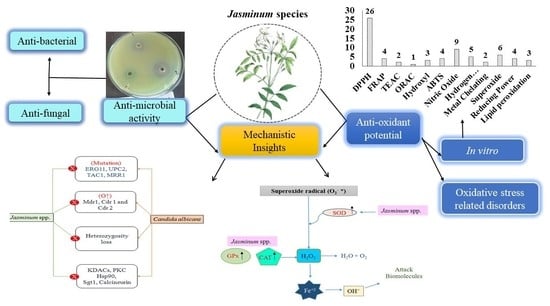
Mechanistic Insight into Antimicrobial and Antioxidant Potential of Jasminum Species: A Herbal Approach for Disease Management
Abstract
:1. Introduction
2. Search Strategy
3. Distribution of Genus Jasminum
4. Antimicrobial Profile of Jasminum spp.
5. Role of Jasminum Plants in Combating Resistance
5.1. Bacterial Antibiotic Resistance
5.2. Antibiotic Resistance in Fungi
5.3. Protective Role of Jasminum Species
6. Antioxidant Potential of Jasminum spp.
7. Oxidative Stress Related Diseases
8. Impact of Jasminum Plants against Oxidative Stress In Vivo
9. Mechanistic Basis of ROS Neutralization
10. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. WHO Methods and Data Sources for Global Burden of Disease Estimates 2000–2011; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- Gupta, M.; Sharma, R.; Kumar, A. Comparative potential of Simvastatin, Rosuvastatin and Fluvastatin against bacterial infection: An in silico and in vitro study. Orient. Pharm. Exp. Med. 2019, 19, 259–275. [Google Scholar] [CrossRef]
- WHO. WHO Antimicrobial Resistance: Global Report on Surveillance; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Baym, M.; Stone, L.K.; Kishony, R. Multidrug evolutionary strategies to reverse antibiotic resistance. Science 2016, 351, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. WHO Traditional Medicine Strategy; WHO: Geneva, Switzerland, 2002. [Google Scholar]
- Djeussi, D.E.; Noumedem, J.A.K.; Seukep, J.A.; Fankam, A.G.; Voukeng, I.K.; Tankeo, S.B.; Nkuete, A.H.L.; Kuete, V. Antibacterial activities of selected edible plants extracts against multidrug-resistant Gram-negative bacteria. BMC Complement. Altern. Med. 2013, 13, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duraipandiyan, V.; Ayyanar, M.; Ignacimuthu, S. Antimicrobial activity of some ethnomedicinal plants used by Paliyar tribe from Tamil Nadu, India. BMC Complement. Altern. Med. 2006, 6, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwu, M.W.; Duncan, A.R.; Okunji, C.O. New antimicrobials of plant origin. In Perspectives on New Crops and New Uses; Janick, J., Ed.; ASHS Press: Alexandria, VA, USA, 1999; pp. 457–462. [Google Scholar]
- Kumari, A.; Verma, R.; Sharma, M.; Chauhan, P.; Kumar, A. Evaluation of phytochemical, antioxidant, antibacterial and anti-cancerous activity of Ficus auriculata Lour. and Osyris wightiana Wall. ex Wight. Bull. Environ. Pharmacol. Life Sci. 2018, 7, 64–70. [Google Scholar]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Ind. J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, J.; Das, A.; Sinha, M.; Saha, S. Biological Efficacy of Medicinal Plant Extracts in Preventing Oxidative Damage. Oxidative Med. Cell. Longev. 2018, 2018, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Antioxidants and Human Disease: A General Introduction. Nutr. Rev. 1997, 55, S44–S49. [Google Scholar] [CrossRef]
- Khan, F.; Garg, V.K.; Singh, A.K.; Kumar, T. Role of free radicals and certain antioxidants in the management of huntington’s disease: A review. J. Anal. Pharm. Res. 2018, 7, 386–392. [Google Scholar] [CrossRef] [Green Version]
- Parmar, J.; Sharma, P.; Verma, P.; Goyal, P.K. Chemopreventive action of Syzygium cumini on DMBA-induced skin papillomagenesis in mice. Asian Pac. J. Cancer Prev. 2010, 11, 261–265. [Google Scholar]
- Kumar, A.; Kumar, D. Development of antioxidant rich fruit supplemented probiotic yogurts using free and microencapsulated Lactobacillus rhamnosus culture. J. Food Sci. Technol. 2016, 53, 667–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raghuvanshi, D.; Dhalaria, R.; Sharma, A.; Kumar, D.; Kumar, H.; Valis, M.; Kuča, K.; Verma, R.; Puri, S. Ethnomedicinal Plants Traditionally Used for the Treatment of Jaundice (Icterus) in Himachal Pradesh in Western Himalaya—A Review. Plants 2021, 10, 232. [Google Scholar] [CrossRef]
- Priya, J.; Raja, D.P. Anti-bacterial activity studies of Jasminum grandiflorum and Jasminum sambac. Ethnobot. Leafl. 2008, 12, 481–483. [Google Scholar]
- KewScience-Plants of the World Online Home Page. Available online: http://www.plantsoftheworldonline.org/ (accessed on 10 January 2021).
