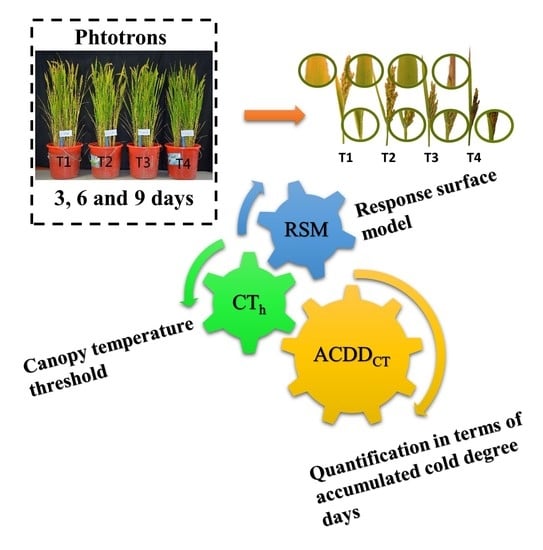
Responses of Grain Yield and Yield Related Parameters to Post-Heading Low-Temperature Stress in Japonica Rice
Abstract
:1. Introduction
2. Results
2.1. Effects of Post-Heading LTS on Grain Yield and Related Parameters
2.2. Effects of Post-Heading LTS on Grain Filling
2.3. The Correlation between Yield as Well as Yield and Grain Filling Related Parameters
2.4. Response Surface Model for the Association between LTS and Grain Yields as Well as Yield Related Parameters
2.5. Quantitative Effects of Post-Heading LTS on Yield and Related Parameters
3. Discussion
3.1. Effect of Post-Heading LTS on Yields and Related Parameters in Rice
3.2. Quantification of Post-Heading LTS Effects on Rice Yield
4. Materials and Methods
4.1. Crop Husbandry and Experimental Design
4.2. Ambient and Phytotron Environment
4.3. Yield as Well as Yield and Grain FillingRelated Parameters
4.4. Response Surface Model
4.5. Quantification of the Effect of Post-Heading LTS on Yields and Related Parameters
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shelton, A.M.; Zhao, J.-Z.; Roush, R.T. Economic, Ecological, Food Safety, and Social Consequences of the Deployment of Bt. Transgenic Plants. Ann. Rev. Entomol. 2002, 47, 845–881. [Google Scholar] [CrossRef]
- Yoshida, S. Fundamentals of Rice Crop Science; International Rice Research Institute: Los Baños, Philippines, 1981; pp. 65–109. [Google Scholar]
- Shimono, H.; Hasegawa, T.; Iwama, K. Response of Growth and Grain Yield in Paddy Rice to Cool Water at Different Growth Stages. Field Crop. Res. 2002, 73, 67–79. [Google Scholar] [CrossRef]
- Galiba, G.; Tóth, B. Cold Stress. In Encyclopedia of Applied Plant Sciences, 2nd ed.; Brian, T., Brian, G.M., Denis, J.M., Eds.; Academic Press: Cambridge, MA, USA; London, UK, 2016; pp. 1–7. [Google Scholar] [CrossRef]
- Huang, X.; Kurata, N.; Wei, X.; Wang, Z.X.; Wang, A.; Zhao, Q.; Zhao, Y.; Liu, K.; Lu, H.; Li, W.; et al. A Map of Rice Genome Variation Reveals the Origin of Cultivated Rice. Nature 2012, 490, 497–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimono, H.; Hasegawa, T.; Iwama, K. Modeling the Effects of Water Temperature on Rice Growth and Yield under a Cool Climate: I. Model Development. Agron. J. 2007, 99, 1327–1337. [Google Scholar] [CrossRef]
- Lee, M.H. Low Temperature Tolerance in Rice: The Korean Experience In Increased Lowland Rice Production in the Mekong Region, Proceedings of the International Workshop, Vientiane, Laos, 30 October–2 November 2000; Australian Centre for International Ag-ricultural Research: Canberra, Australia, 2001; pp. 109–117.
