Invasive Plant Species Distribution Is Structured by Soil and Habitat Type in the City Landscape
Abstract
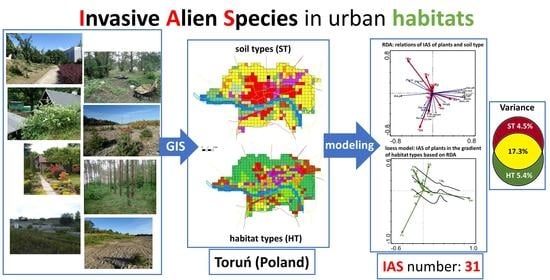
:1. Introduction
2. Results
2.1. Invasive Species Flora
2.2. Invasive Species Distribution Patterns
2.2.1. Species—Soil-Type Relations
2.2.2. Species—Habitat-Type Relations
2.2.3. Invasive Species Richness
3. Discussion
4. Materials and Methods
4.1. The Research Area
4.2. Data Collection
4.3. Data Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tokarska-Guzik, B.; Dajdok, Z.; Zając, M.; Zając, A.; Urbisz, A.; Danielewicz, W.; Hołdyński, C. Rośliny Obcego Pochodzenia w Polsce ze Szczególnym Uwzględnieniem Gatunków Inwazyjnych; GDOŚ: Krzywe, Poland, 2014. [Google Scholar]
- Ramírez-Albores, J.E.; Badano, E.I.; Flores, J.; Flores-Flores, J.L.; Yáñez-Espinosa, L. Scientific literature on invasive alien species in a megadiverse country: Advances and challenges in Mexico. NeoBiota 2019, 48, 113–127. [Google Scholar] [CrossRef]
- Colautti, R.I.; MacIsaac, H.J. A neutral terminology to define ‘invasive’ species. Divers. Distrib. 2004, 10, 135–141. [Google Scholar] [CrossRef]
- IUCN (World Conservation Union). IUCN Guidelines for the Prevention of Biodiversity Loss Caused by Alien Invasive Species. In Proceedings of the SSC Invasive Species Specialist Group, Approved by the 51st Meeting of the IUCN Council, Gland, Switzerland, February 2000. [Google Scholar]
- Independent Evaluation Group. The Global Invasive Species Program. Global Program Review; World Bank: Washington, DC, USA, 2009. [Google Scholar]
- Perrino, E.V.; Calabrese, G. Endangered segetal species in southern Italy: Distribution, conservation status, trends, actions and ethnobotanical notes. Genet. Resour. Crop Evol. 2018, 65, 2107–2134. [Google Scholar] [CrossRef]
- Lazzaro, L.; Bolpagni, R.; Buffa, G.; Gentili, R.; Lonati, M.; Stinca, A.; Acosta, A.T.R.; Adorni, M.; Aleffi, M.; Allegrezza, M.; et al. Impact of invasive alien plants on native plant communities and Natura 2000 Habitats: State of the art, gap analysis and perspectives in Italy. J. Environ. Manag. 2020, 274, 111140. [Google Scholar] [CrossRef]
- Jordan, N.R.; Larson, D.L.; Huerd, S.C. Soil modification by invasive plants: Effects on native and invasive species of mixed-grass prairies. Biol. Invasions 2007, 10, 177–190. [Google Scholar] [CrossRef]
- Litt, A.R.; Cord, E.E.; Fulbright, T.E.; Schuster, G.L. Effects of Invasive Plants on Arthropods. Conserv. Biol. 2014, 28, 1532–1549. [Google Scholar] [CrossRef] [Green Version]
- EPPO (European and Mediterranean Plant Protection Organization). Data sheet on Invasive Alien Plants: Heracleum mantegazzianum, Heracleum sosnowskyi and Heracleum persicum. Bull. OEPP 2009, 39, 489–499. [Google Scholar] [CrossRef]
- Hoffmann, B.D.; Broadhurst, L.M. The economic cost of managing invasive species in Australia. NeoBiota 2016, 31, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Simberloff, D. Invasive Species: What Everyone Needs to Know; Oxford University Press: New York, NY, USA, 2013. [Google Scholar]
- Kowarik, I.; von der Lippe, M. Pathways in Plant Invasions. In Biological Invasions; Nentwig, W., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 193. [Google Scholar]
- Bhowmik, P.C. Characteristics, significance, and human dimension of global invasive weeds. In Invasive Plants: Ecological and Agricultural Aspects; Inderjit, Ed.; Birkhäuser: Basel, Switzerland, 2005. [Google Scholar] [CrossRef]