- Ali, S.T.; Ayub, A.; Ali, S.N. Antibacterial activity of methanolic extracts from some selected medicinal plants. FUUAST J. Biol. 2017, 7, 123–125. [Google Scholar]
- Prakkash, M.J.; Ragunathan, R.; Jesteena, J. Evaluation of bioactive compounds from Jasminum polyanthum and its medicinal properties. J. Drug Deliv. Ther. 2019, 9, 303–310. [Google Scholar] [CrossRef] [Green Version]
- Rani, B.; Yadav, M.; Pachauri, G. Awesome medicinal benefits of jasmine plant. J. Biol. Chem. Res. 2017, 34, 918–922. [Google Scholar]
- Upaganlawar, A.B.; Bhagat, A.; Tenpe, C.R.; Yeole, P.G. Effect of Jasminum sambac leaves extracts on serum glucose and lipid profile rats treated with alloxan. Pharmacologyonline 2003, 1, 1–6. [Google Scholar]
- Lis-Balchin, M.; Hart, S.; Lo, W.H. Jasmine absolute (Jasminum grandiflora L.) and its mode of action on guinea pig ileum in vitro. Phytother. Res. 2002, 16, 437–439. [Google Scholar] [CrossRef]
- Kunhachan, P.; Banchonglikitkul, C.; Kajsongkram, T. Chemical composition, toxicity and vasodilatation effect of the flowers extract of Jasminum sambac (L.) Ait. “G. Duke of Tuscany”. Evid. Based Complement. Alter. Med. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.H.; Yadava, P.S. Ethno Medicinal Plants of Manipur, North-East India (Thoubal District); Bishen Singh Mahendra Pal Singh: Dehradun, India, 2014; pp. 242–261. [Google Scholar]
- Bhutya, R.K. Ayurvedic Medicinal Plant of India; Scientific Publishers: Jodhpur, India, 2011; Volume 1, pp. 253–254. [Google Scholar]
- Geyid, A.; Abebe, D.; Debella, A.; Makonnen, Z.; Aberra, F.; Teka, F.; Kebede, T.; Urga, K.; Yersaw, K.; Biza, T.; et al. Screening of some medicinal plants of Ethiopia for their anti-microbial properties and chemical profiles. J. Ethnopharmacol. 2005, 97, 421–427. [Google Scholar] [CrossRef]
- Lulekal, E.; Rondevaldova, J.; Bernaskova, E.; Cepkova, J.; Asfaw, Z.; Kelbessa, E.; Kokoska, L.; van Damme, P. Antimicrobial activity of traditional medicinal plants from Ankober District, North Shewa Zone, Amhara Region, Ethiopia. Pharm. Biol. 2014, 52, 614–620. [Google Scholar] [CrossRef] [Green Version]
- Ramya, V.; Dhayalan, V.D.; Umamaheswari, S. In vitro studies on antibacterial activity and separation of active compounds of selected flower extracts by HPTLC. J. Chem. Pharm. Res. 2010, 2, 86–91. [Google Scholar]
- Moe, T.S.; Win, H.H.; Hlaing, T.T.; Lwin, W.W.; Htet, Z.M.; Mya, K.M. Evaluation of in vitro antioxidant, antiglycation and antimicrobial potential of indigenous Myanmar medicinal plants. J. Integr. Med. 2018, 16, 358–366. [Google Scholar] [CrossRef]
- Abhipsa, V.; Manasa, M.; Poornima, G.; Rekha, C.; Kekuda, T.R. In vitro antibacterial efficacy of selected plant extracts, streptomycin and their combination. Asian J. Res. Chem. 2012, 5, 791–793. [Google Scholar]
- Mittal, A.; Satish, S.; Anima, P. Evaluation of wound healing, antioxidant and antimicrobial efficacy of Jasminum auriculatum Vahl. leaves. Avicenna J. Phytomed. 2016, 6, 295–304. [Google Scholar]
- Thiruvengadam, S.; Nivedha, S.; Pujita, V.; Romauld, S.I. Detection of Antioxidant and Antimicrobial Activity of Leaf Extract of Jasminum azoricum. Res. J. Pharm. Technol. 2018, 11, 3629–3632. [Google Scholar] [CrossRef]
- SyamSree, K.; Anudeep, M.; Ramana, C.V.; Bhaskar, C. Screening of antimicrobial activity of flower extracts on human bacterial pathogens. J. Pharmacog. Phytochem. 2015, 3, 153–156. [Google Scholar]
- Anoopkumar, A.N.; Aneesh, E.M.; Sudhikumar, A.V. Exploring the mode of action of isolated bioactive compounds by induced reactive oxygen species generation in Aedes aegypti: A microbes based double-edged weapon to fight against Arboviral diseases. Int. J. Trop. Insect Sci. 2020, 40, 573–585. [Google Scholar] [CrossRef]
- Mamba, P.; Adebayo, S.A.; Tshikalange, T.E. Anti-microbial, anti-inflammatory and HIV-1 reverse transcriptase activity of selected South African plants used to treat sexually transmitted diseases. Int. J. Pharmacog. Phytochem. Res. 2016, 8, 1870–1876. [Google Scholar]
- Nagarajappa, R.; Batra, M.; Sharda, A.J.; Asawa, K.; Sanadhya, S.; Daryani, H.; Ramesh, G. Antimicrobial Effect of Jasminum grandiflorum L. and Hibiscus rosa-sinensis L. Extracts Against Pathogenic Oral Microorganisms—An In Vitro Comparative Study. Oral Health Prev. Dent. 2013, 13, 441–448. [Google Scholar]
- Rahman, M.; Khatun, A.; Khan, S.; Hossain, F.; Khan, A.A. Phytochemical, cytotoxic and antibacterial activity of two medicinal plants of Bangladesh. Pharmacologyonline 2014, 1, 3–10. [Google Scholar]
- Abdel-Sattar, E.; Harraz, F.M.; El-Gayed, S.H. Antimicrobial Activity of Extracts of some Plants Collected from the Kingdom of Saudi Arabia. JKAU Med. Sci. 2008, 15, 25–33. [Google Scholar] [CrossRef]
- Ngan, D.H.; Hoai, H.T.C.; Huong, L.M.; Hansen, P.E.; Vang, O. Bioactivities and chemical constituents of a Vietnamese medicinal plant Che Vang, Jasminum subtriplinerve Blume (Oleaceae). Nat. Prod. Res. 2008, 22, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.M.C.; Seo, D.-J.; Park, R.-D.; Jung, W.-J. Antifungal, Nematicidal and Antioxidant Activity of the Methanol Extracts Obtained from Medicinal Plants. J. Appl. Biol. Chem. 2013, 56, 199–204. [Google Scholar] [CrossRef]
