Corn-Soybean Intercropping Improved the Nutritional Quality of Forage Cultivated on Podzols in Boreal Climate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Corn–Soybean Forage Quality Analysis
2.2. Determination of Forage Mineral Nutrients
2.3. Forage Fatty Acid Extraction
2.4. Fatty Acid Methyl Esters (FAMEs) Analysis
2.5. Microbial Fatty Acid Analysis
2.6. Statistical Analyses
3. Results
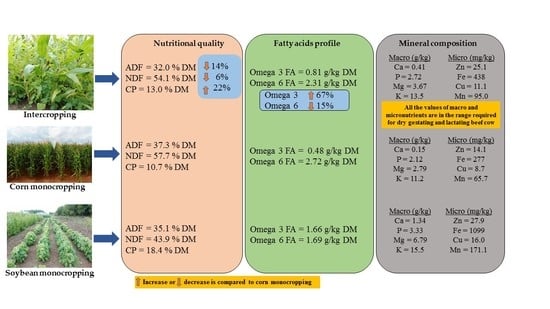
3.1. Forage Nutritional Quality
3.2. Mineral Composition of Corn and Soybean Forage Obtained Following Cultivation as Mono and Intercrop
3.3. Fatty Acid Composition of Forage Obtained from Corn and Soybean Cultivated as Monocrops or Intercrops
3.4. Relationships between the Cropping Systems, Active Microbial Population and Quality of the Forage When Cultivated on Podzols in Cool Climates
4. Discussion
4.1. Effect of Intercropping on Forage Quality
4.2. Effect of Intercropping on Forage Mineral Contents
4.3. Effects of Intercropping on Forage Fatty Acid Composition
4.4. Relationship between Soil Health Status and Forage Nutritional Quality
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eskandari, H.; Ghanbari, A. Intercropping of maize (Zea mays) and cowpea (Vigna sinensis) as whole-crop forage: Effect of different planting pattern on total dry matter production and maize forage quality. Not. Bot. Horti Agrobot. 2009, 37, 152–155. [Google Scholar]
- Eslamizadeh, A.; Kashani, A.; Ata, S.; Siyadat, A.; Modhej, A.; Lak, S. Study of soybean forage at different planting dates intercropped with corn. WALIA J. 2015, 31, 108–112. [Google Scholar]
- Geren, H.; Avcioglu, R.; Soya, H.; Kir, B. Intercropping of corn with cowpea and bean: Biomass yield and silage quality. Afr. J. Biotechnol. 2008, 7, 4100–4104. [Google Scholar]
- Ananthi, T.; Amanullah, M.M.; Al-Tawaha, A.R.M.S. A review on maize-legume intercropping for enhancing the productivity and soil fertility for sustainable agriculture in India. Adv. Environ. Biol. 2017, 11, 49–63. [Google Scholar]
- Nadeau, E.; Rustas, B.O.; Arnesson, A.; Swensson, C. Maize silage quality on Swedish dairy and beef farms. In Proceedings of the 14th International Symposium Forage Conservation, Brno, Czech Republic, 17–19 March 2010; pp. 195–197. [Google Scholar]
- Masoero, F.; Rossi, F.; Pulimeno, A.M. Chemical composition and in vitro digestibility of stalks, leaves and cobs of four corn hybrids at different phenological stages. Ital. J. Anim. Sci. 2006, 5, 215–227. [Google Scholar] [CrossRef]
- Armstrong, K.L.; Albrecht, K.A.; Lauer, J.G.; Riday, H. Intercropping corn with lablab bean, velvet bean, and scarlet runner bean for forage. Crop Sci. 2008, 48, 371–379. [Google Scholar] [CrossRef]
- Filya, I.; Sucu, E.; Karabulut, A. The effect of Lactobacillus buchneri on the fermentation, aerobic stability and ruminal degradability of maize silage. J. Appl. Microbiol. 2006, 101, 1216–1223. [Google Scholar] [CrossRef]
- Tau, M.S. Grazing Management in the Communal Rangelands of the Upper Thukela, KwaZulu-Natal. Master’s Thesis, University of KwaZulu-Natal, Pietermaritzburg, South Africa, 2005, (unpublished). [Google Scholar]
- Jayanegara, A.; Dewi, S.P.; Laylli, N.; Laconi, E.B.; Nahrowi, N.; Ridla, M. Determination of cell wall protein from selected feedstuffs and its relationship with ruminal protein digestibility in vitro. Media Peternak. 2016, 39, 134–140. [Google Scholar] [CrossRef] [Green Version]
- Blount, A.R.S.; Wright, D.L.; Sprenkel, R.K.; Hewitt, T.D.; Myer, R.O. Forage Soybeans for Grazing, Hay and Silage; University of Florida, IFAS Extensión: Gainesville, FL, USA, 2009. [Google Scholar]
- Camacho Barrón, M.; González De Mejía, E. Comparative study of enzymes related to proline metabolism in tepary bean (Phaseolus acutifolius) and common bean (Phaseolus vulgaris) under drought and irrigated conditions, and various urea concentrations. Plant Foods Hum. Nutr. 1998, 52, 119–132. [Google Scholar] [CrossRef]
- Paulson, J.; Jung, H.; Raeth-Knight, M.; Linn, J. Grass vs Legume Forages for Dairy Cattle; University of Minnesota: Saint Paul, MA, USA, 2008; pp. 119–133. [Google Scholar]
- Baghdadi, A.; Halim, R.A.; Ghasemzadeh, A.; Ebrahimi, M.; Othman, R.; Yusof, M.M. Effect of intercropping of corn and soybean on dry matter yield and nutritive value of forage corn. Legum. Res. 2016, 39, 976–981. [Google Scholar]
- Agarwal, D.K.; Billore, S.D.; Sharma, A.N.; Dupare, B.U.; Srivastava, S.K. Soybean: Introduction, improvement, and utilization in India-problems and prospects. Agric. Res. 2013, 2, 293–300. [Google Scholar] [CrossRef]