- Auffret, A.G.; Cousins, S.A.O. Humans as Long-Distance Dispersers of Rural Plant Communities. PLoS ONE 2013, 8, e62763. [Google Scholar] [CrossRef] [Green Version]
- Coughlan, N.E.; Kelly, T.C.; Jansen, M.A.K. Mallard duck (Anas platyrhynchos)—Mediated dispersal of Lemnaceae: A contributing factor in the spread of invasive Lemna minuta? Plant Biol. 2014, 17, 108–114. [Google Scholar] [CrossRef]
- Kowarik, I.; von der Lippe, M. Secondary wind dispersal enhances long-distance dispersal of an invasive species in urban road corridors. NeoBiota 2011, 9, 49–70. [Google Scholar] [CrossRef] [Green Version]
- Štajerová, K.; Šmilauer, P.; Brůna, J.; Pyšek, P. Distribution of invasive plants in urban environment is strongly spatially structured. Landsc. Ecol. 2017, 32, 681–692. [Google Scholar] [CrossRef]
- Shackleton, C.M.; Shackleton, R.T. Knowledge, perceptions and willingness to control designated invasive tree species in urban household gardens in South Africa. Biol. Invasions 2016, 18, 1599–1609. [Google Scholar] [CrossRef]
- Aronson, M.F.J.; Patel, M.V.; O’Neill, K.M.; Ehrenfeld, J.G. Urban riparian systems function as corridors for both native and invasive plant species. Biol. Invasions 2017, 19, 3645–3657. [Google Scholar] [CrossRef]
- Pejchar, L.; Mooney, H.A. Invasive species, ecosystem services and human well-being. Trends Ecol. Evol. 2009, 24, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Gulezian, P.Z.; Nyberg, D.W. Distribution of invasive plants in a spatially structured urban landscape. Landsc. Urban Plan. 2010, 95, 161–168. [Google Scholar] [CrossRef]
- Francis, R.A.; Chadwick, M.A. Urban invasions: Non-native and invasive species in cities. Geography 2015, 100, 144–151. [Google Scholar] [CrossRef]
- von der Lippe, M.; Kowarik, I. Do cities export biodiversity? Traffic as dispersal vector across urban-rural gradients. Divers. Distrib. 2008, 14, 18–25. [Google Scholar] [CrossRef]
- Cutway, H.B.; Ehrenfeld, J.G. Exotic plant invasions in forested wetlands: Effects of adjacent urban land use type. Urban Ecosyst. 2009, 12, 371–390. [Google Scholar] [CrossRef]
- Padayachee, A.L.; Irlich, U.M.; Faulkner, K.T.; Gaertner, M.; Procheş, Ş.; Wilson, J.R.U.; Rouget, M. How do invasive species travel to and through urban environments? Biol. Invasions 2017, 19, 3557–3570. [Google Scholar] [CrossRef]
- Godefroid, S.; Ricotta, C. Alien plant species do have a clear preference for different land uses within urban environments. Urban Ecosyst. 2018, 21, 1189–1198. [Google Scholar] [CrossRef]
- Rendeková, A.; Mičieta, K.; Hrabovský, M.; Eliašová, M.; Miškovic, J. Effects of invasive plant species on species diversity: Implications on ruderal vegetation in Bratislava City, Slovakia, Central Europe. Acta Soc. Bot. Pol. 2019, 88, 3621. [Google Scholar] [CrossRef] [Green Version]
- La Sorte, F.A.; Aronson, M.F.J.; Williams, N.S.G.; Celesti-Grapow, L.; Cilliers, S.; Clarkson, B.D.; Dolan, R.W.; Hipp, A.; Klotz, S.; Kühn, I.; et al. Beta diversity of urban floras among European and non-European cities. Glob. Ecol. Biogeogr. 2014, 23, 769–779. [Google Scholar] [CrossRef]