- Dai, L.; Lin, X.; Ye, C.; Ise, K.; Saito, K.; Kato, A.; Xu, F.; Yu, T.; Zhang, D. Identification of Quantitative Trait Loci Controlling Cold Tolerance at the Reproductive Stage in Yunnan Landrace of Rice, Kunmingxiaobaigu. Breed. Sci. 2004, 54, 253–258. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.M.; Zhou, L.; Zeng, Y.W.; Wang, F.M.; Zhang, H.L.; Shen, S.Q.; Li, Z.C. Identification and Mapping of Quantitative Trait Loci for Cold Tolerance at the Booting Stage in a Japonica Rice Near-Isogenic Line. Plant Sci. 2008, 174, 340–347. [Google Scholar] [CrossRef]
- Jiang, W.; Lee, J.; Chu, S.H.; Ham, T.H.; Woo, M.O.; Cho, Y., II; Chin, J.H.; Han, L.; Xuan, Y.; Yuan, D.; et al. Genotype×environment Interactions for Chilling Tolerance of Rice Recombinant Inbred Lines under Different Low Temperature Environments. Field Crop. Res. 2010, 117, 226–236. [Google Scholar] [CrossRef]
- Wang, P.; Hu, T.; Kong, F.; Xu, J.; Zhang, D. Rice Exposure to Cold Stress in China: How Has Its Spatial Pattern Changed under Climate Change? Eur. J. Agron. 2019, 103, 73–79. [Google Scholar] [CrossRef]
- Farell, T.C.; Fox, K.M.; Williams, R.L.; Fukai, S.; Reinke, R.F.; Lewin, L.G. Temperature constraints to rice production in Australia and Laos: A shared problem. In Increased lowland rice production in the Mekong Region, Proceedings of the International Workshop, Vientiane, Laos, 30 October–2 November 2000; Australian Centre for International Agricultural Research: Canberra, Australia, 2001; pp. 129–137. [Google Scholar]
- Wang, J.; Lin, X.; Sun, Q.; Jena, K.K. Evaluation of Cold Tolerance for Japonica Rice Varieties from Different Country. Adv. J. Food Sci. Technol. 2013, 5, 54–56. [Google Scholar] [CrossRef]
- Li, T.G.; Visperas, R.M.; Vergara, B.S. Correlation of Cold Tolerance at Different Growth Stages in Rice. J. Integ. Plant Biol. 1981, 23, 203–207. [Google Scholar]
- Shimono, H.; Hasegawa, T.; Moriyama, M.; Fujimura, S.; Nagata, T. Modeling Spikelet Sterility Induced by Low Temperature in Rice. Agron. J. 2005, 97, 1524–1536. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, L.; Asseng, S.; Xia, Y.; Tang, L.; Liu, B.; Cao, W.; Zhu, Y. Estimating Spring Frost and Its Impact on Yield across Winter Wheat in China. Agric. For. Meteorol. 2018, 260–261, 154–164. [Google Scholar] [CrossRef]
- Neelima, P.; Rani, K.J.; Raju, C.D.; Keshavulu, K. Evaluation of Rice Genotypes for Cold Tolerance. Agric. Sci. Res. J. 2015, 5, 124–133. [Google Scholar]
- Khatun, H.; Biswas, P.S.; Hwang, H.G.; Kim, K.-M. A Quick and Simple In-House Screening Protocol for Cold-Tolerance at Seedling Stage in Rice. Plant Breed. Biotechnol. 2016, 4, 373–378. [Google Scholar] [CrossRef]