- Lithourgidis, A.S.; Vasilakoglou, I.B.; Dhima, K.V.; Dordas, C.A.; Yiakoulaki, M.D. Forage yield and quality of common vetch mixtures with oat and triticale in two seeding ratios. Field Crop. Res. 2006, 99, 106–113. [Google Scholar] [CrossRef]
- Ross, S.M.; King, J.R.; O’Donovan, J.T.; Spaner, D. Intercropping berseem clover with barley and oat cultivars for forage. Agron. J. 2004, 96, 1719–1729. [Google Scholar] [CrossRef]
- Javanmard, A.; Nasab, A.D.M.; Javanshir, A.; Moghaddam, M.; Janmohammadi, H. Forage yield and quality in intercropping of maize with different legumes as double-cropped. J. Food Agric. Environ. 2009, 7, 163–166. [Google Scholar]
- Zhou, X.; Yu, G.; Wu, F. Effects of intercropping cucumber with onion or garlic on soil enzyme activities, microbial communities and cucumber yield. Eur. J. Soil Biol. 2011, 47, 279–287. [Google Scholar] [CrossRef]
- Brooker, R.W.; Jones, H.G.; Paterson, E.; Watson, C.; Brooker, R.W.; Bennett, A.E.; Cong, W.; Daniell, T.J.; George, T.S.; Hallett, P.D.; et al. Improving intercropping: A synthesis of research in agronomy, plant physiology and ecology. New Phytol. 2015, 206, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, L.; Zhang, F. Crop mixtures and the mechanisms of overyielding. Encycl. Biodivers. 2013, 2, 382–395. [Google Scholar]
- Jahanzad, E.; Sadeghpour, A.; Hosseini, M.B.; Barker, A.V.; Hashemi, M.; Zandvakili, O.R. Silage yield and nutritive value of millet–soybean intercrops as influenced by nitrogen application. Agron. J. 2014, 106, 1993–2000. [Google Scholar] [CrossRef]
- Htet, M.N.S.; Ya-qin, P.; Ya-dong, X.; Soomro, R.N.; Jiang-bo, H. Effect of intercropping maize (Zea mays L.) with soybean (Glycine max L.) on green forage yield, and quality evaluation. IOSR J. Agric. Vet. Sci. 2016, 9, 2319–2372. [Google Scholar]
- Htet, M.N.S.; Soomro, R.N.; Jiang-bo, H. Effects of different planting pattern of maize (Zea mays L.) and soybean (Glycine max (L.) Merrill) intercropping in resource consumption on fodder yield, and silage quality. Am. J. Plant Sci. 2017, 08, 666–679. [Google Scholar] [CrossRef] [Green Version]
- Serbester, U.; Akkaya, M.R.; Yucel, C.; Gorgulu, M. Comparison of yield, nutritive value, and in vitro digestibility of monocrop and intercropped corn-soybean silages cut at two maturity stages. Ital. J. Anim. Sci. 2015, 14, 66–70. [Google Scholar] [CrossRef]
- Reta Sánchez, D.G.; Espinosa Silva, J.T.; Palomo Gil, A.; Serrato Corona, J.S.; Cueto Wong, J.A.; Gaytán Mascorro, A. Forage yield and quality of intercropped corn and soybean in narrow strips. Span. J. Agric. Res. 2010, 8, 713–721. [Google Scholar] [CrossRef] [Green Version]
- Yucel, C.; Avcı, M.; Inal, I.; Yucel, D. Yield and silage quality of soybean-maize intercrop under different mixing ratios and harvest stages. Int. J. Agron. Agric. Res. 2017, 10, 95–105. [Google Scholar]
- Sanborn, P.; Lamontagne, L.; Hendershot, W. Podzolic soils of Canada: Genesis, distribution, and classification. Can. J. Soil Sci. 2011, 91, 843–880. [Google Scholar] [CrossRef]
- Harris, L.; Hiller, J. Newfoundland and Labrador—Climate. 2018. Available online: Britannica.com (accessed on 18 June 2018).
- Van Der Heijden, M.G.A.; Bardgett, R.D.; Van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef]
- Richardson, A.E.; Barea, J.M.; McNeill, A.M.; Prigent-Combaret, C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 2009, 321, 305–339. [Google Scholar] [CrossRef]
- Bonkowski, M. Protozoa and plant growth: The microbial loop in soil revisited. New Phytol. 2004, 162, 617–631. [Google Scholar] [CrossRef]
- Elgersma, A.; Tamminga, S.; Ellen, G. Modifying milk composition through forage. Anim. Feed Sci. Technol. 2006, 131, 207–225. [Google Scholar] [CrossRef]
- Dewhurst, R.J.; Shingfield, K.J.; Lee, M.A.; Scollan, N.D. Increasing the concentrations of beneficial polyunsaturated fatty acids in milk produced by dairy cows in high-forage systems. Anim. Feed Sci. Technol. 2006, 131, 168–206. [Google Scholar] [CrossRef]
- Scollan, N.D.; Choi, N.-J.; Kurt, E.; Fisher, A.V.; Enser, M.; Wood, J.D. Manipulating the fatty acid composition of muscle and adipose tissue in beef cattle. Br. J. Nutr. 2001, 85, 115. [Google Scholar] [CrossRef] [Green Version]
- Pariza, M.W. Perspective on the safety and effectiveness of conjugated linoleic acid. Am. J. Clin. Nutr. 2004, 79, 1132S–1136S. [Google Scholar] [CrossRef] [Green Version]
- Ali, W.; Nadeem, M.; Ashiq, W.; Zaeem, M.; Thomas, R.; Kavanagh, V.; Cheema, M. Forage yield and quality indices of silage-corn following organic and inorganic phosphorus amendments in podzol soil under boreal climate. Agronomy 2019, 9, 489. [Google Scholar] [CrossRef] [Green Version]