- Saxena, S.; Uniyal, V.; Bhatt, R.P. Inhibitory effect of essential oils against Trichosporon ovoides causing Piedra Hair Infection. Braz. J. Microbiol. 2012, 43, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Chander, M.P.; Pillai, C.R.; Sunish, I.P.; Vijayachari, P. Antimicrobial and antimalarial properties of medicinal plants used by the indigenous tribes of Andaman and Nicobar Islands, India. Microb. Pathog. 2016, 96, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Lambert, P.A. Cellular impermeability and uptake of biocides and antibiotics in Gram-positive bacteria and mycobacteria. J. Appl. Microbiol. 2002, 92, 46S–54S. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, S.; Kumar, D. Evaluation of antimicrobial potential of cadmium sulphide nanoparticles against bacterial pathogens. Int. J. Pharm. Sci. Rev. Res. 2014, 24, 202–207. [Google Scholar]
- Brown, E.D.; Wright, G.D. Antibacterial drug discovery in the resistance era. Nature 2016, 529, 336–343. [Google Scholar] [CrossRef]
- Sekyere, J.O.; Asante, J. Emerging mechanisms of antimicrobial resistance in bacteria and fungi: Advances in the era of genomics. Futur. Microbiol. 2018, 13, 241–262. [Google Scholar] [CrossRef]
- Sekyere, J.O. Current State of Resistance to Antibiotics of Last-Resort in South Africa: A Review from a Public Health Perspective. Front. Public Health 2016, 4, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Sekyere, J.O.; Govinden, U.; Bester, L.A.; Essack, S.Y. Colistin and tigecycline resistance in carbapenemase-producing Gram-negative bacteria: Emerging resistance mechanisms and detection methods. J. Appl. Microbiol. 2016, 121, 601–617. [Google Scholar] [CrossRef]
- Sekyere, J.O.; Govinden, U.; Essack, S. The molecular epidemiology and genetic environment of carbapenemases detected in Africa. Microb. Drug Resist. 2016, 22, 59–68. [Google Scholar] [CrossRef]
- Voss-Rech, D.; Potter, L.; Vaz, C.S.L.; Pereira, D.I.B.; Sangioni, L.A.; Vargas, A.C.; de Avila Botton, S. Antimicrobial resistance in non-typhoidal Salmonella isolated from human and poultry-related samples in Brazil: 20-year meta-analysis. Foodborne Pathog. Dis. 2017, 14, 116–124. [Google Scholar] [CrossRef]
- Maxwell, A. DNA gyrase as a drug target. Trends Microbiol. 1997, 5, 102–109. [Google Scholar] [CrossRef]
- Tenover, F.C. Mechanisms of Antimicrobial Resistance in Bacteria. Am. J. Med. 2006, 119, S3–S10. [Google Scholar] [CrossRef]
- Schneider, T.; Sahl, H.-G. An oldie but a goodie—Cell wall biosynthesis as antibiotic target pathway. Int. J. Med. Microbiol. 2010, 300, 161–169. [Google Scholar] [CrossRef]
- Sekyere, J.O.; Amoako, D.G. Carbonyl Cyanide m-Chlorophenylhydrazine (CCCP) Reverses Resistance to Colistin, but Not to Carbapenems and Tigecycline in Multidrug-Resistant Enterobacteriaceae. Front. Microbiol. 2017, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Sekyere, J.O.; Amoako, D.G. Genomic and phenotypic characterisation of fluoroquinolone resistance mechanisms in Enterobacteriaceae in Durban, South Africa. PLoS ONE 2017, 12, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Levy, S.B. Active efflux mechanisms for antimicrobial resistance. Antimicrob. Agents Chemother. 1992, 36, 695–703. [Google Scholar] [CrossRef] [Green Version]
- Paulsen, I.T.; Brown, M.H.; Skurray, R.A. Proton-dependent multidrug efflux systems. Microbiol. Rev. 1996, 60, 575–608. [Google Scholar] [CrossRef] [PubMed]
- Khameneh, B.; Diab, R.; Ghazvini, K.; Bazzaz, B.S.F. Breakthroughs in bacterial resistance mechanisms and the potential ways to combat them. Microb. Pathog. 2016, 95, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Kongkham, B.; Prabakaran, D.; Puttaswamy, H. Opportunities and challenges in managing antibiotic resistance in bacteria using plant secondary metabolites. Fitoterapia 2020, 147, 104762. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C. Molecular mechanisms that confer antibacterial drug resistance. Nature 2000, 406, 775–781. [Google Scholar] [CrossRef]
- Savjani, J.K.; Gajjar, A.K.; Savjani, K.T. Mechanisms of Resistance: Useful Tool to Design Antibacterial Agents for Drug—Resistant Bacteria. Mini Rev. Med. Chem. 2009, 9, 194–205. [Google Scholar] [CrossRef]
- Fink, A.L. The molecular basis of β-lactamase catalysis and inhibition. Pharm. Res. 1985, 2, 55–61. [Google Scholar] [CrossRef]
- Blair, J.M.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Olsen, I. New promising β-lactamase inhibitors for clinical use. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1303–1308. [Google Scholar] [CrossRef]
- Stavri, M.; Piddock, L.J.V.; Gibbons, S. Bacterial efflux pump inhibitors from natural sources. J. Antimicrob. Chemother. 2007, 59, 1247–1260. [Google Scholar] [CrossRef] [Green Version]
- Berkow, E.L.; Angulo, D.; Lockhart, S.R. In Vitro Activity of a Novel Glucan Synthase Inhibitor, SCY-078, against Clinical Isolates of Candida auris. Antimicrob. Agents Chemother. 2017, 61, 1–2. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Li, S.; Kaminskyj, S.G.W. Using Aspergillus nidulans To Identify Antifungal Drug Resistance Mutations. Eukaryot. Cell 2014, 13, 288–294. [Google Scholar] [CrossRef] [Green Version]
- Cowen, L.; Sanglard, D.; Howard, S.J.; Rogers, P.D.; Perlin, D.S. Mechanisms of Antifungal Drug Resistance. Cold Spring Harb. Perspect. Med. 2015, 5, 1–2. [Google Scholar] [CrossRef]
- Cowen, L.E. The evolution of fungal drug resistance: Modulating the trajectory from genotype to phenotype. Nat. Rev. Microbiol. 2008, 6, 187–198. [Google Scholar] [CrossRef]
- Cowen, L.E.; Steinbach, W.J. Stress, Drugs, and Evolution: The Role of Cellular Signaling in Fungal Drug Resistance. Eukaryot. Cell 2008, 7, 747–764. [Google Scholar] [CrossRef] [Green Version]
