Aging Interv Ther. 2006, 1067, 323–331. [Google Scholar] [CrossRef]
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. Ultraviolet radiation and skin cancer. Int. J. Dermatol. 2010, 49, 978–986. [Google Scholar] [CrossRef]
- Gaweł-Bęben, K.; Kukula-Koch, W.; Hoian, U.; Czop, M. Characterization of Cistus × incanus L. and Cistus ladanifer L. extracts as potential multifunctional antioxidant ingredients for skin protecting cosmetics. Antioxidants 2020, 9, 202. [Google Scholar] [CrossRef] [Green Version]
- Radice, M.; Manfredini, S.; Ziosi, P.; Dissette, V.; Buso, P.; Fallacara, A.; Vertuani, S. Herbal extracts, lichens and biomolecules as natural photo-protection alternatives to synthetic UV filters. A systematic review. Fitoterapia 2016, 114, 144–162. [Google Scholar] [CrossRef] [PubMed]
- Mansur, J.D.S.; Breder, M.N.R.; Mansur, M.C.D.A.; Azulay, R.D. Determinação do fator de radiação solar por espectrofotometria. An. Bras Dermatol. 1986, 61, 121–124. [Google Scholar]
- Madan, K.; Nanda, S. In-vitro evaluation of antioxidant, anti-elastase, anti-collagenase, anti-hyaluronidase activities of safranal and determination of its sun protection factor in skin photoaging. Bioorg. Chem. 2018, 77, 159–167. [Google Scholar] [CrossRef]
- Baldisserotto, A.; Buso, P.; Radice, M.; Dissette, V.; Lampronti, I.; Gambari, R.; Vertuani, S. Moringa oleifera leaf extracts as multifunctional ingredients for “natural and organic” sunscreens and photoprotective preparations. Molecules 2018, 23, 664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Sultana, S. Effect of Hibiscus rosa-sinensis extract on hyperproliferation and oxidative damage caused by benzoyl peroxide and ultraviolet radiations in mouse skin. Basic Clin. Pharmacol. 2004, 95, 115–220. [Google Scholar] [CrossRef] [PubMed]
- Robbins, R.J. Phenolic acids in foods: An overview of analytical methodology. J. Agric. Food Chem. 2003, 51, 2866–2887. [Google Scholar] [CrossRef] [PubMed]
- Taofiq, O.; González-Paramás, A.M.; Barreiro, M.F.; Ferreira, I.C. Hydroxycinnamic acids and their derivatives: Cosmeceutical significance, challenges and future perspectives, a review. Molecules 2017, 22, 281. [Google Scholar] [CrossRef] [PubMed]
- Bodiga, V.L.; Bodiga, S. Ascorbic acid is a potential inhibitor of collagenases—In Silico and In Vitro biological studies. In Silico Drug Design: Repurposing Techniques and Methodologies, 1st ed.; Roy, K., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 649–677. [Google Scholar]
- Azzi, A.; Ricciarelli, R.; Zingg, J.M. Non-antioxidant molecular functions of α-tocopherol (vitamin E). FEBS Lett. 2002, 519, 8–10. [Google Scholar] [CrossRef] [Green Version]
- Geeta, G.; Widodo, W.S.; Widowati, W.; Ginting, C.N.; Lister, I.N.E.; Armansyah, A.; Girsang, E. Comparison of antioxidant and anti-collagenase activity of genistein and epicatechin. Pharm. Sci. Res. 2019, 6, 111–117. [Google Scholar] [CrossRef]
- Sin, B.Y.; Kim, H.P. Inhibition of collagenase by naturally-occurring flavonoids. Arch. Pharml. Res. 2005, 28, 1152–1155. [Google Scholar] [CrossRef]
- Boran, R. Investigations of anti-aging potential of Hypericum origanifolium Willd for skincare formulations. Ind. Crops. Prod. 2018, 118, 290–295. [Google Scholar] [CrossRef]