- Jacobs, B.C.; Pearson, C.J. Growth, Development and Yield of Rice in Response to Cold Temperature. J. Agron. Crop Sci. 1999, 182, 79–88. [Google Scholar] [CrossRef]
- Gunawardena, T.A.; Fukai, S.; Blamey, F.P.C. Low Temperature Induced Spikelet Sterility in Rice. II. Effects of Panicle and Root Temperatures. Aust. J. Agric. Res. 2003, 54, 947–956. [Google Scholar] [CrossRef]
- Satake, T.; Koike, S. Sterility Caused by Cooling Treatment at the Flowering Stage in Rice Plants I. The Stage and Organ Susceptible to Cool Temperature. Jap. J. Crop Sci. 1983, 52, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, S.; Hara, T.; Hara, T. Effects of Air Temperature and Light on Grain Filling of an Indica and a Japonica Rice (Oryza sativa L.) under Controlled Environmental Conditions. Soil Sci. Plant Nutr. 1977, 23, 93–107. [Google Scholar] [CrossRef]
- Arshad, M.S.; Farooq, M.; Asch, F.; Krishna, J.S.V.; Prasad, P.V.V.; Siddique, K.H.M. Thermal Stress Impacts Reproductive Development and Grain Yield in Rice. Plant Physiol. Biochem. 2017, 115, 57–72. [Google Scholar] [CrossRef]
- Siddik, M.A.; Zhang, J.; Chen, J.; Qian, H.; Jiang, Y.; Raheem, A.k.; Deng, A.; Song, Z.; Zheng, C.; Zhang, W. Responses of Indica Rice Yield and Quality to Extreme High and Low Temperatures during the Reproductive Period. Eur. J. Agron. 2019, 106, 30–38. [Google Scholar] [CrossRef]
- Martínez-Eixarch, M.; Ellis, R.H. Temporal Sensitivities of Rice Seed Development from Spikelet Fertility to Viable Mature Seed to Extreme-Temperature. Crop Sci. 2015, 55, 354. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Xia, Y.; Liu, B.; Chang, C.; Xiao, L.; Shen, J.; Tang, L.; Cao, W.; Zhu, Y. Individual and Combined Effects of Jointing and Booting Low-Temperature Stress on Wheat Yield. Eur. J. Agron. 2020, 113. [Google Scholar] [CrossRef]
- Shi, P.; Tang, L.; Lin, C.; Liu, L.; Wang, H.; Cao, W.; Zhu, Y. Modeling the Effects of Post-Anthesis Heat Stress on Rice Phenology. Field Crop. Res. 2015, 177, 26–36. [Google Scholar] [CrossRef]
- Liu, L.; Song, H.; Shi, K.; Liu, B.; Zhang, Y.; Tang, L.; Cao, W.; Zhu, Y. Response of Wheat Grain Quality to Low Temperature during Jointing and Booting Stages—On the Importance of Considering Canopy Temperature. Agric. For. Meteorol. 2019, 278, 107658. [Google Scholar] [CrossRef]
- Ji, H.; Xiao, L.; Xia, Y.; Song, H.; Liu, B.; Tang, L.; Cao, W.; Zhu, Y.; Liu, L. Effects of Jointing and Booting Low Temperature Stresses on Grain Yield and Yield Components in Wheat. Agric. For. Meteorol. 2017, 243, 33–42. [Google Scholar] [CrossRef]