- Ali, W.; Nadeem, M.; Ashiq, W.; Zaeem, M.; Gilani, S.S.M.; Rajabi-Khamseh, S.; Pham, T.H.; Kavanagh, V.; Thomas, R.; Cheema, M. The effects of organic and inorganic phosphorus amendments on the biochemical attributes and active microbial population of agriculture podzols following silage corn cultivation in boreal climate. Sci. Rep. 2019, 9, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Nadeem, M.; Pham, T.H.; Thomas, R.; Galagedara, L.; Kavanagh, V.; Zhu, X.; Ali, W.; Cheema, M. Potential role of root membrane phosphatidic acid in superior agronomic performance of silage-corn cultivated in cool climate cropping systems. Physiol. Plant. 2019, 167, 585–596. [Google Scholar] [CrossRef]
- Zaeem, M.; Nadeem, M.; Pham, T.H.; Ashiq, W.; Ali, W.; Gilani, S.S.M.; Elavarthi, S.; Kavanagh, V.; Cheema, M.; Galagedara, L.; et al. The potential of corn-soybean intercropping to improve the soil health status and biomass production in cool climate boreal ecosystems. Sci. Rep. 2019, 9, 1–17. [Google Scholar]
- Weiss, W.P.; Conrad, H.R.; St. Pierre, N.R. A theoretically-based model for predicting total digestible nutrient values of forages and concentrates. Anim. Feed Sci. Technol. 1992, 39, 95–110. [Google Scholar] [CrossRef]
- Council, N.R. Nutrient Requirements of Dairy Cattle; The National Academies Press: Washington, DC, USA, 2001; ISBN 978-0-309-06997-7. [Google Scholar]
- Van Soest, P.J. Nutritional Ecology of the Ruminant; O & B Books: Corvallis, OR, USA, 1982. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Fried, B.; Sherma, J.; Fried, B. Handbook of Thin-Layer Chromatography, 3rd ed.; Marcel Dekker: New York, NY, USA, 2003; ISBN 0824708954. [Google Scholar]
- Liu, J.H.; Zeng, Z.H.; Jiao, L.X.; Hu, Y.G.; Wang, Y.; Li, H. Intercropping of different silage maize cultivars and alfalfa. Acta Agron. Sin. 2006, 32, 125–137. [Google Scholar]
- Lithourgidis, A.S.; Dhima, K.V.; Vasilakoglou, I.B.; Dordas, C.A.; Yiakoulaki, M.D. Sustainable production of barley and wheat by intercropping common vetch. Agron. Sustain. Dev. 2007, 27, 95–99. [Google Scholar] [CrossRef]
- Abdulraheem, M.I.; Ojeniyi, S.O.; Charles, E.F. Effect of different planting pattern on total dry matter production and maize forage quality in maize (Zea mays) and cowpea (Vigna sinensis) intercropped as whole-crop forage. IOSR J. Agric. Vet. Sci. 2012, 1, 42–46. [Google Scholar]
- Anil, L.; Park, J.; Phipps, R.H. The potential of forage-maize intercrops in ruminant nutrition. Anim. Feed Sci. Technol. 2000, 86, 157–164. [Google Scholar] [CrossRef]
- Dahmardeh, M.; Ghanbari, A.; Syasar, B.; Ramroudi, M.; Zhu, Y.; Bai, C.S.; Guo, X.S.; Xue, Y.L.; Ataku, K. Effect of intercropping maize (Zea mays L.) with cow pea (Vigna unguiculata L.) on green forage yield and quality evaluation. Asian J. Plant Sci. 2009, 8, 235–239. [Google Scholar] [CrossRef] [Green Version]
- Strydhorst, S.M.; King, J.R.; Lopetinsky, K.J.; Harker, K.N. Forage potential of intercropping barley with faba bean, lupin, or field pea. Agron. J. 2008, 100, 182–190. [Google Scholar] [CrossRef]
- Bingol, N.T.; Karsli, M.A.; Yilmaz, I.H.; Bolat, D. The effects of planting time and combination on the nutrient composition and digestible dry matter yield of four mixtures of vetch varieties intercropped with barley. Turkish J. Vet. Anim. Sci. 2007, 31, 297–302. [Google Scholar]
- Contreras-Govea, F.E.; Albrecht, K.A.; Muck, R.E. Spring yield and silage characteristics of kura clover, winter wheat, and in mixtures. Agron. J. 2006, 98, 781–787. [Google Scholar] [CrossRef] [Green Version]
- Aasen, A.; Baron, V.S.; Clayton, G.W.; Dick, A.C.; McCartney, D.H. Swath grazing potential of spring cereals, field pea and mixtures with other species. Can. J. Plant Sci. 2004, 84, 1051–1058. [Google Scholar]
- Lauriault, L.M.; Kirksey, R.E. Yield and nutritive value of irrigated winter cereal forage grass-legume intercrops in the Southern High Plains, USA. Agron. J. 2004, 96, 352–358. [Google Scholar]
- Sleugh, B.; Moore, K.J.; George, J.R.; Brummer, E.C. Binary legume-grass mixture improve forage yield, quality, and seasonal distribution. Agron. J. 2000, 92, 24–29. [Google Scholar] [CrossRef]
- Costa, P.M.; Villela, S.D.J.; de Paula Leonel, F.; do Carmo Araújo, S.A.; Araújo, K.G.; Ruas, J.R.M.; Coelho, F.S.; Andrade, V.R. Intercropping of corn, brachiaria grass and leguminous plants: Productivity, quality and composition of silages. Rev. Bras. Zootec. 2012, 41, 2144–2149. [Google Scholar] [CrossRef] [Green Version]
- Gill, K.S.; Omokanye, A.T. Potential of spring barley, oat and triticale intercrops with field peas for forage production, nutrition quality and beef cattle diet. J. Agric. Sci. 2018, 10, 1. [Google Scholar] [CrossRef] [Green Version]