- Mohsen, E.; Younis, I.Y.; Farag, M.A. Metabolites profiling of Egyptian Rosa damascena Mill flowers as analyzed via ultra-high-performance liquid chromatography-mass spectrometry and solid-phase microextraction gas chromatography-mass spectrometry in relation to its anti-collagenase skin effect. Ind. Crops. Prod. 2020, 155, 112818. [Google Scholar] [CrossRef]
- Roy, A.; Sahu, R.K.; Matlam, M.; Deshmukh, V.K.; Dwivedi, J.; Jha, A.K. In vitro techniques to assess the proficiency of skin care cosmetic formulations. Pharmacogn. Rev. 2013, 7, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roda, G.; Marinello, C.; Grassi, A.; Picozzi, C.; Aldini, G.; Carini, M.; Regazzoni, L. Ripe and raw pu-erh tea: LC-MS profiling, antioxidant capacity and enzyme inhibition activities of aqueous and hydro-alcoholic extracts. Molecules 2019, 24, 473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Martino, O.; Tito, A.; De Lucia, A.; Cimmino, A.; Cicotti, F.; Apone, F.; Calabrò, V. Hibiscus syriacus extract from an established cell culture stimulates skin wound healing. BioMed. Res. Int. 2017. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.E.; Ngo, H.T.; Hwang, E.; Seo, S.A.; Park, S.W.; Yi, T.H. Dietary enzyme-treated Hibiscussyriacus L. protects skin against chronic UVB-induced photoaging via enhancement of skin hydration and collagen synthesis. Arch. Biochem. Biophys. 2019, 662, 190–200. [Google Scholar] [CrossRef]
- Kandi, S.; Charles, A.L. Statistical comparative study between the conventional DPPH spectrophotometric and dropping DPPH analytical method without spectrophotometer: Evaluation for the advancement of antioxidant activity analysis. Food Chem. 2019, 287, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N.; Cumbes, Q.J. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 1989, 28, 1057–1060. [Google Scholar] [CrossRef]
- Gori, A.; Tattini, M.; Centritto, M.; Ferrini, F.; Marino, G.; Mori, J.; Guidi, L.; Brunetti, C. Seasonal and daily variations in primary and secondary metabolism of three maquis shrubs unveil different adaptive responses to Mediterranean climate. Conserv. Physiol. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Sayre, R.M.; Agin, P.P.; Levee, G.J.; Marlowe, E. Comparison of in vivo and in vitro testing of sunscreening formulas. Photochem. Photobiol. 1979, 29, 559–566. [Google Scholar] [CrossRef] [PubMed]


| Peak | Retention Time (min) | Extract | Putative Identification | Authentic Standard Identification |
|---|---|---|---|---|
| 1 | 2.9 | HL/HF | chlorogenic acid derivative | No |
| 2 | 4.4 | HL | p-coumaric acid derivative | No |
| 3 | 4.7 | HF | p-coumaric acid derivative | No |
| 4 | 5.7 | HF | p-coumaric acid derivative | No |
| 5 | 6.3 | HL | p-coumaric acid derivative | No |
| 6 | 6.4 | HL/HF | trans-ferulic acid derivative | No |
| 7 | 6.5 | HF | chlorogenic acid derivative | No |
| 8 | 6.7 | HF | p-coumaric acid derivative | No |
| 9 | 7.5 | HL | p-coumaric acid derivative | No |
| 10 | 7.8 | HF | p-coumaric acid derivative | No |
| 11 | 8.0 | HL | trans-ferulic acid derivative | No |
| 12 | 8.4 | HL | p-coumaric acid derivative | No |
| 13a | 8.7 | HF/HL | p-coumaric acid derivative | No |
| 13b | 8.7 | HF/HL | chlorogenic acid derivative | No |
| 14 | 9.0 | HF | trans-ferulic acid derivative | No |