- Cowen, L.E. Hsp90 Orchestrates Stress Response Signaling Governing Fungal Drug Resistance. PLOS Pathog. 2009, 5, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Cowen, L.E. The fungal Achilles’ heel: Targeting Hsp90 to cripple fungal pathogens. Curr. Opin. Microbiol. 2013, 16, 377–384. [Google Scholar] [CrossRef]
- Naidu, A.; Davidson, P.M. Phyto-phenols. In Natural Food Antimicrobial Systems; Naidu, A.S., Ed.; CRC Press: Boca Raton, FL, USA, 2000; pp. 278–307. [Google Scholar]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Gill, A.O.; Holley, R.A. Mechanisms of Bactericidal Action of Cinnamaldehyde against Listeria monocytogenes and of Eugenol against L. monocytogenes and Lactobacillus sakei. Appl. Environ. Microbiol. 2004, 70, 5750–5755. [Google Scholar] [CrossRef] [Green Version]
- Gill, A.O.; Holley, R.A. Disruption of Escherichia coli, Listeria monocytogenes and Lactobacillus sakei cellular membranes by plant oil aromatics. Int. J. Food Microbiol. 2006, 108, 1–9. [Google Scholar] [CrossRef]
- Negi, P.S. Plant extracts for the control of bacterial growth: Efficacy, stability and safety issues for food application. Int. J. Food Microbiol. 2012, 156, 7–17. [Google Scholar] [CrossRef]
- Ozfenerci, M.; Calıskan, U.K. Tea tree oil and its use in aromatherapy. Curr. Pers. Maps. 2018, 2, 90–102. [Google Scholar]
- Gallo, F.R.; Palazzino, G.; Federici, E.; Iurilli, R.; Monache, F.D.; Chifundera, K.; Galeffi, C. Oligomeric secoiridoid glucosides from Jasminum abyssinicum. Phytochemistry 2006, 67, 504–510. [Google Scholar] [CrossRef]
- Tadiwos, Y.; Nedi, T.; Engidawork, E. Analgesic and anti-inflammatory activities of 80% methanol root extract of Jasminum abyssinicum Hochst. ex. Dc. (Oleaceae) in mice. J. Ethnopharmacol. 2017, 202, 281–289. [Google Scholar] [CrossRef]
- Lakshmanan, P.; Gabriel, J.J. Comparative qualitative analysis of callus extracts of in-vitro and in-vivo plants of Jasminum angustifolium, a wild and medicinal plant. World J. Pharm. Sci. 2015, 3, 1421–1425. [Google Scholar]
- Kathiresan, K.; Philip, R. Phytochemical screening and in vitro antioxidant activity of extracts of Jasminum sessiliflorum. Int. J. Pharmacol. Clin. Res. 2018, 2, 117–123. [Google Scholar]
- Philip, R.; Krishnasamy, K.; Abraham, E. Evaluation of anti-inflammatory activity of Jasminum sessiliflorum extracts. Int. J. Res. Pharm. Sci. 2019, 10, 2515–2518. [Google Scholar] [CrossRef]
- Gupta, A.; Chaphalkar, S.R. Use of flow cytometry to measure the immunostimulatory activity of aqueous extract of Jasminum auriculatum. Int. J. Curr. Adv. Res. 2015, 4, 87–91. [Google Scholar]
- Bahuguna, Y.; Juyal, V.; Rawat, M.S.M.; Jalalpure, S. Diuretic activity of flowers of Jasminum auriculatum Vahl. J. Pharm. Res. 2009, 2, 215–216. [Google Scholar]
- Rastogi, R.P.; Mehrotra, B.N.; Sinha, S.; Pant, P.; Seth, R. Compendium of Indian Medicinal Plants; Central Drug Research Institute: Lucknow, India, 2001; pp. 395–396. [Google Scholar]
- Arivoli, S.; Divya, S.; Arumugam, B.; Meeran, M.; Jayakumar, M.; Raveen, R.; Samuel, T. Phytochemical constituents of Jasminum fluminense Linnaeus (Oleaceae): An additional tool in the ecofriendly management of mosquitoes. J. Pharmacog. Phytochem. 2018, 7, 548–556. [Google Scholar]
- Prajapati, N.D.; Purohit, S.S.; Sharma, A.K.; Kumar, T. A Handbook of Medicinal Plants: A Complete Source Book; Agrobios: Jodhpur, India, 2003; p. 554. [Google Scholar]
- Zhao, G.-Q.; Yin, Z.-F.; Liu, Y.-C.; Li, H.-B. Iridoid glycosides from buds of Jasminum officinale L. var grandiflorum. Yao Xue Xue Bao Acta Pharm. Sin. 2011, 46, 1221–1224. [Google Scholar]
- Zhao, G.-Q.; Xia, J.-J.; Dong, J.-X. Glycosides from flowers of Jasminum officinale L. var grandiflorum. Yao Xue Xue Bao Acta Pharm. Sin. 2007, 42, 1066–1069. [Google Scholar]
- Singh, B.; Sharma, R.A. Secondary Metabolites of Medicinal Plants, 4 Volume Set: Ethnopharmacological Properties, Biological Activity and Production Strategies; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 574–584. [Google Scholar]
- Dubey, P.; Tiwari, A.; Gupta, S.K.; Watal, G. Phytochemical and biochemical studies of Jasminum officinale leaves. Int. J. Pharm. Sci. Res. 2016, 7, 2632–2640. [Google Scholar]
- El-Hawary, S.S.; El-Hefnawy, H.M.; Osman, S.M.; El-Raey, M.A.; Ali, F.A.M. Phenolic profiling of different Jasminum species cultivated in Egypt and their antioxidant activity. Nat. Prod. Res. 2019, 1–6. [Google Scholar] [CrossRef]
- Lu, Y.; Han, Z.-Z.; Zhang, C.-G.; Ye, Z.; Wu, L.-L.; Xu, H. Four new sesquiterpenoids with anti-inflammatory activity from the stems of Jasminum officinale. Fitoterapia 2019, 135, 22–26. [Google Scholar] [CrossRef]
- Tauchen, J.; Doskocil, I.; Caffi, C.; Lulekal, E.; Marsik, P.; Havlik, J.; van Damme, P.; Kokoska, L. In vitro antioxidant and anti-proliferative activity of Ethiopian medicinal plant extracts. Ind. Crop. Prod. 2015, 74, 671–679. [Google Scholar] [CrossRef]
- Bhagath, K.; Kekuda, P.T.R.; Raghavendra, H.L.; Swarnalatha, S.P.; Preethi, H.R.; Surabhi, K.S. In vitro antioxidant and anthelmintic activity of extracts of Jasminum arborescens (Roxb.). Int. J. Drug. Dev. Res. 2010, 2, 89–95. [Google Scholar]
- Ferreres, F.; Grosso, A.C.; Gil-Izquierdo, A.; Valentão, P.; Andrade, P.B. Assessing Jasminum grandiflorum L. authenticity by HPLC-DAD-ESI/MSn and effects on physiological enzymes and oxidative species. J. Pharm. Biomed. Anal. 2014, 88, 157–161. [Google Scholar] [CrossRef]