- Mugweni, B.Z.; Titterton, M.; Maasdorp, B.V.; Gandiya, A.F. Effect of mixed cereal-legume silages on milk production from lactating holstein dairy cows (R7010). In Proceedings of the 3rd Workshop Livestock Production Programme Projects, Matobo, Zimbabwe, 26–28 September 2000; pp. 82–89. [Google Scholar]
- Htet, M.N.S.; Soomro, R.N.; Jiang-bo, H. Intercropping of maize and climbing bean: Fodder yield, quality and nutrient composition of silages. Int. J. Agron. Agric. Res. 2016, 9, 25–31. [Google Scholar]
- Sadeghpour, A.; Jahanzad, E.; Esmaeili, A.; Hosseini, M.B.; Hashemi, M. Forage yield, quality and economic benefit of intercropped barley and annual medic in semi-arid conditions: Additive series. Field Crop. Res. 2013, 148, 43–48. [Google Scholar] [CrossRef]
- Salama, H.S.A.; Zeid, M.M.K. Hay quality evaluation of summer grass and legume forage monocultures and mixtures grown under irrigated conditions. Aust. J. Crop Sci. 2016, 10, 1543–1550. [Google Scholar] [CrossRef]
- Yurchak, T.; Orkine, E. Beef ration rules of thumb agdex (420/52-4). Alberta Agric. Food Rural Dev. Publ. 2004, 2004, 1–2. [Google Scholar]
- Rostamza, M.; Chaichi, M.R.; Jahansouz, M.R.; Alimadadi, A. Forage quality, water use and nitrogen utilization efficiencies of pearl millet (Pennisetum americanum L.) grown under different soil moisture and nitrogen levels. Agric. Water Manag. 2011, 98, 1607–1614. [Google Scholar] [CrossRef]
- Sadeghpour, A.; Jahanzad, E.; Lithourgidis, A.; Hashemi, M. Forage yield & quality of barley-annual medic intercrops in semi-arid environments. Int. J. Plant Prod. 2014, 8, 1735–8043. [Google Scholar]
- Stoltz, E.; Nadeau, E.; Wallenhammar, A.C. Intercropping maize and faba bean for silage under Swedish climate conditions. Agric. Res. 2013, 2, 90–97. [Google Scholar] [CrossRef] [Green Version]
- Ullah, M.A. Forage Production in Panicum Grass-Legumes Intercropping by Combining Geometrical Configuration, Inoculation and Fertilizer under Rainfed Conditions. Ph.D. Thesis, Kassel University, Kassel, Germany, 2010. [Google Scholar]
- Caballero, R.; Goicoechea, E.L.; Hernaiz, P.J. Forage yields and quality of common vetch and oat sown at varying seeding ratios and seeding rates of vetch. Field Crop. Res. 1995, 41, 135–140. [Google Scholar] [CrossRef]
- Schroeder, J.W. Quality Forage for Maximum Production and Return. North Dakota State Univ. Coop. Ext. Serv. Pub. 1996, AS-1117, 1–12. [Google Scholar]
- Carr, P.M.; Horsley, R.D.; Poland, W.W. Barley, oat, and cereal-pea mixtures as dryland forages in the northern Great Plains. Agron. J. 2004, 96, 677–684. [Google Scholar] [CrossRef] [Green Version]
- National Research Council. Nutrient Requirements of Beef Cattle, 7th ed.; The National Academies Press: Washington, DC, USA, 2000; ISBN 978-0-309-06934-2. [Google Scholar]
- Iba, K. Acclimative response to temperature stress in higher plants: Approaches of gene engineering for temperature tolerance. Annu. Rev. Plant Biol. 2002, 53, 225–245. [Google Scholar] [CrossRef] [Green Version]
- Routaboul, J.M.; Skidmore, C.; Wallis, J.G.; Browse, J. Arabidopsis mutants reveal that short-and long-term thermotolerance have different requirements for trienoic fatty acids. J. Exp. Bot. 2012, 63, 1435–1443. [Google Scholar] [CrossRef] [Green Version]
- Falcone, D.L.; Ogas, J.P.; Somerville, C.R. Regulation of membrane fatty acid composition by temperature in mutants of Arabidopsis with alterations in membrane lipid composition. BMC Plant Biol. 2004, 4, 1–45. [Google Scholar] [CrossRef] [Green Version]
- Elgersma, A. Grazing increases the unsaturated fatty acid concentration of milk from grass-fed cows: A review of the contributing factors, challenges and future perspectives. Eur. J. Lipid Sci. Technol. 2015, 117, 1345–1369. [Google Scholar] [CrossRef]
- Khan, N.A.; Cone, J.W.; Fievez, V.; Hendriks, W.H. Causes of variation in fatty acid content and composition in grass and maize silages. Anim. Feed Sci. Technol. 2012, 174, 36–45. [Google Scholar] [CrossRef]
- Hatfield, R.D.; Jung, H.J.G.; Broderick, G.; Jenkins, T.C. Nutritional chemistry of forages. In Forages: The Science of Grassland Agriculture; Blackwell Publishing: Ames, IA, USA, 2007; pp. 467–485. [Google Scholar]
- Kalac, P.; Samkova, E. The effects of feeding various forages on fatty acid composition of bovine milk fat: A review. Czech J. Anim. Sci. 2010, 55, 521–537. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.A.; Yu, P.; Ali, M.; Cone, J.W.; Hendriks, W.H. Nutritive value of maize silage in relation to dairy cow performance and milk quality. J. Sci. Food Agric. 2015, 95, 238–252. [Google Scholar] [CrossRef] [PubMed]