- Lososová, Z.; Chytrý, M.; Tichý, L.; Danihelka, J.; Fajmon, K.; Hájek, O.; Kintrová, K.; Láníková, D.; Otýpková, Z.; Řehořek, V. Biotic homogenization of Central European urban floras depends on residence time of alien species and habitat types. Biol. Conserv. 2012, 145, 179–184. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, J.; Liu, J.; Xiao, H.; Wang, L. Functional traits and reproductive allocation strategy of Conyza canadensis as they vary by invasion degree along a latitude gradient. Pol. J. Environ. Stud. 2017, 26, 1289–1297. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Jagodziński, A.M. Patterns of plant invasions at small spatial scale correspond with that at the whole country scale. Urban Ecosyst. 2016, 19, 983–998. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Feng, L.; Zhao, Y.; Feng, L.; Zhu, C.; Qu, Y.; Wang, H. Predicting the potential distribution of an invasive species, Erigeron canadensis L., in China with a maximum entropy model. Glob. Ecol. Conserv. 2020, 21, e00822. [Google Scholar] [CrossRef]
- Shah, M.A.; Callaway, R.M.; Shah, T.; Houseman, G.R.; Pal, R.W.; Xiao, S.; Luo, W.; Rosche, C.; Reshi, Z.A.; Khasa, D.P.; et al. Conyza canadensis suppresses plant diversity in its nonnative ranges but not at home: A transcontinental comparison. New Phytol. 2014, 202, 1286–1296. [Google Scholar] [CrossRef] [PubMed]
- Straigytė, L.; Cekstere, G.; Laivins, M.; Marozas, V. The spread, intensity and invasiveness of the Acer negundo in Riga and Kaunas. Dendrobiology 2015, 74, 157–168. [Google Scholar] [CrossRef] [Green Version]
- Erfmeier, A.; Böhnke, M.; Bruelheide, H. Secondary invasion of Acer negundo: The role of phenotypic responses versus local adaptation. Biol. Invasions 2010, 13, 1599–1614. [Google Scholar] [CrossRef]
- Tokarska-Guzik, B. The Establishment and Spread of Alien Plant Species (Kenophytes) in the Flora of Poland; Wydawnictwo Uniwersytetu Śląskiego: Katowice, Poland, 2005; p. 192. [Google Scholar]
- Dyderski, M.K.; Gdula, A.K.; Jagodziński, A.M. “The rich get richer” concept in riparian woody species—A case study of the Warta River Valley (Poznań, Poland). Urban For. Urban Green. 2015, 14, 107–114. [Google Scholar] [CrossRef]
- Sabo, A.E. Robinia pseudoacacia invasions and control in North America and Europe. Restor. Reclam. Rev. 2000, 6, 1–9. [Google Scholar]
- Benesperi, R.; Giuliani, C.; Zanetti, S.; Gennai, M.; Lippi, M.M.; Guidi, T.; Nascimbene, J.; Foggi, B. Forest plant diversity is threatened by Robinia pseudoacacia (black-locust) invasion. Biodivers. Conserv. 2012, 21, 3555–3568. [Google Scholar] [CrossRef]
- Vítková, M.; Müllerová, J.; Sádlo, J.; Pergl, J.; Pyšek, P. Black locust (Robinia pseudoacacia) beloved and despised: A story of an invasive tree in Central Europe. For. Ecol. Manag. 2017, 384, 287–302. [Google Scholar] [CrossRef]
- Lazzaro, L.; Mazza, G.; d’Errico, G.; Fabiani, A.; Giuliani, C.; Inghilesi, A.F.; Lagomarsino, A.; Landi, S.; Lastrucci, L.; Pastorelli, R.; et al. How ecosystems change following invasion by Robinia pseudoacacia: Insights from soil chemical properties and soil microbial, nematode, microarthropod and plant communities. Sci. Total Environ. 2018, 622–623, 1509–1518. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Jagodziński, A.M. Seedling survival of Prunus serotina Ehrh., Quercus rubra L. and Robinia pseudoacacia L. in temperate forests of Western Poland. For. Ecol. Manag. 2019, 450, 117498. [Google Scholar] [CrossRef]
- Damalas, C.A. Distribution, biology, and agricultural importance of Galinsoga parviflora (Asteraceae). Weed Biol. Manag. 2008, 8, 147–153. [Google Scholar] [CrossRef]
- Węgrzynek, B.; Tokarska-Guzik, B.; Trueman, I.C.; Cohn, E. Galinsoga species in Poland: History of spread and habitat preferences of two successful alien weeds. In Biological Invasions—From Ecology to Conservation; Rabitsch, W., Essl, F., Klingenstein, F., Eds.; NEOBIOTA: Berlin, Germany, 2008; Volume 7, pp. 200–209. [Google Scholar]