- Shi, P.; Zhu, Y.; Tang, L.; Chen, J.; Sun, T.; Cao, W.; Tian, Y. Differential Effects of Temperature and Duration of Heat Stress during Anthesis and Grain Filling Stages in Rice. Environ. Exp. Bot. 2016, 132, 28–41. [Google Scholar] [CrossRef]
- Espe, M.B.; Hill, J.E.; Hijmans, R.J.; McKenzie, K.; Mutters, R.; Espino, L.A.; Leinfelder-Miles, M.; van Kessel, C.; Linquist, B.A. Point Stresses during Reproductive Stage Rather than Warming Seasonal Temperature Determine Yield in Temperate Rice. Glob. Chang. Biol. 2017, 23, 4386–4395. [Google Scholar] [CrossRef]
- Shimono, H. Earlier Rice Phenology as a Result of Climate Change Can Increase the Risk of Cold Damage during Reproductive Growth in Northern Japan. Agric. Ecosyst. Environ. 2011, 144, 201–207. [Google Scholar] [CrossRef]
- Espe, M.B.; Yang, H.; Cassman, K.G.; Guilpart, N.; Sharifi, H.; Linquist, B.A. Estimating Yield Potential in Temperate High-Yielding, Direct-Seeded US Rice Production Systems. Field Crop. Res. 2016, 193, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Zhang, Z.; Chen, Y.; Wei, X.; Feng, B.; Tao, F. How Much Yield Loss Has Been Caused by Extreme Temperature Stress to the Irrigated Rice Production in China? Clim. Chang. 2016, 134, 635–650. [Google Scholar] [CrossRef]
- Da Cruz, R.P.; Kothe Milach, S.C.; Federizzi, L.C. Rice Cold Tolerance at the Reproductive Stage in a Controlled Environment. Sci. Agric. 2006, 63, 255–261. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.; Hasegawa, T.; Tang, L.; Wang, W.; Zhou, J.; Liu, L.; Liu, B.; Cao, W.; Zhu, Y. Stage-Dependent Temperature Sensitivity Function Predicts Seed-Setting Rates under Short-Term Extreme Heat Stress in Rice. Agric. For. Meteorol. 2018, 256–257, 196–206. [Google Scholar] [CrossRef]
- Sánchez, B.; Rasmussen, A.; Porter, J.R. Temperatures and the Growth and Development of Maize and Rice: A Review. Glob. Chang. Biol. 2014, 20, 408–417. [Google Scholar] [CrossRef]
- Mitchell, J.H.; Zulkafli, S.L.; Bosse, J.; Campbell, B.; Snell, P.; Mace, E.S.; Godwin, I.D.; Fukai, S. Rice-Cold Tolerance across Reproductive Stages. Crop Past. Sci. 2016, 67, 823–833. [Google Scholar] [CrossRef]
- Ahmed, N.; Maekawa, M.; Tetlow, I.J. Effects of Low Temperature on Grain Filling, Amylose Content, and Activity of Starch Biosynthesis Enzymes in Endosperm of Basmati Rice. Aus. J. Agric. Res. 2008, 59, 599. [Google Scholar] [CrossRef]
- Meng, F.; Zhou, Y.; Wang, S.; Duan, J.; Zhang, Z.; Niu, H.; Jiang, L.; Cui, S.; Li, X.; Luo, C.; et al. Temperature Sensitivity Thresholds to Warming and Cooling in Phenophases of Alpine Plants. Clim. Chang. 2016, 139, 579–590. [Google Scholar] [CrossRef]