- Umamaheswari, M.; Asokkumar, K.; Rathidevi, R.; Sivashanmugam, A.T.; Subhadradevi, V.; Ravi, T.K. Antiulcer and in vitro antioxidant activities of Jasminum grandiflorum L. J. Ethnopharmacol. 2007, 110, 464–470. [Google Scholar] [CrossRef]
- Chaturvedi, A.P.; Tripathi, Y.B. Methanolic extract of leaves of Jasminum grandiflorum Linn modulates oxidative stress and inflammatory mediators. Inflammopharmacology 2011, 19, 273–281. [Google Scholar] [CrossRef]
- Dessai, P.; Sawant, R.P. In-vitro pharmacological activities of Jasminum malabaricum Wight. J. Glob. Trends Pharm. Sci. 2018, 9, 5076–5082. [Google Scholar]
- Poonia, P.; Niazi, J.; Chaudhary, G.; Kalia, A.N. In vitro antioxidant potential of Jasminum mesnyi Hance (Leaves) extracts. Res. J. Pharm. Biol. Chem. Sci. 2011, 2, 348–357. [Google Scholar]
- Borar, S.; Punia, P.; Kalia, A.N. Antioxidant potential of n-butanol fraction from extract of Jasminum mesnyi Hance leaves. Indian J. Exp. Boil. 2011, 49, 39–43. [Google Scholar]
- Guo, Z.-Y.; Li, P.; Huang, W.; Wang, J.-J.; Liu, Y.-J.; Liu, B.; Wang, Y.-L.; Wu, S.-B.; Kennelly, E.J.; Long, C.-L. Antioxidant and anti-inflammatory caffeoyl phenylpropanoid and secoiridoid glycosides from Jasminum nervosum stems, a Chinese folk medicine. Phytochemistry 2014, 106, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Li, A.-N.; Li, S.; Li, H.-B.; Xu, D.-P.; Xu, X.-R.; Chen, F. Total phenolic contents and antioxidant capacities of 51 edible and wild flowers. J. Funct. Foods 2014, 6, 319–330. [Google Scholar] [CrossRef]
- Khidzir, K.M.; Cheng, S.-F.; Chuah, C.-H. Interspecies variation of chemical constituents and antioxidant capacity of extracts from Jasminum sambac and Jasminum multiflorum grown in Malaysia. Ind. Crop. Prod. 2015, 74, 635–641. [Google Scholar] [CrossRef]
- He, F.; Zuo, L. Redox Roles of Reactive Oxygen Species in Cardiovascular Diseases. Int. J. Mol. Sci. 2015, 16, 27770–27780. [Google Scholar] [CrossRef] [Green Version]
- Dias, V.; Junn, E.; Mouradian, M.M. The role of oxidative stress in Parkinson’s disease. J. Parkinson’s Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef] [Green Version]
- Zuo, L.; Zhou, T.; Pannell, B.K.; Ziegler, A.C.; Best, T.M. Biological and physiological role of reactive oxygen species—The good, the bad and the ugly. Acta Physiol. 2015, 214, 329–348. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Huynh, K.; Heshu, S.R.; Yeap, S.K.; Hazilawati, H.; Roselina, K. Water extract of brewers’ rice induces apoptosis in human colorectal cancer cells via activation of caspase-3 and caspase-8 and downregulates the Wnt/β-catenin downstream signaling pathway in brewers’ rice-treated rats with azoxymethane-induced colon carcinogenesis. BMC Complement. Altern. Med. 2015, 15, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative Stress in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Applications. Oxidative Med. Cell. Longev. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Hercberg, S.; Galan, P.; Preziosi, P.; Bertrais, S.; Mennen, L.; Malvy, D.; Roussel, A.M.; Favier, A.; Briançon, S. The SU. VI. MAX Study: A randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch. Intern. Med. 2004, 164, 2335–2342. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B.; Rafter, J.; Jenner, A. Health promotion by flavonoids, tocopherols, tocotrienols, and other phenols: Direct or indirect effects? Antioxidant or not? Am. J. Clin. Nutr. 2005, 81, 268S–276S. [Google Scholar] [CrossRef] [Green Version]
- Dysken, M.W.; Sano, M.; Asthana, S.; Vertrees, J.E.; Pallaki, M.; Llorente, M.; Love, S.; Schellenberg, G.D.; McCarten, J.R.; Malphurs, J.; et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: The TEAM-AD VA cooperative randomized trial. Jama 2014, 311, 33–44. [Google Scholar] [CrossRef]
- Yuan, G.; Sun, B.; Yuan, J.; Wang, Q. Effect of 1-methylcyclopropene on shelf life, visual quality, antioxidant enzymes and health-promoting compounds in broccoli florets. Food Chem. 2010, 118, 774–781. [Google Scholar] [CrossRef]
- Kolanjiappan, K.; Manoharan, S. Chemopreventive efficacy and anti-lipid peroxidative potential of Jasminum grandiflorum Linn. on 7,12-dimethylbenz(a)anthracene-induced rat mammary carcinogenesis. Fundam. Clin. Pharmacol. 2005, 19, 687–693. [Google Scholar] [CrossRef]
- Chaturvedi, A.P.; Kumar, M.; Tripathi, Y.B. Efficacy of Jasminum grandiflorum L. leaf extract on dermal wound healing in rats. Int. Wound J. 2012, 10, 675–682. [Google Scholar] [CrossRef]
- Sengar, N.; Joshi, A.; Prasad, S.K.; Hemalatha, S. Anti-inflammatory, analgesic and anti-pyretic activities of standardized root extract of Jasminum sambac. J. Ethnopharmacol. 2015, 160, 140–148. [Google Scholar] [CrossRef]
- Ho, C.C.; Ng, S.C.; Chuang, H.L.; Wen, S.Y.; Kuo, C.H.; Mahalakshmi, B.; Huang, C.Y.; Kuo, W.W. Extracts of Jasminum sambac flowers fermented by Lactobacillus rhamnosus inhibit H2O2-and UVB-induced aging in human dermal fibroblasts. Environ. Toxicol. 2020, 36, 607–619. [Google Scholar] [CrossRef]
- Abdoul-Latif, F.; Edou, P.; Eba, F.; Mohamed, N.; Ali, A.; Djama, S.; Obame, L.; Bassolé, I.; Dicko, M. Antimicrobial and antioxidant activities of essential oil and methanol extract of Jasminum sambac from Djibouti. Afr. J. Plant Sci. 2010, 4, 38–43. [Google Scholar]