- Stadler, J.; Mungai, G.; Brandl, R. Weed invasion in East Africa: Insights from herbarium records. Afr. J. Ecol. 1998, 36, 15–22. [Google Scholar] [CrossRef]
- Török, K.; Botta-Dukát, Z.; Dancza, I.; Németh, I.; Kiss, J.; Mihály, B.; Magyar, D. Invasion Gateways and Corridors in the Carpathian Basin: Biological Invasions in Hungary. Biol. Invasions 2003, 5, 349–356. [Google Scholar] [CrossRef]
- Shen, S.; Xu, G.; Li, D.; Jin, G.; Liu, S.; Clements, D.R.; Yang, Y.; Rao, J.; Chen, A.; Zhang, F.; et al. Potential Use of Sweet Potato (Ipomoea batatas (L.) Lam.) to Suppress Three Invasive Plant Species in Agroecosystems (Ageratum conyzoides L., Bidens pilosa L., and Galinsoga parviflora Cav.). Agronomy 2019, 9, 318. [Google Scholar] [CrossRef] [Green Version]
- Eichler, B.; Łapczyński, K. Korespondencja do Wszechświata. Wszechświat 1892, 11, 814–815. [Google Scholar]
- Stosik, T. Rumex confertus Willd-biologia a ekspansja gatunku. Ann. Univ. Mariae Curie-Skłodowska E Agric. 2008, 63, 81–95. [Google Scholar]
- Raycheva, T. Rumex confertus (Polygonaceae) in the Bulgarian flora. Bot. Serb. 2011, 35, 55–59. [Google Scholar]
- Abramova, L.M.; Golovanov, Y.M. Invasions of Alien Plant Species in the South Urals: Current State of the Problem. KnE Life Sci. 2018, 4, 1–9. [Google Scholar] [CrossRef]
- Maltseva, S.Y.; Bobrov, A.A. Alien species of vascular plants in the Rybinsk Reservoir (Upper Volga, Russia). Russ. J. Biol. Invasions 2017, 8, 321–326. [Google Scholar] [CrossRef]
- Ronzhina, D.A. Distribution, competitive ability, and seed production of Bidens frondosa L. in the Middle Urals. Russ. J. Biol. Invasions 2017, 8, 351–359. [Google Scholar] [CrossRef]
- Sukopp, H. On the study of anthropogenic plant migrations in central Europe. In Plant Invasions: Ecological Mechanisms and Human Responses; Starfinger, U., Edwards, K., Kowarik, I., Williamson, M., Eds.; Backhuys Publishers: Leiden, The Netherlands, 1998; pp. 43–56. [Google Scholar]
- Sudnik-Wójcikowska, B.; Guzik, J. The spread and habitats of Eragrostis pilosa (Poaceae) in the Vistula valley. Fragm. Florist. Geobot. Pol. 1996, 41, 753–769. [Google Scholar]
- Glatkova, G.; Surek, H.; Andov, D.; Andreevska, D.; Pacanoski, Z. Eragrostis pilosa (L.) P. Beauv. a New Invasive and Economically Important Weed in the Rice Fields in the Kocani Region. Int. J. Innov. Approaches Agric. Res. 2019, 3, 671–679. [Google Scholar] [CrossRef]
- Carloto, B.W.; Buriol, G.A.; Dornelles, S.H.B.; Trivisiol, V.S.; Peripolli, M.; Escobar, O.S. Morphological and phenological responses of Eragrostis plana Nees and Eragrostis pilosa (L.) P. Beauv. plants subjected to different soil moisture conditions. Planta daninha 2019, 37, e019217246. [Google Scholar] [CrossRef]
- Carloto, B.W.; Escobar, O. dos S.; Trivisiol, V.S.; Peripolli, M.; Pivetta, M.; Posser, T.; Barreto, E.P.M.; Dornelles, S.H.B. Response of Eragrostis plana and Eragrostis pilosa (L.) P. Beauv. submitted on flooded soil. Acta Sci. Biol. Sci. 2020, 42, e47557. [Google Scholar] [CrossRef]
- Gentili, R.; Ferrè, C.; Cardarelli, E.; Montagnani, C.; Bogliani, G.; Citterio, S.; Comolli, R. Comparing Negative Impacts of Prunus serotina, Quercus rubra and Robinia pseudoacacia on Native Forest Ecosystems. Forests 2019, 10, 842. [Google Scholar] [CrossRef] [Green Version]
- Dyderski, M.K.; Chmura, D.; Dylewski, Ł.; Horodecki, P.; Jagodziński, A.M.; Pietras, M.; Robakowski, P.; Woziwoda, B. Biological Flora of the British Isles: Quercus rubra. J. Ecol. 2020, 108, 1199–1225. [Google Scholar] [CrossRef] [Green Version]