- Kliem, K.E.; Morgan, R.; Humphries, D.J.; Shingfield, K.J.; Givens, D.I. Effect of replacing grass silage with maize silage in the diet on bovine milk fatty acid composition. Animal 2008, 2, 1850–1858. [Google Scholar] [CrossRef] [Green Version]
- Mach, N.; Zom, R.L.G.; Widjaja, H.C.A.; van Wikselaar, P.G.; Weurding, R.E.; Goselink, R.M.A.; van Baal, J.; Smits, M.A.; van Vuuren, A.M. Dietary effects of linseed on fatty acid composition of milk and on liver, adipose and mammary gland metabolism of periparturient dairy cows. J. Anim. Physiol. Anim. Nutr. 2013, 97, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Enser, M.; Hallett, K.G.; Hewett, B.; Fursey, G.A.J.; Wood, J.D.; Harrington, G. Fatty acid content and composition of UK beef and lamb muscle in relation to production system and implications for human nutrition. Meat Sci. 1998, 49, 329–341. [Google Scholar] [CrossRef]
- Harfoot, C.G.; Hazlewood, G.P. Lipid Metabolism in the Rumen. In The Rumen Microbial Ecosystem; Hobson, P.N., Stewart, C.S., Eds.; Springer: Dordrecht, The Netherlands; Chapman and Hall: London, UK, 1997; pp. 382–426. ISBN 978-3-319-30531-8. [Google Scholar]
- Lopes, C.N.; Scarpa, A.B.; Cappellozza, B.I.; Cooke, R.F.; Vasconcelos, J.L.M. Effects of rumen-protected polyunsaturated fatty acid supplementation on reproductive performance of Bos indicus beef cows. J. Anim. Sci. 2009, 87, 3935–3943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Veth, M.J.; Bauman, D.E.; Koch, W.; Mann, G.E.; Pfeiffer, A.M.; Butler, W.R. Efficacy of conjugated linoleic acid for improving reproduction: A multi-study analysis in early-lactation dairy cows. J. Dairy Sci. 2009, 92, 2662–2669. [Google Scholar] [CrossRef] [PubMed]
- Belury, M.A. Inhibition of carcinogenesis by conjugated linoleic acid: Potential mechanisms of action. J. Nutr. 2002, 132, 2995–2998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rochfort, S.; Parker, A.J.; Dunshea, F.R. Plant bioactives for ruminant health and productivity. Phytochemistry 2008, 69, 299–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, N.A.; Farooq, M.W.; Ali, M.; Suleman, M.; Ahmad, N.; Sulaiman, S.M.; Hendriks, W.H. Effect of species and harvest maturity on the fatty acids profile of tropical forages. J. Anim. Plant Sci. 2015, 25, 739–746. [Google Scholar]






| pH | Organic Matter (%) | N (%) | P ppm | K ppm | Na ppm | Zn ppm | Mn ppm | S ppm | Fe ppm | Ca ppm | Mg ppm |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2016 | |||||||||||
| 6.4 | 2.98 | 0.2 | 81 | 38 | 7 | 0.6 | 18 | 14 | 150 | 1256 | 265 |
| 2017 | |||||||||||
| 6.8 | 3.38 | 0.2 | 68 | 35 | 5 | 1.0 | 10 | 17 | 233 | 1426 | 322 |
| Growing Season 2016 | Growing Season 2017 | |||||
|---|---|---|---|---|---|---|
| Month | Rainfall (mm) | Tmax (°C) | Tmin (°C) | Rainfall (mm) | Tmax (°C) | Tmin (°C) |
| May (15–30) | 47 | 16.3 | 2.8 | 50 | 13.5 | −0.1 |
| June | 148 | 19.1 | 6.1 | 80 | 18.4 | 6.0 |
| July | 82 | 23.0 | 9.5 | 41 | 23.8 | 8.5 |
| August | 139 | 22.4 | 10 | 85 | 22.9 | 8.1 |
| September | 133 | 16.3 | 5.4 | 156 | 17.2 | 5.2 |
| October (1–25) | 182 | 13.0 | 0.8 | 78 | 12.6 | 1.6 |
| 731 | 18.3 | 5.7 | 490 | 18.1 | 4.9 | |
| Steps | Ramp (mm:ss) | Temperature (°C) | Hold (mm:ss) |
|---|---|---|---|
| 1 | 10:00 | 140 | 5:00 |
| 2 | 1:00 | 160 | 15:00 |
| Growing Seasons (2016–17) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatments | CP (%DM) | AP (%DM) | SP (%CP) | ADF (%DM) | NDF (%DM) | WSC (%DM) | SS (%DM) | Ash (%DM) |
| C1 | 11.1 ± 0.30 de | 10.3 ± 0.29 ef | 39.8 ± 1.48 cdef | 37.7 ± 1.83 a | 57.7 ± 0.61 a | 13.9 ± 0.66 b | 6.1 ± 1.04 d | 6.8 ± 1.41 de |
| C2 | 10.3 ± 0.21 e | 9.7 ± 0.17 f | 40.8 ± 1.74 bcde | 36.8 ± 1.18 ab | 57.7 ± 0.16 a | 17.3 ± 1.49 a | 7.5 ± 1.29 cd | 6.0 ± 0.49 e |
| S1 | 19.4 ± 1.16 a | 17.3 ± 0.92 a | 35.7 ± 1.54 f | 34.7 ± 1.36 abcde | 41.4 ± 2.64 f | 7.0 ± 0.53 d | 6.0 ± 0.47 d | 14.0 ± 1.48 ab |
| S2 | 18.1 ± 0.74 ab | 15.9 ± 0.42 b | 38.5 ± 1.78 def | 35.1 ± 0.98 abcd | 43.1 ± 2.07 ef | 7.7 ± 0.33 d | 5.7 ± 0.80 d | 12.9 ± 1.14 bc |
| S3 | 17.6 ± 0.51 b | 14.9 ± 0.50 b | 36.2 ± 3.53 ef | 35.6 ± 1.01 abc | 47.3 ± 1.39 de | 5.4 ± 0.86 d | 5.9 ± 1.14 d | 17.9 ± 2.60 a |
| S1C1 | 12.5 ± 0.21 cd | 11.3 ± 0.21 de | 46.8 ± 1.47 a | 33.6 ± 1.11 bcde | 55.4 ± 0.97 ab | 11.3 ± 0.76 c | 10.2 ± 0.36 ab | 10.1 ± 1.44 bcd |
| S2C1 | 13.1 ± 0.24 cd | 11.9 ± 0.19 cd | 44.3 ± 0.80 abc | 33.1 ± 0.94 cdef | 55.4 ± 1.01 ab | 11.6 ± 1.05 c | 10.5 ± 0.24 ab | 8.8 ± 0.91 cde |
| S3C1 | 12.4 ± 0.30 cd | 11.7 ± 0.21 cd | 47.8 ± 1.01 a | 32.5 ± 0.79 cdef | 56.3 ± 1.02 ab | 12.3 ± 0.99 bc | 11.4 ± 0.53 a | 8.9 ± 1.16 cde |