- Barlow, K.M.; Christy, B.P.; O’Leary, G.J.; Riffkin, P.A.; Nuttall, J.G. Simulating the Impact of Extreme Heat and Frost Events on Wheat Crop Production: A Review. Field Crop. Res. 2015, 171, 109–119. [Google Scholar] [CrossRef] [Green Version]
- Julia, C.; Dingkuhn, M. Predicting Temperature Induced Sterility of Rice Spikelets Requires Simulation of Crop-Generated Microclimate. Eur. J. Agron. 2013, 49, 50–60. [Google Scholar] [CrossRef]
- Zhang, W.; Han, Y.; Du, H. Relationship between Canopy Temperature at Flowering Stage and Soil Water Content, Yield Components in Rice. Rice Sci. 2007, 14, 67–70. [Google Scholar] [CrossRef]
- Liu, W.; Yu, K.; He, T.; Li, F.; Zhang, D.; Liu, J. The Low Temperature Induced Physiological Responses of Avena nuda L., a Cold-Tolerant Plant Species. Sci. World J. 2013, 658793. [Google Scholar] [CrossRef] [Green Version]
- Cai, C.; Yin, X.; He, S.; Jiang, W.; Si, C.; Struik, P.C.; Luo, W.; Li, G.; Xie, Y.; Xiong, Y.; et al. Responses of Wheat and Rice to Factorial Combinations of Ambient and Elevated CO 2 and Temperature in FACE Experiments. Glob. Chang. Biol. 2016, 22, 856–874. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Shon, J.; Lee, C.K.; Yang, W.; Yoon, Y.; Yang, W.H.; Kim, Y.G.; Lee, B.W. Relationship between Grain Filling Duration and Leaf Senescence of Temperate Rice under High Temperature. Field Crop. Res. 2011, 122, 207–213. [Google Scholar] [CrossRef]
- Tavares, O.C.H.; Santos, L.A.; Filho, D.F.; Ferreira, L.M.; García, A.C.; Castro, T.A.V.T.; Zonta, E.; Pereira, M.G.; Fernandes, M.S. Response Surface Modeling of Humic Acid Stimulation of the Rice (Oryza Sativa L.) Root System. Arch. Agron. Soil Sci. 2020, 67, 1046–1059. [Google Scholar] [CrossRef]
- Liu, L.; Ji, H.; An, J.; Shi, K.; Ma, J.; Liu, B.; Tang, L.; Cao, W.; Zhu, Y. Response of Biomass Accumulation in Wheat to Low-Temperature Stress at Jointing and Booting Stages. Environ. Exp. Bot. 2019, 157, 46–57. [Google Scholar] [CrossRef]








| Cultivar | LTS | Flowering Stage | Grain Filling Stage | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SF (%) | TGW (g) | YPP (g plant−1) | SNPP | SF (%) | TGW (g) | YPP (g plant−1) | SNPP | ||
| Huaidao 5 | Control | 94.6 a | 29.8 | 17.2 a | 122.1 | 96.5 | 29.5 | 17.7 | 124.9 |
| T2D1 | 96.2a | 31.0 | 18.3 a | 123.0 | 97.3 | 30.5 | 18.7 | 126.0 | |
| T2D2 | 94.6 a | 30.4 | 17.7 a | 123.1 | 96.2 | 29.5 | 18.0 | 126.8 | |
| T2D3 | 92.5 a | 29.8 | 16.9 a | 123.1 | 96.1 | 30.1 | 18.3 | 126.9 | |
| T3D1 | 96.5 a | 30.3 | 17.7 a | 120.9 | 96.3 | 30.2 | 18.2 | 125.6 | |