- AlRashdi, A.S.; Salama, S.M.; Alkiyumi, S.S.; Abdulla, M.A.; Hadi, A.H.A.; Abdelwahab, S.I.; Taha, M.M.; Hussiani, J.; Asykin, N. Mechanisms of gastroprotective effects of ethanolic leaf extract of Jasminum sambac against HCl/ethanol-induced gastric mucosal injury in rats. Evid. Based Compl. Alt. Med. 2012, 2012, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Bhagat, A.D.; Khairnar, A.U.; Tenpe, C.R.; Upaganalwar, A.B.; Yeole, P.G. Anti-inflammatory activity of Jasminum sambac leaf extract against carrageenan induced rat paw edema. Indian J. Nat. Prod. 2007, 23, 25–28. [Google Scholar]
- Rahman, M.A.; Hasan, M.; Hossain, S.M.A.; Biswas, N.N. Analgesic and cytotoxic activities of Jasminum sambac (L.) Aiton. Pharmacologyonline 2011, 1, 124–131. [Google Scholar]
- Kumar, M.; Randhava, N.K. Jasminum mesnyi Hance: Review at a Glance. J. Drug Deliv. Ther. 2014, 4, 44–47. [Google Scholar] [CrossRef] [Green Version]
- Bhushan, B.; Sardana, S.; Bansal, G. Phytochemical and pharmacognostical studies of leaves of Jasminum mesyni Hance. J. Chem. Pharma. Res. 2015, 7, 922–926. [Google Scholar]
- Kumaresan, M.; Kannan, M.; Sankari, A.; Chandrasekhar, C.N.; Vasanthi, D. Phytochemical screening and antioxidant activity of Jasminum multiflorum (pink Kakada) leaves and flowers. J. Pharmacog. Phytochem. 2019, 8, 1168–1173. [Google Scholar]
- Jain, A.; Sharma, R.; Kumar, A.; Sharma, S. Jasminum species: An overview. Int. J. Inst. Pharm. Life Sci. 2011, 1, 251–266. [Google Scholar]
- Shekhar, S.; Prasad, M.P. Evaluation of antimicrobial activity of Jasminum species using solvent extracts against clinical pathogens. World J. Pharm. Pharm. Sci. 2015, 4, 1247–1256. [Google Scholar]
- Yuniarto, A.; Kurnia, I.; Ramadhan, M. Anti-obesity effect of ethanolic extract of jasmine flowers (Jasminum sambac (L.) Ait.) in high fat diet induced mice: Potent inhibitor of pancreatic lipase enzyme. Int. J. Adv. Phar. Biol. Chem. 2015, 4, 18–22. [Google Scholar]
- Dhalaria, R.; Verma, R.; Kumar, D.; Puri, S.; Tapwal, A.; Kumar, V.; Nepovimova, E.; Kuca, K. Bioactive Compounds of Edible Fruits with Their Anti-Aging Properties: A Comprehensive Review to Prolong Human Life. Antioxidants 2020, 9, 1123. [Google Scholar] [CrossRef]
- Nagmoti, D.M.; Khatri, D.K.; Juvekar, P.R.; Juvekar, A.R. Antioxidant activity free radical-scavenging potential of Pithecellobium dulce Benth seed extracts. Free Radic. Antioxid. 2012, 2, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Bernatchez, P.N.; de Haan, J.B. Targeting Endothelial Dysfunction in Vascular Complications Associated with Diabetes. Int. J. Vasc. Med. 2012, 2012, 1–12. [Google Scholar] [CrossRef]
- Boora, F.; Chirisa, E.; Mukanganyama, S. Evaluation of Nitrite Radical Scavenging Properties of Selected Zimbabwean Plant Extracts and Their Phytoconstituents. J. Food Process. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Tehrani, H.S.; Moosavi-Movahedi, A.A. Catalase and its mysteries. Prog. Biophys. Mol. Biol. 2018, 140, 5–12. [Google Scholar] [CrossRef]
- Rakotoarisoa, M.; Angelov, B.; Espinoza, S.; Khakurel, K.; Bizien, T.; Angelova, A. Cubic Liquid Crystalline Nanostructures Involving Catalase and Curcumin: BioSAXS Study and Catalase Peroxidatic Function after Cubosomal Nanoparticle Treatment of Differentiated SH-SY5Y Cells. Molecules 2019, 24, 3058. [Google Scholar] [CrossRef] [Green Version]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Devasagayam, T.P.A.; Tilak, J.C.; Boloor, K.K.; Sane, K.S.; Ghaskadbi, S.S.; Lele, R.D. Free radicals and antioxidants in human health: Current status and future prospects. J. Assoc. Physicians India 2004, 52, 794–804. [Google Scholar]
- Wills, E.D. Effects of lipid peroxidation on membrane-bound enzymes of the endoplasmic reticulum. Biochem. J. 1971, 123, 983–991. [Google Scholar] [CrossRef] [Green Version]
- Farooqui, A.A.; Horrocks, L.A. Lipid Peroxides in the Free Radical Pathophysiology of Brain Diseases. Cell. Mol. Neurobiol. 1998, 18, 599–608. [Google Scholar] [CrossRef]
- Cheeseman, K. Mechanisms and effects of lipid peroxidation. Mol. Asp. Med. 1993, 14, 191–197. [Google Scholar] [CrossRef]
- Yu, B.P.; Suescun, E.A.; Yang, S.Y. Effect of age-related lipid peroxidation on membrane fluidity and phospholipase A2: Modulation by dietary restriction. Mech. Ageing Dev. 1992, 65, 17–33. [Google Scholar] [CrossRef]
- Sies, H.; Sharov, V.S.; Klotz, L.O.; Briviba, K. Glutathione peroxidase protects against peroxynitrite-mediated oxidations: A new function for selenoproteins as peroxynitrite reductase. J. Biol. Chem. 1997, 272, 27812–27817. [Google Scholar] [CrossRef] [Green Version]
- Szabó, C.; Ischiropoulos, H.; Radi, R. Peroxynitrite: Biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 2007, 6, 662–680. [Google Scholar] [CrossRef] [PubMed]





| Botanical Name | Extract/Solvent (Conc.) | Microbes | ZOI (mm)/MIC (μg/mL) | References |
|---|---|---|---|---|
Jasminum abyssinicum Hochst. ex DC. | Aerial parts extract/Methanol (250–2000 μg/mL) Positive control | Staphylococcus aureus Streptococcus pyogenes S. pneumonia Neisseria gonorrhoea Escherichia coli Bacillus cereus Shigella dysenteriae S. flexineri Salmonella typhi S. typhimuriumAspergillus flavus A. niger Candida albicans Trichophyton mentagrophytes T. violacum Cryptococcus neoformas (Tetracycline, Co-trimoxazole, Gentamycin, Chloroamphenicol, Sulphadaizine, Cephalotin) | N.A. N.A. N.A. Active N.A. N.A. N.A. N.A. N.A. N.A. N.A. N.A. N.A. N.A. N.A. N.A. D.N.S. | [27] |
| Leaves extract/Ethanol Positive control | Bacillus cereus Clostridium perfringens, Listeria monocytogenes Staphylococcus epidermidis Enterococcus faecalis Staphylococcus aureus Streptococcus pyogenes Bacteroides fragilis Escherichia coli, Pseudomonas aeruguinosa Salmonella enteritidis Candida albicans (Ciprofloxacin, Tioconazole, Penicillin) | MIC 512 N.A. 512 512 N.A. N.A. 256 N.A. N.A. N.A. N.A. N.A. 0.015–8 | [28] | |