- Stanek, M.; Piechnik, Ł.; Stefanowicz, A.M. Invasive red oak (Quercus rubra L.) modifies soil physicochemical properties and forest understory vegetation. For. Ecol. Manag. 2020, 472, 118253. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Jagodziński, A.M. How do invasive trees impact shrub layer diversity and productivity in temperate forests? Ann. For. Sci. 2021, 78, 1–14. [Google Scholar] [CrossRef]
- Florianová, A.; Münzbergová, Z. Drivers of natural spread of invasive Impatiens parviflora differ between life-cycle stages. Biol. Invasions 2018, 20, 2121–2140. [Google Scholar] [CrossRef]
- Bomanowska, A.; Adamowski, W.; Kirpluk, I.; Otręba, A.; Rewicz, A. Invasive alien plants in Polish national parks—threats to species diversity. PeerJ 2019, 7, e8034. [Google Scholar] [CrossRef] [Green Version]
- Šipek, M.; Šajna, N. Public opinions and perceptions of peri-urban plant invasion: The role of garden waste disposal in forest fragments. Manag. Biol. Invasions 2020, 11, 733–746. [Google Scholar] [CrossRef]
- Ferus, P.; Hoťka, P.; Košútová, D.; Konôpková, J. Invasions of alien woody plant taxa across a cluster of villages neighbouring the Mlyňany Arboretum (SW Slovakia). Folia Oecologica 2020, 47, 121–130. [Google Scholar] [CrossRef]
- Hansen, M.J.; Clevenger, A.P. The influence of disturbance and habitat on the presence of non-native plant species along transport corridors. Biol. Conserv. 2005, 125, 249–259. [Google Scholar] [CrossRef]
- Christen, D.C.; Matlack, G.R. The habitat and conduit functions of roads in the spread of three invasive plant species. Biol. Invasions 2008, 11, 453–465. [Google Scholar] [CrossRef]
- Manier, D.J.; Aldridge, C.L.; O’Donnell, M.; Schell, S.J. Human Infrastructure and Invasive Plant Occurrence Across Rangelands of Southwestern Wyoming, USA. Rangel. Ecol. Manag. 2014, 67, 160–172. [Google Scholar] [CrossRef]
- Wagner, V.; Chytrý, M.; Jiménez-Alfaro, B.; Pergl, J.; Hennekens, S.; Biurrun, I.; Knollová, I.; Berg, C.; Vassilev, K.; Rodwell, J.S.; et al. Alien plant invasions in European woodlands. Divers. Distrib. 2017, 23, 969–981. [Google Scholar] [CrossRef]
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species: A Selection from the Global Invasive Species Database; The Invasive Species Specialist Group (ISSG) a specialist group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN): Auckland, New Zealand, 2000; p. 12. [Google Scholar]
- Wójcik, G.; Marciniak, K. Klimat. In Toruń i Jego Okolice. Monografia Przyrodnicza; Andrzejewski, L., Weckwerth, P., Burak, S., Eds.; Wydawnictwo Uniwersytetu Mikołaja Kopernika w Toruniu: Toruń, Poland, 2006; pp. 99–127. [Google Scholar]
- Przybylak, R.; Uscka-Kowalkowska, J.; Araźny, A.; Kejna, M.; Kunz, M.; Maszewski, R. Spatial distribution of air temperature in Toruń (Central Poland) and its causes. Theor. Appl. Clim. 2017, 127, 441–463. [Google Scholar] [CrossRef]
- Zając, A. Atlas of distribution of vascular plants in Poland (ATPOL). Taxon 1978, 27, 481–484. [Google Scholar] [CrossRef]
- Komsta, Ł. Rewizja matematyczna siatki geobotanicznej ATPOL—Propozycja algorytmów konwersji współrzędnych. [ATPOL geobotanical grid revisited—A proposal of coordinate conversion algorithms. Ann. Univ. Mariae Curie-Skłodowska E Agric. 2016, 71, 31–37. [Google Scholar]
- World Flora Online. Available online: http://www.worldfloraonline.org/ (accessed on 25 February 2021).