| S1C2 | 13.8 ± 0.38 c | 12.7 ± 0.38 c | 47.4 ± 1.04 a | 31.2 ± 1.32 ef | 52.6 ± 1.00 bc | 12.0 ± 0.56 bc | 11.0 ± 0.47 ab | 8.7 ± 1.30 de |
| S2C2 | 13.3 ± 0.48 c | 12.5 ± 0.56 cd | 41.7 ± 1.33 bcd | 29.9 ± 1.82 f | 50.9 ± 2.28 cd | 12.0 ± 0.36 bc | 9.0 ± 0.59 bc | 10.4 ± 1.73 bcd |
| S3C2 | 13.3 ± 0.28 c | 11.9 ± 0.31 cd | 45.1 ± 1.13 ab | 31.7 ± 0.98 def | 54.2 ± 1.01 abc | 12.4 ± 0.38 bc | 11.3 ± 0.30 a | 9.4 ± 1.24 cde |
| Average | 14.1 ± 0.38 | 12.7 ± 0.30 | 42.2 ± 1.70 | 33.8 ± 0.45 | 52.0 ± 0.79 | 11.2 ± 0.46 | 8.6 ± 0.35 | 10.4 ± 0.57 |
| Mono-(US) | 18.8 ± 0.68 | 16.6 ± 0.53 | 37.1 ± 1.20 | 34.9 ± 0.80 | 42.3 ± 1.62 | 7.3 ± 0.32 A | 5.9 ± 0.45 | 13.4 ± 0.91 B |
| Mono-(VS) | 17.6 ± 0.51 | 14.9 ± 0.50 | 36.2 ± 3.53 | 35.6 ± 1.01 | 47.3 ± 1.39 | 5.4 ± 0.86 B | 5.9 ± 1.14 | 17.9 ± 2.60 A |
| Inter-(US) | 13.1 ± 0.19 | 12.1 ± 0.20 | 45.1 ± 0.73 | 31.9 ± 0.69 | 53.6 ± 0.78 | 11.7 ± 0.34 | 10.2 ± 0.25 B | 9.5 ± 0.66 |
| Inter-(VS) | 12.8 ± 0.24 | 11.8 ± 0.18 | 46.4 ± 0.83 | 32.1 ± 0.61 | 55.2 ± 0.75 | 12.4 ± 0.51 | 11.4 ± 0.29 A | 9.1 ± 0.81 |
| Mono-S | 18.4 ± 0.49 A | 15.3 ± 0.40 A | 36.8 ± 1.36 C | 35.1 ± 0.62 A | 43.9 ± 1.28 C | 6.7 ± 0.41 C | 5.9 ± 0.46 B | 14.9 ± 1.13 A |
| Mono-C | 10.7 ± 0.20 C | 10.2 ± 0.33 C | 40.3 ± 1.10 B | 37.3 ± 1.02 A | 57.7 ± 0.30 B | 15.6 ± 0.93 A | 6.8 ± 0.82 B | 6.4 ± 0.72 C |
| Inter-(C + S) | 13.0 ± 0.15 B | 12.1 ± 0.28 B | 45.5 ± 0.56 A | 32.0 ± 0.50 B | 54.1 ± 0.58 A | 11.9 ± 0.29 B | 10.6 ± 0.21 A | 9.4 ± 0.51 B |
| Growing Seasons (2016–17) | |||||||
|---|---|---|---|---|---|---|---|
| Treatments | TDN % | NEL (Mcal/kg) | NEM (Mcal/kg) | NEG (Mcal/kg) | DDM % | DMI 3 | RFV % |
| C1 | 55.6 ± 1.9 ab | 1.17 ± 0.04 bcd | 1.08 ± 0.05 ab | 0.53 ± 0.05 ab | 59.5 ± 1.37 f | 2.06 ± 0.02 d | 95 ± 2.8 f |
| C2 | 54.2 ± 1.5 b | 1.13 ± 0.04 cd | 1.14 ± 0.05 b | 0.48 ± 0.05 b | 60.2 ± 0.92 ef | 2.08 ± 0.00 d | 97 ± 1.5 f |
| S1 | 56.7 ± 1.9 ab | 1.30 ± 0.03 a | 1.17 ± 0.04 ab | 0.60 ± 0.03 ab | 61.9 ± 1.06 bcdef | 2.99 ± 0.17 a | 144 ± 10.1 a |
| S2 | 54.7 ± 1.4 ab | 1.30 ± 0.03 a | 1.14 ± 0.04 ab | 0.59 ± 0.03 ab | 61.6 ± 0.77 cdef | 2.80 ± 0.08 ab | 134 ± 5.6 ab |
| S3 | 47.8 ± 2.0 c | 1.08 ± 0.05 d | 0.86 ± 0.07 c | 0.32 ± 0.07 c | 61.2 ± 0.78 def | 2.64 ± 0.04 b | 125 ± 3.4 bc |
| S1C1 | 59.8 ± 1.7 a | 1.27 ± 0.04 ab | 1.22 ± 0.07 a | 0.64 ± 0.06 ab | 62.8 ± 0.86 bcde | 2.18 ± 0.03 d | 106 ± 3.0 ef |
| S2C1 | 59.2 ± 2.0 ab | 1.26 ± 0.04 ab | 1.22 ± 0.06 a | 0.65 ± 0.05 a | 63.1 ± 0.73 abcd | 2.20 ± 0.03 d | 108 ± 2.6 ef |
| S3C1 | 59.7 ± 1.3 ab | 1.22 ± 0.03 abc | 1.21 ± 0.05 ab | 0.64 ± 0.04 ab | 63.6 ± 0.61 abcd | 2.10 ± 0.04 d | 104 ± 2.8 ef |
| S1C2 | 60.2 ± 2.4 a | 1.26 ± 0.05 ab | 1.22 ± 0.08 a | 0.66 ± 0.08 a | 64.6 ± 1.03 ab | 2.25 ± 0.02 cd | 113 ± 2.5 cde |
| S2C2 | 60.2 ± 2.9 a | 1.27 ± 0.05 ab | 1.23 ± 0.07 a | 0.66 ± 0.07 a | 65.6 ± 1.42 a | 2.41 ± 0.10 c | 123 ± 7.7 bcd |
| S3C2 | 59.2 ± 2.1 ab | 1.24 ± 0.05 abc | 1.19 ± 0.07 ab | 0.64 ± 0.07 ab | 64.2 ± 0.76 abc | 2.23 ± 0.05 cd | 111 ± 3.5 de |
| Average | 57.0 ± 0.7 | 1.22 ± 0.01 | 1.14 ± 0.02 | 0.58 ± 0.02 | 62.6 ± 0.35 | 2.36 ± 0.04 | 115 ± 2.3 |
| Mono-(US) | 55.7 ± 1.2 A | 1.30 ± 0.02 A | 1.15 ± 0.03 A | 0.59 ± 0.02 A | 61.7 ± 0.62 | 2.90 ± 0.09 | 139 ± 5.7 |
| Mono-(VS) | 47.8 ± 2.0 B | 1.08 ± 0.05 B | 0.86 ± 0.07 B | 0.32 ± 0.07 B | 61.2 ± 0.78 | 2.64 ± 0.04 | 125 ± 4.7 |
| Inter-(US) | 59.8 ± 1.1 | 1.26 ± 0.02 | 1.22 ± 0.03 | 0.65 ± 0.03 | 64.0 ± 0.54 | 2.26 ± 0.03 | 112 ± 2.5 |
| Inter-(VS) | 59.4 ± 1.2 | 1.23 ± 0.03 | 1.20 ± 0.04 | 0.64 ± 0.04 | 63.9 ± 0.48 | 2.17 ± 0.03 | 107 ± 2.4 |
| Mono-S | 53.1 ± 1.3 B | 1.22 ± 0.03 AB | 1.05 ± 0.04 B | 0.50 ± 0.04 B | 61.5 ± 0.48 B | 2.81 ± 0.07 A | 135 ± 4.5 A |
| Mono-C | 54.9 ± 1.2 B | 1.15 ± 0.03 B | 1.06 ± 0.04 B | 0.51 ± 0.03 B | 59.9 ± 0.79 B | 2.07 ± 0.01 C | 96 ± 1.2 C |
| Inter-(C + S) | 59.7 ± 0.8 A | 1.25 ± 0.02 A | 1.21 ± 0.02 A | 0.65 ± 0.02 A | 64.0 ± 0.39 A | 2.23 ± 0.03 B | 111 ± 1.9 B |
| Growing Seasons (2016–17) | |||||
|---|---|---|---|---|---|
| Treatments | Ca (g/kg) | P (g/kg) | Mg (g/kg) | K (g/kg) | Na (mg/kg) |
| C1 | 0.14 ± 0.03 e | 2.10 ± 0.16 e | 2.93 ± 0.19 ef | 13.0 ± 1.12 cd | 26.5 ± 8.69 de |
| C2 | 0.16 ± 0.03 e | 2.14 ± 0.15 e | 2.64 ± 0.15 f | 9.4 ± 0.88 e | 13.4 ± 3.12 e |
| S1 | 1.42 ± 0.05 b | 3.61 ± 0.11 a | 7.07 ± 0.11 a | 16.8 ± 1.11 ab | 51.7 ± 8.50 ab |
| S2 | 1.60 ± 0.08 a | 3.55 ± 0.07 ab | 7.02 ± 0.28 a | 17.5 ± 1.19 a | 59.2 ± 4.19 a |
| S3 | 1.01 ± 0.04 c | 2.85 ± 0.39 cd | 6.27 ± 0.14 b | 12.3 ± 0.95 cde | 54.3 ± 6.36 ab |
| S1C1 | 0.37 ± 0.06 d | 2.38 ± 0.16 de | 3.29 ± 0.31 def | 14.2 ± 1.12 bc | 41.8 ± 7.02 bcd |
| S2C1 | 0.43 ± 0.04 d | 2.76 ± 0.25 cd | 3.55 ± 0.13 de | 13.7 ± 0.89 bcd | 51.4 ± 7.00 ab |