| T3D2 | 90.7 a | 29.5 | 16.9 a | 125.8 | 95.9 | 30.9 | 17.9 | 121.3 | |
| T3D3 | 78.4 b | 30.7 | 14.5 c | 120.6 | 96.2 | 30.9 | 18.7 | 126.2 | |
| T4D1 | 95.6 a | 30.1 | 17.8 a | 123.4 | 97.7 | 30.4 | 19.2 | 129.2 | |
| T4D2 | 79.9 b | 30.5 | 15.0 bc | 122.5 | 96.4 | 29.9 | 18.4 | 127.4 | |
| T4D3 | 55.6 c | 30.5 | 10.6 d | 125.1 | 95.5 | 29.1 | 17.2 | 123.9 | |
| Statistical Significance | Year (Y) | ns | ns | ns | ns | ns | ns | ns | ns |
| Temp (T) | ** | ns | ** | ns | ns | ns | ns | ns | |
| Duration (D) | ** | ns | ** | ns | ns | ns | ns | ns | |
| Y*T | ns | ns | ns | ns | ns | ns | ns | ns | |
| Y*D | ns | ns | ns | ns | ns | ns | ns | ns | |
| T*D | ** | ns | ** | ns | ns | ns | ns | ns | |
| Y*T*D | ns | ns | ns | ns | ns | ns | ns | ns | |
| Nanjing 46 | Control | 91.4 a | 27.3 | 16.7 a | 134 | 94.6 | 26.3 | 16.8 | 134.9 |
| T2D1 | 94.1 a | 26.9 | 16.7 a | 131.8 | 93.4 | 26.4 | 17.0 | 138.0 | |
| T2D2 | 86.8 abc | 26.8 | 16.0 ab | 137.9 | 94.4 | 26.1 | 17.3 | 140.7 | |
| T2D3 | 78.8 cd | 27.2 | 14.6 c | 136.1 | 95 | 26.6 | 17.7 | 139.9 | |
| T3D1 | 94.4 a | 26.9 | 17.3 a | 135.9 | 94.1 | 26 | 16.1 | 131.6 | |
| T3D2 | 82.7 bc | 26.8 | 15.4 ab | 139.2 | 95.3 | 26 | 17.2 | 138.9 | |
| T3D3 | 68.7 de | 26.7 | 12.2 c | 133.8 | 95.7 | 26.1 | 16.5 | 132.3 | |
| T4D1 | 90.1 ab | 26.8 | 16.3 ab | 135.3 | 94.1 | 25.9 | 17.1 | 140.4 | |
| T4D2 | 66.6 e | 27.2 | 12.0 c | 132.0 | 94.7 | 25.3 | 16.5 | 138.0 | |
| T4D3 | 23.9 f | 26.8 | 04.4 d | 137.0 | 94.6 | 25.0 | 16.4 | 138.5 | |
| Statistical | Y | ns | ns | ns | ns | ns | ns | ns | ns |
| Significance | T | ** | ns | ** | ns | ns | * | ns | ns |
| D | ** | ns | ** | ns | ns | ns | ns | ns | |
| Y*T | ns | ns | ns | ns | ns | ns | ns | ns | |
| Y*D | ns | ns | ns | ns | ns | ns | ns | ns | |
| T*D | ** | ns | ** | ns | ns | ns | ns | ns | |
| Y*T*D | ns | ns | ns | ns | ns | ns | ns | ns | |
| Cultivar | Stage | Treatment | SWm (mg spike−1) | B | t50 (d) | p Value | R2 | D (d) | Rmean (mg spike−1 d−1) | Rmax (mg spike−1 d−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| Huaidao 5 | Flowering | Control | 27.4 | 5.2 | 15.4 | <0.0001 | 0.981 | 31.1 | 0.88 | 1.32 |
| T2D1 | 27.1 | 5.0 | 15.0 | <0.0001 | 0.976 | 30.0 | 0.90 | 1.36 | ||
| T2D2 | 27.5 | 5.6 | 16.3 | <0.0001 | 0.983 | 33.1 | 0.83 | 1.23 | ||
| T2D3 | 27.9 | 6.2 | 17.8 | <0.0001 | 0.987 | 36.4 | 0.77 | 1.13 | ||
| T3D1 | 26.7 | 5.3 | 15.6 | <0.0001 | 0.981 | 31.3 | 0.85 | 1.26 | ||
| T3D2 | 27.4 | 6.2 | 17.7 | <0.0001 | 0.986 | 36.4 | 0.75 | 1.10 | ||
| T3D3 | 24.9 | 6.3 | 18.8 | <0.0001 | 0.973 | 37.6 | 0.66 | 0.99 | ||