Jasminum angustifolium (L.) Willd. | Flower extract/ Methanol (500 ppm) Positive control | Bacillus sp. Escherichia coli Staphylococcus sp. Klebsiella pneumoniae Lactobacillus sp. Yersinia sp. Enterococcus sp. Pseudomonas sp. | ZOI N.A. N.A. N.A. N.A. 6 5.5 5 6 D.N.S. | [29] |
| Jasminum angustifolium var. sessiliflorum (Vahl) P.S.Green | Leaves and Stem extracts/Ethanol (25 mg/50 µL) Positive control | Escherichia coli Pseudomonas aeruginosa Staphylococcus aureus Enterococcus faecalis Bacillus cereus Candida albicans [Chloramphenicol (30 μg/well)] | ZOI S: N.A; L: N.A S: 22; L: 17 S: 12; L: N.A S: 14; L: 11 S: 12; L: 11 S: 13; L: 15 24–30 except Pseudomonas aeruginosa | [30] |
Jasminum arborescens Roxb. | Leaves extract / Methanol (50 mg/mL) Positive control | Escherichia coli Pseudomonas aeruginosa Staphylococcus aureus Bacillus subtilis [Streptomycin (1mg/mL)] | ZOI 2.8 3.1 3.7 3.6 2.8–3.6 | [31] |
Jasminum auriculatum Vahl | Leaves extract /Ethanol Positive control | Bacillus subtilis, Staphyloccocus aureus Pseudomonas aeruginosa Micrococcus luteus Escherichia coli Aspergillius niger Candida albicans (Ciprofloxacin against bacterial strains Fluconazole against fungi) | MIC 1560 6250 780 3125 12500 N.A. N.A. 1.25–2.5 2.5 | [32] |
| Jasminum azoricum L. | Leaves extract/ Acetone (30 mg/mL) Positive control | Staphylococcus aureus Bacillus cereus B. subtilis Escherichia coli Pseudomonas sp. | ZOI 20 24 9 14 17 D.N.S. | [33] |
| Flowers extract/Butanol (500 mg/mL) Positive control | Salmonella typhi Staphylococcus aureus Pseudomonas sp. Vibrio cholerae Streptococcus sp. Corynebacterium sp. Enterobacter aerogenes Proteus vulgaris Escherichia coli (Ampicillin) | 22 15 20 18 17 14 N.A. 18 21 D.N.S | [34] | |
Jasminum brevilobum DC. | Leaves extract/Acetone, Water, Methanol, Petroleum ether, Jatamansone | Staphylococcus aureus Bacillus subtilis Escherichia coli Klebsiella pneumoniae Proteus mirabilis Positive control | MIC D: 0.44; E: 0.92; F: 1.17; G: 1.56; H: 0.05 D: 0.42; E: 0.62; F: 1.04; G: 1.36; H: 0.15 D: 0.89; E: 1.24; F: 1.09; G: 1.08; H: 0.07 D: 0.54; E: 0.66; F: 0.95; G: 1.00; H: 0.14 D: 0.49; E: 0.51; F: 0.60; G: 0.92; H: 0.09 D.N.S. | [35] |
| Jasminum fluminense Vell. | Root extracts/ Methanol Positive control | Candida albicans Gardnerella vaginalis Neisseria gonorrhoeae Oligella ureolytica (Ciprofloxacin) | MIC 3100 <12,500 6300 3100 10–< 10 | [36] |
| Jasminum grandiflorum L. | Leaves extract/Aqueous and Ethanol (hot solvent) Positive control | Streptococcus mutans Lactobacillus acidophilus (Ciprofloxacin) | MIC J: 6.25 E: 50 J: 25 E: 50 10–< 10 | [37] |
| Plant extract/Ethanol (500 μg/μL) Positive control | Enterococcus faecalis Hafnia alvei Pseudomonas aeruginosa Proteus vulgaris Plesiomonas shigelloides Staphylococcus epidermidis S. aureus, S. saprophyticus S. pyogenes Salmonella typhi Shigella flexneri S. sonnie S. boydii S. dysenteriae | ZOI N.A. N.A. 7 N.A. N.A. 15 7 7 7 N.A. 10 7 7 6 D.N.S. | [38] | |
| Jasminum grandiflorum subsp. floribundum (R.Br. ex Fresen.) P.S.Green | Plant extract/Methanol (10 mg/mL) Positive control | Escherichia coli Proteus vulgaris Pseudomonas aeruginosa Staphylococcus aureus Sarcina lutea Bacillus subtilis Mycobacterium phlei Candida albicans (Ofloxacin, Amphotericin B) | ZOI 14 12 22 20 20 15 N.A. 22 D.N.S. | [39] |
| Jasminum nervosum Lour. (Synonym Jasminum subtriplinerve Blume) | Stem and leaves extract/petroleum ether, ethyl acetate, ethanol, methanol and water Positive control | Escherichia coli Pseudomonas aeruginosa Bacillus subtilis Staphylococcus aureus Aspergillus Niger Fusarium oxysporum Candida albicans Saccharomyces cerevisiae | MIC F: 200 AE: N.A G: 100 I and J: 200 AE: N.A. AE: N.A. AE: N.A. AE: N.A. D.N.S. | [40] |
| Leaves extract/ Methanol [80% methanol at a ratio of 1:5 (v/v, dry plant material/solvent)] Positive control | Fusarium solani F. oxysporum Rhizoctonia solani | N.A. N.A. Active D.N.S. | [41] | |
| Jasminum officinale L. | Essential oil from flowers extract Positive control | Trichosporon ovoides [Imidazole (50 µg/disc) Nystatin B (100 µg/disc)] | MIC 3.1 12.5 6.2 | [42] |
| Jasminum polyanthum Franch. | Flower and leaf extracts/ water extract (2 g flowers as well as leaves used for extract preparation) Positive control | Escherichia coli Klebsiella pneumoniae Staphylococcus aureus Pseudomonas aeruginosa Aspergillus flavus A. niger (Gentamicin for bacterial strains) | ZOI Fl: 8; L: 7 Fl: 9; L: 8 Fl: 13; L: 11 Fl: 13; L: 12 Fl: 8; L: 10 Fl: N.A; L: N.A 10 | [20] |
| Jasminum syringifolium Wall. ex G.Don | Leaves extract/ Methanol (100 g leaves in 95% methanol) Positive control | Escherichia coli Pseudomonas aeruginosa Staphylococcus aureus Bacillus cereus Staphylococcus epidermidis Vibrio cholerae Proteus mirabilis Shigella flexneri Salmonella enterica typhi Klebsiella pneumoniae Aspergillus niger Candida albicans (Gentamycin for bacterial strains Nystatin for fungi) | ZOI 21.33 16.67 21.67 22.33 16.33 18.67 15.33 22.67 19.33 18.33 17.33 15.33 12.67–22.67 17.67–21.33 | [43] |
| Botanical Name | Part Used | Solvent/Compound/Conc. | Method Used and Major Findings (IC50 and EC50- μg/mL) | References |
|---|---|---|---|---|
| Jasminum abyssinicum Hochst. ex DC. | L | E | DPPH (IC50) = 26.3 ORAC = 1023.7 μg TE/mg | [96] |