| S3C1 | 0.45 ± 0.03 d | 2.62 ± 0.13 cde | 4.28 ± 0.16 c | 14.6 ± 0.77 abc | 41.2 ± 4.22 bcd |
| S1C2 | 0.36 ± 0.06 d | 2.71 ± 0.17 cd | 3.66 ± 0.43 cd | 14.0 ± 2.14 bc | 31.1 ± 3.44 cd |
| S2C2 | 0.45 ± 0.03 d | 3.04 ± 0.08 bc | 3.72 ± 0.31 cd | 13.7 ± 0.88 bcd | 46.8 ± 2.88 abc |
| S3C2 | 0.40 ± 0.03 d | 2.82 ± 0.13 cd | 3.51 ± 0.19 de | 10.5 ± 0.84 de | 40.7 ± 4.25 bcd |
| Average | 0.62 ± 0.06 | 2.78 ± 0.08 | 4.36 ± 0.20 | 13.6 ± 0.42 | 41.7 ± 2.27 |
| Mono-(US) | 1.51 ± 0.05 B | 3.58 ± 0.06 A | 7.05 ± 0.14 A | 17.2 ± 0.78 A | 55.5 ± 4.66 |
| Mono-(VS) | 1.01 ± 0.04 A | 2.85 ± 0.39 B | 6.27 ± 0.14 B | 12.3 ± 0.95 B | 54.3 ± 6.36 |
| Inter-(US) | 0.40 ± 0.02 | 2.72 ± 0.10 | 3.55 ± 0.15 | 13.9 ± 0.64 | 42.8 ± 2.98 |
| Inter-(VS) | 0.43 ± 0.02 | 2.72 ± 0.09 | 3.89 ± 0.16 | 12.6 ± 0.82 | 41.0 ± 2.86 |
| Mono-S | 1.34 ± 0.07 A | 3.33 ± 0.15 A | 6.79 ± 0.14 A | 15.5 ± 0.82 A | 55.1 ± 3.65 A |
| Mono-C | 0.15 ± 0.02 C | 2.12 ± 0.11 B | 2.79 ± 0.12 C | 11.2 ± 0.87 C | 20.0 ± 4.82 C |
| Inter-(C + S) | 0.41 ± 0.02 B | 2.72 ± 0.07 A | 3.67 ± 0.12 B | 13.5 ± 0.51 B | 42.2 ± 2.19 B |
| Growing Seasons (2016–17) | ||||||
|---|---|---|---|---|---|---|
| Treatments | Zn (mg/kg) | Fe (mg/kg) | B (mg/kg) | Mn (mg/kg) | Cu (mg/kg) | Co (mg/kg) |
| C1 | 17.5 ± 0.57 cde | 307 ± 51.3 b | 5.2 ± 0.56 e | 74.6 ± 20.3 cde | 10.1 ± 1.86 cde | 0.92 ± 0.37 ab |
| C2 | 10.8 ± 1.23 e | 247 ± 54.2 b | 5.5 ± 0.70 e | 56.9 ± 13.5 e | 7.3 ± 1.49 e | 0.36 ± 0.14 b |
| S1 | 35.4 ± 1.71 a | 1092 ± 213.6 a | 29.5 ± 1.85 a | 206.0 ± 43.7 a | 14.7 ± 1.58 abc | 1.85 ± 0.54 a |
| S2 | 17.1 ± 1.97 de | 1244 ± 318.3 a | 32.1 ± 1.13 a | 142.4 ± 8.9 abc | 16.8 ± 1.14 a | 1.07 ± 0.16 ab |
| S3 | 31.4 ± 1.90 ab | 963 ± 92.5 a | 22.8 ± 1.56 b | 164.9 ± 2.6 ab | 16.4 ± 2.58 ab | 1.93 ± 0.75 a |
| S1C1 | 26.5 ± 6.86 abcd | 474 ± 74.3 b | 10.2 ± 1.69 cd | 83.3 ± 18.8 cde | 14.1 ± 3.38 abcd | 0.97 ± 0.39 ab |
| S2C1 | 26.6 ± 2.96 abc | 386 ± 39.2 b | 9.0 ± 0.56 cd | 85.6 ± 14.0 cde | 12.0 ± 1.25 abcde | 0.78 ± 0.24 ab |
| S3C1 | 19.6 ± 1.07 cde | 377 ± 36.6 b | 7.2 ± 0.74 de | 128.8 ± 35.4 bcd | 11.5 ± 1.77 bcde | 1.25 ± 0.49 ab |
| S1C2 | 18.7 ± 0.55 cde | 372 ± 42.3 b | 7.6 ± 0.38 de | 58.4 ± 8.3 de | 9.0 ± 1.50 de | 0.50 ± 0.18 b |
| S2C2 | 25.4 ± 2.68 bcd | 524 ± 60.3 b | 11.0 ± 0.50 c | 119.8 ± 25.0 bcde | 9.4 ± 1.10 de | 1.47 ± 0.53 ab |
| S3C2 | 33.6 ± 6.59 ab | 495 ± 26.8 b | 9.2 ± 0.69 cd | 93.8 ± 11.1 bcde | 10.9 ± 0.83 cde | 0.77 ± 0.24 ab |
| Average | 23.9 ± 1.30 | 589 ± 53.6 | 13.6 ± 1.19 | 110.4 ± 8.8 | 12.0 ± 0.62 | 1.08 ± 0.13 |
| Mono-(US) | 26.2 ± 3.03 | 1168 ± 184.2 | 30.8 ± 1.11 A | 174.2 ± 23.4 | 15.8 ± 0.98 | 1.46 ± 0.29 |
| Mono-(VS) | 31.4 ± 1.90 | 963 ± 92.5 | 22.8 ± 1.56 B | 164.9 ± 39.3 | 16.4 ± 2.58 | 1.93 ± 0.75 |
| Inter-(US) | 24.3 ± 1.98 | 439 ± 29.1 | 9.5 ± 0.51 | 86.8 ± 9.4 | 11.1 ± 1.04 | 0.93 ± 0.18 |
| Inter-(VS) | 26.6 ± 3.82 | 436 ± 28.0 | 8.2 ± 0.57 | 111.3 ± 18.5 | 11.2 ± 0.94 | 1.01 ± 0.27 |
| Mono-S | 27.9 ± 2.16 A | 1099 ± 126.6 A | 28.2 ± 1.26 A | 171.1 ± 19.7 A | 16.0 ± 1.03 A | 1.62 ± 0.31 A |
| Mono-C | 14.1 ± 1.21 B | 277 ± 36.7 B | 5.4 ± 0.43 C | 65.7 ± 11.9 B | 8.7 ± 1.21 B | 0.64 ± 0.21 B |
| Inter-(C + S) | 25.1 ± 1.81 A | 438 ± 21.3 B | 9.0 ± 0.40 B | 95.0 ± 8.8 B | 11.1 ± 0.75 B | 0.96 ± 0.15 B |
| Growing Seasons (2016–17) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | C16-0 | C16-1n-7 | C18-0 | C18-1n-9cis | C18-2n-6 | C18:3n-3 | Total FA | SFA % | MUFA % | PUFA % |
| C1 | 1.73 ± 0.03 f | 0.12 ± 0.03 b | 0.15 ± 0.02 c | 0.98 ± 0.11 cd | 2.54 ± 0.48 ab | 0.46 ± 0.03 d | 5.98 ± 0.33 d | 31.8 ± 1.58 cd | 19.2 ± 3.38 ab | 49.0 ± 4.93 |
| C2 | 1.75 ± 0.08 ef | 0.12 ± 0.03 b | 0.17 ± 0.03 c | 0.93 ± 0.09 cd | 2.90 ± 0.47 a | 0.50 ± 0.01 cd | 6.37 ± 0.25 cd | 30.3 ± 1.34 d | 16.9 ± 2.52 ab | 52.9 ± 3.63 |
| S1 | 2.30 ± 0.10 a | 0.42 ± 0.04 a | 0.47 ± 0.04 a | 0.66 ± 0.12 d | 1.84 ± 0.19 b | 1.84 ± 0.09 a | 7.53 ± 0.37 ab | 36.8 ± 0.69 a | 15.0 ± 2.73 b | 48.2 ± 3.04 |
| S2 | 1.98 ± 0.06 bc | 0.38 ± 0.04 a | 0.40 ± 0.05 ab | 0.50 ± 0.07 d | 1.59 ± 0.11 b | 1.58 ± 0.04 a | 6.43 ± 0.30 cd | 37.0 ± 0.21 a | 14.2 ± 2.31 b | 48.8 ± 2.29 |
| S3 | 1.81 ± 0.04 def | 0.38 ± 0.09 a | 0.35 ± 0.02 b | 0.55 ± 0.05 d | 1.63 ± 0.32 b | 1.55 ± 0.05 a | 6.27 ± 0.43 cd | 35.4 ± 2.64 ab | 15.9 ± 3.31 ab | 48.7 ± 5.92 |
| S1C1 | 1.78 ± 0.05 def | 0.17 ± 0.04 b | 0.19 ± 0.02 c | 1.26 ± 0.11 bc | 2.36 ± 0.43 ab | 0.64 ± 0.01 bcd | 6.39 ± 0.30 cd | 31.1 ± 1.12 cd | 23.1 ± 3.31 ab | 45.8 ± 4.40 |
| S2C1 | 1.87 ± 0.04 cdef | 0.17 ± 0.04 b | 0.22 ± 0.03 c | 1.36 ± 0.23 abc | 2.47 ± 0.38 ab | 0.75 ± 0.03 bcd | 6.84 ± 0.09 bc | 30.6 ± 0.68 cd | 22.6 ± 4.12 ab | 46.8 ± 4.72 |