| T4D1 | 26.7 | 5.8 | 16.6 | <0.0001 | 0.980 | 33.9 | 0.79 | 1.15 | ||
| T4D2 | 27.6 | 6.4 | 19.7 | <0.0001 | 0.975 | 39.0 | 0.71 | 1.08 | ||
| T4D3 | 23.3 | 6.8 | 20.3 | <0.0001 | 0.951 | 40.8 | 0.57 | 0.86 | ||
| Grain Filling | Control | 27.8 | 6.4 | 17.0 | <0.0001 | 0.957 | 36.3 | 0.77 | 1.09 | |
| T2D1 | 27.2 | 6.5 | 17.9 | <0.0001 | 0.971 | 37.4 | 0.73 | 1.05 | ||
| T2D2 | 26.9 | 6.2 | 16.7 | <0.0001 | 0.974 | 35.2 | 0.76 | 1.08 | ||
| T2D3 | 27.2 | 6.7 | 17.6 | <0.0001 | 0.965 | 37.7 | 0.72 | 1.01 | ||
| T3D1 | 27.6 | 6.6 | 18.7 | <0.0001 | 0.969 | 38.4 | 0.72 | 1.05 | ||
| T3D2 | 27.5 | 6.7 | 20.1 | <0.0001 | 0.970 | 40.2 | 0.68 | 1.03 | ||
| T3D3 | 28.4 | 7.1 | 21.5 | <0.0001 | 0.976 | 42.7 | 0.67 | 1.00 | ||
| T4D1 | 27.1 | 6.5 | 19.3 | <0.0001 | 0.986 | 38.9 | 0.70 | 1.04 | ||
| T4D2 | 26.7 | 6.5 | 20.7 | <0.0001 | 0.972 | 40.2 | 0.66 | 1.03 | ||
| T4D3 | 27.1 | 7.0 | 23.2 | <0.0001 | 0.969 | 44.2 | 0.61 | 0.97 | ||
| Nanjing 46 | Flowering | Control | 24.3 | 5.2 | 17.3 | <0.0001 | 0.983 | 32.9 | 0.74 | 1.17 |
| T2D1 | 24.4 | 5.5 | 18.4 | <0.0001 | 0.979 | 35.0 | 0.70 | 1.11 | ||
| T2D2 | 24.1 | 6.6 | 19.1 | <0.0001 | 0.965 | 38.8 | 0.62 | 0.91 | ||
| T2D3 | 21.9 | 6.3 | 20.5 | <0.0001 | 0.936 | 39.8 | 0.55 | 0.87 | ||
| T3D1 | 23.4 | 5.4 | 17.6 | <0.0001 | 0.977 | 33.8 | 0.69 | 1.08 | ||
| T3D2 | 23.5 | 6.5 | 19.6 | <0.0001 | 0.971 | 39.1 | 0.60 | 0.90 | ||
| T3D3 | 19.8 | 5.5 | 20.3 | <0.0001 | 0.939 | 37.0 | 0.54 | 0.90 | ||
| T4D1 | 23.3 | 5.9 | 17.9 | <0.0001 | 0.975 | 35.4 | 0.66 | 0.99 | ||
| T4D2 | 22.0 | 6.3 | 20.7 | <0.0001 | 0.971 | 39.5 | 0.56 | 0.87 | ||
| T4D3 | 16.2 | 6.9 | 20.8 | <0.0001 | 0.951 | 41.3 | 0.39 | 0.59 | ||
| Grain Filling | Control | 23.9 | 5.0 | 16.8 | <0.0001 | 0.978 | 31.6 | 0.76 | 1.20 | |
| T2D1 | 23.4 | 4.9 | 17.0 | <0.0001 | 0.976 | 31.6 | 0.74 | 1.19 | ||
| T2D2 | 23.3 | 5.3 | 17.9 | <0.0001 | 0.981 | 33.7 | 0.69 | 1.10 | ||
| T2D3 | 23.9 | 6.3 | 19.2 | <0.0001 | 0.964 | 38.1 | 0.63 | 0.95 | ||
| T3D1 | 23.7 | 6.1 | 18.4 | <0.0001 | 0.983 | 36.6 | 0.65 | 0.97 | ||
| T3D2 | 23.7 | 6.8 | 19.4 | <0.0001 | 0.985 | 39.9 | 0.59 | 0.87 | ||
| T3D3 | 23.0 | 6.6 | 19.8 | <0.0001 | 0.964 | 39.6 | 0.58 | 0.87 | ||
| T4D1 | 24.1 | 6.1 | 18.9 | <0.0001 | 0.981 | 37.3 | 0.65 | 0.99 | ||
| T4D2 | 23.4 | 6.1 | 19.6 | <0.0001 | 0.978 | 37.7 | 0.62 | 0.96 | ||
| T4D3 | 22.8 | 6.5 | 20.8 | <0.0001 | 0.961 | 40.2 | 0.57 | 0.88 |
| Cultivar | Air Temperature (AT) | Soil Temperature (ST) | Canopy Temperature (CT) | ||||
|---|---|---|---|---|---|---|---|
| Model Parameters | Estimate | Significance | Estimate | Significance | Estimate | Significance | |