| Jasminum angustifolium var. sessiliflorum (Vahl) P.S. Green (Synonym: Jasminum sessiliflorum) | L S | E (0.5 mg/mL) E (0.5 mg/mL) | DPPH = 11.12% NO = 51.49% O−2 = 51.29% O−2 =53.93% | [30] |
| Jasminum arborescens Roxb. | L | E, CH and PE (0.025–0.4 mg/mL) | DPPH= 40–90% Fe +3 reducing power (absorbance at 700 nm) = 0.2 to 0.45 | [97] |
| Jasminum auriculatum Vahl | L | E | DPPH (IC50) = 33.39 | [32] |
| Jasminum azoricum L. | L | 80% M | DPPH (IC50) = 199.2 | [94] |
| Jasminum grandiflorum L. | F | BWE HME | DPPH (IC50) = 150.57 O−2 (IC50) = 327.89 NO (IC50) = 38.27 H2O2 (IC50) = 397.09 DPPH (IC50) = 189.93 O−2 (IC50) = 1354.30 NO (IC50) = 225.51 H2O2 (IC50) = 403.31 | [98] |
| L | E | DPPH (IC50) = 15 Reducing power (IC50) = 19.5 NO (IC50) = 98 | [99] | |
| L | M | Iron-induced lipid peroxidation (EC50) = 667.53 ABTS•+ (EC50) = 222.50 O−2 (EC50) = 207 OH (EC50) = 288.19 (+EDTA) and 102.16 (−EDTA) | [100] | |
| Jasminum humile L. | L | 80% M | DPPH (IC50) = 94.6 | [94] |
| Jasminum malabaricum Wight | L, R, B | Aq (500, 1000, 1500 and 2000 μg/mL) | H2O2 = 7, 22.2, 44.4, and 66.6% | [101] |
| Jasminum mesnyi Hance | L | EA (25–400 µg/mL) n-but (25–400 µg/mL) | DPPH (IC50) = 153.45 NO (IC50) = 141.54 FRAP= concentration-dependent Reducing power (absorbance range) = 0.05–1.11 DPPH (IC50) = 6.22 NO (IC50) = 35.12 FRAP= concentration-dependent Reducing power (absorbance range) = 0.07–2.76 | [103] |
| L | M Aq | DPPH (IC50) = 25.27 Lipid peroxidation assay (IC50) = 84.69 DPPH (IC50) = 71.84 Lipid peroxidation assay (IC50) = 145.62 | [102] | |
| Jasminum multiflorum (Burm.f.) Andrews | L | M | DPPH (IC50) = 34.8 | [94] |
| F | M | DPPH (IC50) = 81 | [106] | |
| Jasminum nervosum Lour. | S | Jasnervosides A * Jasnervoside B * Jasnervoside D * Jasnervoside G * | DPPH (IC50) = 0.22 DPPH (IC50) = 0.09 DPPH (IC50) = 0.19 DPPH (IC50) = 1.21 | [104] |
| Jasminum nudiflorum Lindl. | F | Water-soluble (tetrahydrofuran) Fat soluble (methanol–acetic acid–water mixture; 0:3.7:46.3) | FRAP = 11.05 μmol Fe(II)/g TEAC = 3.85 μmol trolox/g FRAP = 3.71 μmol Fe(II)/g TEAC = 0.79 μmol trolox/g | [105] |
| Jasminum officinale L. | L | Aq | DPPH (IC50) = 41.16 NO (IC50) = 30.29 O−2 (IC50) = 20.19 ABTS•+ (IC50) = 29.48 | [93] |
| L | 80% M | DPPH (IC50) =76.6 | [94] | |
| Jasminum sambac (L.) Aiton | F | M | DPPH (IC50) = 208 | [106] |
| L (Arabian nights) L (Grand Duke of Tuskany) | 80% M 80% M | DPPH (IC50) = 130.7 DPPH (IC50) = 155.5 | [94] |
| Botanical Name | Plant Part | Solvent/Dose | Activity | Model | Biomarkers Affected | References |
|---|---|---|---|---|---|---|
| Jasminum grandiflorum L. | F | E (300 mg/kg p.o.) | Chemo preventive | 7,12-dimethylbenz(a)anthracene (DMBA)-induced rat mammary carcinogenesis | ↑ vitamin E (plasma and erythrocytes) ↑ vitamin C (plasma) ↑reduced glutathione (plasma and erythrocytes) ↑ SOD, CAT (plasma, erythrocytes and mammary tissues) ↑ glutathione peroxidase (plasma, erythrocytes) ↓TBARS ↓ reduced glutathione (tissue) ↓glutathione peroxidase (tissue) | [116] |
| L | M (100–800 μg/mL) | Anti-inflammatory | LPS (20 ng/mL)-induced nitric oxide in rat peritoneal macrophage | ↓ NO production (13.26 μ M/1 x 105 cells to 4.41 μM/1 x 105 cells) | [100] | |
| L | O | Wound healing | Cutaneous wound healing in diabetic rats | ↑ wound contraction ↑total hydroxyl proline, ↑ hexosamine ↑ protein ↑ DNA content ↑ Tensile strength ↑collagen & fibrous tissue ↑ number of blood vessels ↑SOD, CAT and GSH content ↓ lipid peroxidation | [117] | |
| Jasminum sambac (L.) Aiton | R | E | Anti-inflammatory | Carrageenan-induced rat paw edema model and cotton pellet-induced granuloma in rats | ↓ paw edema ↓ granuloma formation ↓ AST, ALT, LPO, ↑ SOD and CAT | [118] |
| F | LFE | Anti-aging | UVB (40 mJ/cm2 ) or H2O2 (200 μM) -induced HS68 dermal fibroblast cell | ↓ ROS production ↓ aging markers, such as p16, p21, and p53, ↓ MMP-1 ↓ SA-β-Gal -positive cells ↓ p-ERK, p-JNK, p-P38, and p-c-jun protein levels ↑ p-smad2/3 in the nuclear fraction ↑ TGFβ, p-smad2/3, COL1A1, and COL3A1 protein levels ↑ phoshpho-Nuclear respiratory factor 2 and antioxidant gene expression (HO-1) | [119] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balkrishna, A.; Rohela, A.; Kumar, A.; Kumar, A.; Arya, V.; Thakur, P.; Oleksak, P.; Krejcar, O.; Verma, R.; Kumar, D.; et al. Mechanistic Insight into Antimicrobial and Antioxidant Potential of Jasminum Species: A Herbal Approach for Disease Management. Plants 2021, 10, 1089. https://doi.org/10.3390/plants10061089
Balkrishna A, Rohela A, Kumar A, Kumar A, Arya V, Thakur P, Oleksak P, Krejcar O, Verma R, Kumar D, et al. Mechanistic Insight into Antimicrobial and Antioxidant Potential of Jasminum Species: A Herbal Approach for Disease Management. Plants. 2021; 10(6):1089. https://doi.org/10.3390/plants10061089
Chicago/Turabian StyleBalkrishna, Acharya, Akansha Rohela, Abhishek Kumar, Ashwani Kumar, Vedpriya Arya, Pallavi Thakur, Patrik Oleksak, Ondrej Krejcar, Rachna Verma, Dinesh Kumar, and et al. 2021. "Mechanistic Insight into Antimicrobial and Antioxidant Potential of Jasminum Species: A Herbal Approach for Disease Management" Plants 10, no. 6: 1089. https://doi.org/10.3390/plants10061089
APA StyleBalkrishna, A., Rohela, A., Kumar, A., Kumar, A., Arya, V., Thakur, P., Oleksak, P., Krejcar, O., Verma, R., Kumar, D., & Kuca, K. (2021). Mechanistic Insight into Antimicrobial and Antioxidant Potential of Jasminum Species: A Herbal Approach for Disease Management. Plants, 10(6), 1089. https://doi.org/10.3390/plants10061089