| S3C1 | 1.82 ± 0.05 cdef | 0.15 ± 0.03 b | 0.23 ± 0.01 c | 0.96 ± 0.15 cd | 2.03 ± 0.29 ab | 0.91 ± 0.01 b | 6.10 ± 0.25 cd | 33.9 ± 0.79 abc | 18.9 ± 3.59 ab | 47.3 ± 4.29 |
| S1C2 | 2.05 ± 0.04 b | 0.20 ± 0.05 b | 0.23 ± 0.03 c | 1.85 ± 0.34 a | 2.56 ± 0.30 ab | 0.78 ± 0.03 bcd | 7.67 ± 0.18 a | 29.7 ± 0.27 d | 26.2 ± 4.45 a | 44.1 ± 4.68 |
| S2C2 | 1.89 ± 0.03 bcde | 0.14 ± 0.03 b | 0.20 ± 0.02 c | 1.38 ± 0.18 abc | 1.96 ± 0.30 ab | 0.84 ± 0.01 bc | 6.41 ± 0.17 cd | 32.7 ± 0.92 bcd | 24.1 ± 3.71 ab | 43.2 ± 4.54 |
| S3C2 | 1.94 ± 0.05 bcd | 0.17 ± 0.04 b | 0.21 ± 0.03 c | 1.57 ± 0.31 ab | 2.51 ± 0.44 ab | 0.92 ± 0.01 b | 7.32 ± 0.12 ab | 29.5 ± 1.31 d | 24.1 ± 5.01 ab | 46.4 ± 6.29 |
| Average | 1.90 ± 0.03 | 0.22 ± 0.03 | 0.26 ± 0.01 | 1.09 ± 0.07 | 2.22 ± 0.11 | 0.98 ± 0.09 | 6.67 ± 0.10 | 32.6 ± 0.47 | 20.0 ± 1.11 | 47.4 ± 1.30 |
| Mono-(US) | 2.14 ± 0.07 A | 0.40 ± 0.09 | 0.44 ± 0.03 | 0.58 ± 0.07 | 1.72 ± 0.11 | 1.71 ± 0.14 | 6.98 ± 0.28 | 36.9 ± 0.35 | 14.6 ± 1.71 | 48.5 ± 1.82 |
| Mono-(VS) | 1.81 ± 0.04 B | 0.38 ± 0.01 | 0.35 ± 0.02 | 0.55 ± 0.05 | 1.63 ± 0.32 | 1.55 ± 0.26 | 6.27 ± 0.43 | 35.4 ± 2.64 | 15.9 ± 3.31 | 48.7 ± 5.92 |
| Inter-(US) | 1.90 ± 0.03 | 0.17 ± 0.02 | 0.21 ± 0.01 | 1.46 ± 0.12 | 2.34 ± 0.17 | 0.75 ± 0.02 B | 6.83 ± 0.14 | 31.0 ± 0.44 | 24.0 ± 1.85 | 45.0 ± 2.16 |
| Inter-(VS) | 1.88 ± 0.04 | 0.16 ± 0.02 | 0.22 ± 0.02 | 1.27 ± 0.19 | 2.27 ± 0.26 | 0.92 ± 0.06 A | 6.71 ± 0.23 | 31.7 ± 0.98 | 21.5 ± 3.04 | 46. ± 3.63 |
| Mono-S | 2.03 ± 0.06 A | 0.39 ± 0.03 A | 0.41 ± 0.02 A | 0.57 ± 0.05 C | 1.69 ± 0.12 B | 1.66 ± 0.13 A | 6.74 ± 0.24 AB | 36.4 ± 0.88 A | 15.0 ± 1.53 B | 48.6 ± 2.20 |
| Mono-C | 1.74 ± 0.04 C | 0.12 ± 0.02 B | 0.16 ± 0.02 C | 0.95 ± 0.13 B | 2.72 ± 0.30 A | 0.48 ± 0.02 C | 6.17 ± 0.20 B | 31.0 ± 1.02 B | 18.1 ± 2.04 AB | 50.9 ± 2.98 |
| Inter-(C + S) | 1.89 ± 0.02 B | 0.17 ± 0.01 B | 0.21 ± 0.01 B | 1.40 ± 0.10 A | 2.31 ± 0.14 A | 0.81 ± 0.03 B | 6.79 ± 0.12 A | 31.3 ± 0.44 B | 23.2 ± 1.59 A | 45.6 ± 1.86 |
| C16:0 | C16:1n7 | C18:0 | C18:1n9 | C18:2n-6 | C18:3n-3 | TFA | |
|---|---|---|---|---|---|---|---|
| CP | 0.62 *** | 0.64 *** | 0.86 *** | −0.36 ** | −0.35 ** | 0.86 *** | 0.31 * |
| AP | 0.65 *** | 0.64 *** | 0.83 *** | −0.31 * | −0.37 ** | 0.82 *** | 0.31 * |
| SP | −0.15 ns | −0.46 *** | −0.19 ns | 0.50 *** | 0.05 ns | −0.20 ns | 0.13 ns |
| ADF | −0.28 * | 0.49 *** | 0.19 ns | 0.09 ns | −0.55 *** | −0.14 ns | −0.55 *** |
| NDF | −0.62 *** | −0.38 ** | −0.71 *** | 0.53 *** | 0.07 ns | −0.86 *** | −0.41 *** |
| WSC | −0.40 *** | −0.54 *** | −0.47 *** | 0.44 *** | 0.14 ns | −0.58 *** | −0.17 ns |
| SS | −0.09 ns | −0.45 *** | −0.20 ns | 0.49 *** | 0.08 ns | −0.18 ns | 0.18 ns |
| Ash | 0.10 ns | 0.89 *** | 0.52 *** | 0.07 ns | −0.81 *** | 0.26 * | −0.37 ** |
| TDN | 0.20 ns | −0.68 *** | −0.34 ** | −0.12 ns | 0.70 *** | −0.03 ns | 0.52 *** |
| NEL | 0.40 *** | −0.43 *** | −0.02 ns | −0.37 ** | 0.61 *** | 0.31 * | 0.60 *** |
| NEM | 0.25 * | −0.62 *** | −0.26 * | −0.19 ns | 0.69 *** | 0.06 ns | 0.54 *** |
| NEG | 0.24 * | −0.62 *** | −0.26 * | −0.19 ns | 0.69 *** | 0.07 ns | 0.54 *** |
| DDM | 0.28 * | −0.49 *** | −0.19 ns | −0.10 ns | 0.55 *** | 0.14 ns | 0.55 *** |
| DMI | 0.64 *** | 0.44 *** | 0.77 *** | −0.54 *** | −0.11 ns | 0.90 *** | 0.41 *** |
| RFV | 0.65 *** | 0.26 * | 0.65 *** | -0.51 *** | 0.05 ns | 0.86 *** | 0.53 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaeem, M.; Nadeem, M.; Pham, T.H.; Ashiq, W.; Ali, W.; Gillani, S.S.M.; Moise, E.; Elavarthi, S.; Kavanagh, V.; Cheema, M.; et al. Corn-Soybean Intercropping Improved the Nutritional Quality of Forage Cultivated on Podzols in Boreal Climate. Plants 2021, 10, 1015. https://doi.org/10.3390/plants10051015
Zaeem M, Nadeem M, Pham TH, Ashiq W, Ali W, Gillani SSM, Moise E, Elavarthi S, Kavanagh V, Cheema M, et al. Corn-Soybean Intercropping Improved the Nutritional Quality of Forage Cultivated on Podzols in Boreal Climate. Plants. 2021; 10(5):1015. https://doi.org/10.3390/plants10051015
Chicago/Turabian StyleZaeem, Muhammad, Muhammad Nadeem, Thu Huong Pham, Waqar Ashiq, Waqas Ali, Syed Shah Mohioudin Gillani, Eric Moise, Sathya Elavarthi, Vanessa Kavanagh, Mumtaz Cheema, and et al. 2021. "Corn-Soybean Intercropping Improved the Nutritional Quality of Forage Cultivated on Podzols in Boreal Climate" Plants 10, no. 5: 1015. https://doi.org/10.3390/plants10051015
APA StyleZaeem, M., Nadeem, M., Pham, T. H., Ashiq, W., Ali, W., Gillani, S. S. M., Moise, E., Elavarthi, S., Kavanagh, V., Cheema, M., Galagedara, L., & Thomas, R. (2021). Corn-Soybean Intercropping Improved the Nutritional Quality of Forage Cultivated on Podzols in Boreal Climate. Plants, 10(5), 1015. https://doi.org/10.3390/plants10051015