| Huaidao 5 | a | 0.8599 | 0.0038 ** | 0.8775 | 0.0036 ** | 0.7767 | 0.0146 * |
| b | −0.0772 | 0.0491 * | −0.0787 | 0.0501 | −0.0845 | 0.0499 * | |
| c | 0.0363 | 0.105 | 0.0377 | 0.1201 | 0.0493 | 0.0935 | |
| d | 0.0044 | 0.0019 ** | 0.0049 | 0.0021 ** | 0.0051 | 0.0028 ** | |
| e | −0.0024 | 0.3439 | −0.0024 | 0.3534 | −0.0024 | 0.3757 | |
| f | −0.0014 | 0.0395 * | −0.0016 | 0.0436 * | −0.0019 | 0.0388 * | |
| R2 | 0.9601 | 0.9584 | 0.954 | ||||
| Nanjing 46 | a | 0.8135 | 0.0573 | 0.8395 | 0.0364* | 0.6942 | 0.1168 |
| b | −0.1383 | 0.0542 | −0.1414 | 0.0372* | −0.1531 | 0.0365 * | |
| c | 0.0534 | 0.1797 | 0.0552 | 0.1586 | 0.0725 | 0.1273 | |
| d | 0.0085 | 0.0015 ** | 0.0095 | 0.0009 *** | 0.0099 | 0.0012 ** | |
| e | −0.0047 | 0.3106 | −0.0047 | 0.2707 | −0.0047 | 0.2943 | |
| f | −0.0023 | 0.0597 | −0.0026 | 0.045 * | −0.0031 | 0.0426 * | |
| R2 | 0.9183 | 0.9319 | 0.9241 |
| Cultivar | Stage | Temperature (Tmin/Tmax) (°C) | Duration (days) | Start of Treatment | End of Treatment |
|---|---|---|---|---|---|
| Huaidao 5 | Flowering | T1 (21/27), T2 (17/23), T3 (13/19) and T4 (09/15) | D1 (3), D2 (6) and D3 (9) | 08/23 in 2018 08/26 in 2019 | 09/01 in 2018 09/04 in 2019 |
| Grain Filling | 09/02 in 2018 09/08 in 2019 | 09/11 in 2018 09/17 in 2019 | |||
| Nanjing 46 | Flowering | 09/08 in 2018 09/12 in 2019 | 09/17 in 2018 09/21 in 2019 | ||
| Grain Filling | 09/21 in 2018 09/23 in 2019 | 09/30 in 2018 10/02 in 2019 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, I.; Tang, L.; Dai, J.; Kang, M.; Mahmood, A.; Wang, W.; Liu, B.; Liu, L.; Cao, W.; Zhu, Y. Responses of Grain Yield and Yield Related Parameters to Post-Heading Low-Temperature Stress in Japonica Rice. Plants 2021, 10, 1425. https://doi.org/10.3390/plants10071425
Ali I, Tang L, Dai J, Kang M, Mahmood A, Wang W, Liu B, Liu L, Cao W, Zhu Y. Responses of Grain Yield and Yield Related Parameters to Post-Heading Low-Temperature Stress in Japonica Rice. Plants. 2021; 10(7):1425. https://doi.org/10.3390/plants10071425
Chicago/Turabian StyleAli, Iftikhar, Liang Tang, Junjie Dai, Min Kang, Aqib Mahmood, Wei Wang, Bing Liu, Leilei Liu, Weixing Cao, and Yan Zhu. 2021. "Responses of Grain Yield and Yield Related Parameters to Post-Heading Low-Temperature Stress in Japonica Rice" Plants 10, no. 7: 1425. https://doi.org/10.3390/plants10071425
APA StyleAli, I., Tang, L., Dai, J., Kang, M., Mahmood, A., Wang, W., Liu, B., Liu, L., Cao, W., & Zhu, Y. (2021). Responses of Grain Yield and Yield Related Parameters to Post-Heading Low-Temperature Stress in Japonica Rice. Plants, 10(7), 1425. https://doi.org/10.3390/plants10071425