- Adamska, E.; Juśkiewicz, W. Visualisation of the influence of habitat on lichen occurrence, Toruń, Poland. J. Maps 2018, 14, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Bednarek, R.; Jankowski, M. Gleby. In Toruń i Jego Okolice—Monografia Przyrodnicza; Andrzejewski, L., Weckwerth, P., Burak, S., Eds.; Wydawnictwo Uniwersytetu Mikołaja Kopernika w Toruniu: Toruń, Poland, 2006; pp. 153–175. [Google Scholar]
- Hulisz, P.; Charzyński, P.; Greinert, A. Urban soil resources of medium-sized cities in Poland: A comparative case study of Toruń and Zielona Góra. J. Soils Sediments 2016, 18, 358–372. [Google Scholar] [CrossRef] [Green Version]
- ter Braak, C.J.F.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination (Version 5.0); Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Jongman, R.H.G.; ter Braak, C.J.F.; van Tongeren, O.F.R. Data Analysis in Community and Landscape Ecology; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Šmilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data Using CANOCO 5; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar] [CrossRef] [Green Version]




| Soil-Type Effects | Habitat-Type Effects | ||||||
|---|---|---|---|---|---|---|---|
| Variable | V % | F | p | Variable | V % | F | p |
| Rs | 6.6 | 36.4 | 0.002 | Sw | 11.0 | 63.5 | 0.002 |
| Us | 5.4 | 31.2 | 0.002 | Fo | 6.8 | 42.3 | 0.002 |
| Is | 2.8 | 16.7 | 0.002 | Af | 1.7 | 11.1 | 0.002 |
| Po | 2.4 | 14.9 | 0.002 | Ag | 1.2 | 7.9 | 0.002 |
| As | 1.3 | 7.9 | 0.002 | SaGr | 0.9 | 5.8 | 0.002 |
| Rp | 1.6 | 10.3 | 0.002 | Mh | 0.6 | 4.1 | 0.002 |
| Ho | 1.9 | 12.5 | 0.002 | Ra | 0.4 | 2.3 | 0.02 |
| Ms | 0.8 | 5.4 | 0.002 | Tb | 0.3 | 1.9 | 0.084 |
| Ns | 0.5 | 3.6 | 0.002 | Ia | 0.2 | 1.2 | 0.254 |
| Gp | 0.5 | 3.2 | 0.008 | Ih | 0.2 | 1.2 | 0.334 |
| Gd | 0.5 | 3.3 | 0.004 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szumańska, I.; Lubińska-Mielińska, S.; Kamiński, D.; Rutkowski, L.; Nienartowicz, A.; Piernik, A. Invasive Plant Species Distribution Is Structured by Soil and Habitat Type in the City Landscape. Plants 2021, 10, 773. https://doi.org/10.3390/plants10040773
Szumańska I, Lubińska-Mielińska S, Kamiński D, Rutkowski L, Nienartowicz A, Piernik A. Invasive Plant Species Distribution Is Structured by Soil and Habitat Type in the City Landscape. Plants. 2021; 10(4):773. https://doi.org/10.3390/plants10040773
Chicago/Turabian StyleSzumańska, Ilona, Sandra Lubińska-Mielińska, Dariusz Kamiński, Lucjan Rutkowski, Andrzej Nienartowicz, and Agnieszka Piernik. 2021. "Invasive Plant Species Distribution Is Structured by Soil and Habitat Type in the City Landscape" Plants 10, no. 4: 773. https://doi.org/10.3390/plants10040773
APA StyleSzumańska, I., Lubińska-Mielińska, S., Kamiński, D., Rutkowski, L., Nienartowicz, A., & Piernik, A. (2021). Invasive Plant Species Distribution Is Structured by Soil and Habitat Type in the City Landscape. Plants, 10(4), 773. https://doi.org/10.3390/plants10040773