| 15 | 9.2 | HL | chlorogenic acid derivative | No |
| 16a | 9.3 | HF | catechin | Yes |
| 16b | 9.3 | HF | quercetin derivative | No |
| 17 | 9.6 | HF/HL | trans-ferulic acid derivative | No |
| 18 | 9.7 | HF | chlorogenic acid | Yes |
| 19 | 10.3 | HF | trans-ferulic acid derivative | No |
| 20 | 10.7 | HF/HL | chlorogenic acid derivative | No |
| 21 | 10.8 | HF | peonidin-3-O-glucoside | Yes |
| 22 | 10.9 | HF | oenin | Yes |
| 23 | 11.0 | HF | epicatechin | Yes |
| 24 | 11.7 | HF/HL | quercetin derivative | No |
| 25 | 12.2 | HF/HL | kaempferol derivative | No |
| 26a | 12.7 | HF/HL | quercetin derivative | No |
| 26b | 12.7 | HF/HL | rutin | Yes |
| 27 | 12.8 | HF | trans-ferulic acid | Yes |
| 28a | 13.2 | HF/HL | quercetin3-O-glucoside | Yes |
| 28b | 13.2 | HL | phloridzin derivative | No |
| 29 | 13.4 | HF | quercetin derivative | No |
| 30 | 13.6 | HF | kaempferol-3-O-rutinoside | Yes |
| 31a | 14.1 | HF | phloretin derivative | No |
| 31b | 14.1 | HF | kaempferol-7-O-glucoside | Yes |
| 32 | 14.7 | HF | kaempferol-3-O-glucoside | Yes |
| 33a | 15.6 | HF | phloretin derivative | No |
| 33b | 15.6 | HL | phloridzin | Yes |
| 34 | 17.4 | HF | tiliroside | Yes |
| H. roseus Extracts | THC | TFC | TCD | TDC | TAC | TPC |
|---|---|---|---|---|---|---|
| Leaves (HL) | 5.08 ± 0.48 *** | 3.78 ± 0.22 | nd | nd | nd | 8.86 ± 0.70 |
| Flowers (HF) | 1.31 ± 0.13 | 6.26 ± 0.28 *** | 1.86 ± 0.04 *** | 2.18 ± 0.06 *** | 0.35 ± 0.03 *** | 11.96 ± 0.48 ** |
| EC50 Values (mg mL−1) | ||
|---|---|---|
| H. roseus Extracts | DPPH Assay | HRS Assay |
| Leaves (HL) | 0.38 ± 0.05 | 2.44 ± 0.23 |
| Flowers (HF) | 0.24 ± 0.009 ** | 0.88 ± 0.06 *** |
| Phenolic Content | Pearson Coefficient—r (EC50 Values) | p-Value |
|---|---|---|
| THC | 0.92 | 0.009 ** |
| TFC | −0.87 | 0.02 * |
| TCD | −0.92 | 0.01 ** |
| TDC | −0.91 | 0.01 ** |
| TAC | −0.92 | 0.01 ** |
| TPC | −0.94 | 0.004 ** |
| Wavelenght (λ, nm) | EE × I (Normalized) |
|---|---|
| 290 | 0.0150 |
| 295 | 0.0817 |
| 300 | 0.2874 |
| 305 | 0.3278 |
| 310 | 0.1864 |
| 315 | 0.0839 |
| 320 | 0.0180 |
| Total | 1.0002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos Nascimento, L.B.; Gori, A.; Raffaelli, A.; Ferrini, F.; Brunetti, C. Phenolic Compounds from Leaves and Flowers of Hibiscus roseus: Potential Skin Cosmetic Applications of an Under-Investigated Species. Plants 2021, 10, 522. https://doi.org/10.3390/plants10030522
dos Santos Nascimento LB, Gori A, Raffaelli A, Ferrini F, Brunetti C. Phenolic Compounds from Leaves and Flowers of Hibiscus roseus: Potential Skin Cosmetic Applications of an Under-Investigated Species. Plants. 2021; 10(3):522. https://doi.org/10.3390/plants10030522
Chicago/Turabian Styledos Santos Nascimento, Luana Beatriz, Antonella Gori, Andrea Raffaelli, Francesco Ferrini, and Cecilia Brunetti. 2021. "Phenolic Compounds from Leaves and Flowers of Hibiscus roseus: Potential Skin Cosmetic Applications of an Under-Investigated Species" Plants 10, no. 3: 522. https://doi.org/10.3390/plants10030522
APA Styledos Santos Nascimento, L. B., Gori, A., Raffaelli, A., Ferrini, F., & Brunetti, C. (2021). Phenolic Compounds from Leaves and Flowers of Hibiscus roseus: Potential Skin Cosmetic Applications of an Under-Investigated Species. Plants, 10(3), 522. https://doi.org/10.3390/plants10030522