Mode-Dependent Antiviral Activity of Medicinal Plant Extracts against the Mosquito-Borne Chikungunya Virus
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Plant Samples and Extraction
3.2. Cell Culture and Virus Cultivation
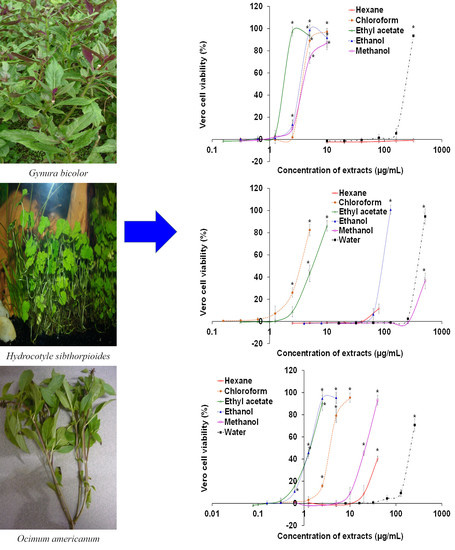
3.3. Cytotoxic Assay
3.4. Cytopathic Effect Inhibitory Assay
3.5. Quantification of Chikungunya Virus RNA Copy Number
3.5.1. Viral RNA Extraction
3.5.2. Generation of Viral RNA Standard
3.5.3. One-Step SYBR Green-Based Real-Time RT-PCR
3.6. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, D.W.; Mackenzie, J.S.; Frolov, I.V.; Weaver, S.C. Alphaviruses. In Clinical Virology, 4th ed.; Richman, D.D., Whitley, R.J., Hayden, F.G., Eds.; ASM Press: Washington, DC, USA, 2017; pp. 1347–1380. [Google Scholar]
- Ross, R.W. The Newala epidemic: III. The virus: Isolation, pathogenic properties and relationship to the epidemic. J. Hyg. 1956, 54, 177–191. [Google Scholar] [CrossRef] [Green Version]
- Vairo, F.; Haider, N.; Kock, R.; Ntoumi, F.; Ippolito, G.; Zumla, A. Chikungunya: Epidemiology, pathogenesis, clinical features, management, and prevention. Infect. Dis. Clin. N. Am. 2019, 33, 1003–1025. [Google Scholar] [CrossRef]
- Silva, L.A.; Dermody, T.S. Chikungunya virus: Epidemiology, replication, disease mechanisms, and prospective intervention strategies. J. Clin. Investig. 2017, 127, 737–749. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention. Chikungunya Virus. Available online: https://www.cdc.gov/chikungunya/index.html (accessed on 3 January 2021).
- Puntasecca, C.J.; King, C.H.; LaBeaud, A.D. Measuring the global burden of chikungunya and Zika viruses: A systematic review. PLoS Negl. Trop. Dis. 2021, 15, e0009055. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Communicable Disease Threats Report, Week 51, 13–19 December 2020. Available online: https://www.ecdc.europa.eu/en/publications-data/communicable-disease-threats-report-13-19-december-2020-week-51 (accessed on 3 January 2021).
- Ministry of Health Malaysia. Kenyataan Akhbar Ketua Pengarah Kesihatan Malaysia Mengenai Situasi Semasa Deman Denggi, Zika Dan Chikungunya Di Malaysia—Minggu Ke 51/2020. Available online: https://www.moh.gov.my/index.php/database_stores/store_view_page/17/1734 (accessed on 3 January 2021). (In Malay)
- Ferreira, F.C.; da Silva, A.S.; Recht, J.; Guaraldo, L.; Moreira, M.E.; de Siqueira, A.M.; Gerardin, P.; Brasil, P. Vertical transmission of chikungunya virus: A systematic review. PLoS ONE 2021, 16, e0249166. [Google Scholar] [CrossRef] [PubMed]
- Natrajan, M.S.; Rojas, A.; Waggoner, J.J. Beyond fever and pain: Diagnostic methods for chikungunya virus. J. Clin. Microbiol. 2019, 57, e00350-19. [Google Scholar] [CrossRef] [Green Version]
- Tanabe, I.S.B.; Tanabe, E.L.L.; Santos, E.C.; Martins, W.V.; Araújo, I.M.T.C.; Cavalcante, M.C.A.; Lima, A.R.V.; Câmara, N.O.S.; Anderson, L.; Yunusov, D.; et al. Cellular and molecular immune response to chikungunya virus infection. Front. Cell. Infect. Microbiol. 2018, 8, 345. [Google Scholar] [CrossRef]
- Van Aalst, M.; Nelen, C.M.; Goorhuis, A.; Stijnis, C.; Grobusch, M.P. Long-term sequelae of chikungunya virus disease: A systematic review. Travel Med. Infect. Dis. 2017, 15, 8–22. [Google Scholar] [CrossRef]
- Powers, A.M. Vaccine and therapeutic options to control chikungunya virus. Clin. Microbiol. Rev. 2017, 31, e00104-16. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Song, S.; Zhang, L. Recent progress in vaccine development against chikungunya virus. Front. Microbiol. 2019, 10, 2881. [Google Scholar] [CrossRef] [Green Version]
- Hucke, F.I.L.; Bugert, J.J. Current and promising antivirals against chikungunya virus. Front. Public Health 2020, 8, 618624. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines on the Conservation of Medicinal Plants; International Union for the Conservation of Nature: Geneva, Switzerland, 1993; pp. 1–38. [Google Scholar]
- Zaynab, M.; Fatima, M.; Abbas, S.; Sharif, Y.; Umair, M.; Zafar, M.H.; Bahadar, K. Role of secondary metabolites in plant defense against pathogens. Microb. Pathog. 2018, 124, 198–202. [Google Scholar] [CrossRef]
- Prinsloo, G.; Marokane, C.K.; Street, R.A. Anti-HIV activity of southern African plants: Current developments, phytochemistry and future research. J. Ethnopharmacol. 2018, 210, 133–155. [Google Scholar] [CrossRef]
- Salehi, B.; Kumar, N.V.A.; Şener, B.; Sharifi-Rad, M.; Kılıç, M.; Mahady, G.B.; Vlaisavljevic, S.; Iriti, M.; Kobarfard, F.; Setzer, W.N.; et al. Medicinal plants used in the treatment of human immunodeficiency virus. Int. J. Mol. Sci. 2018, 19, 1459. [Google Scholar] [CrossRef] [Green Version]
- Akram, M.; Tahir, I.M.; Shah, S.M.A.; Mahmood, Z.; Altaf, A.; Ahmad, K.; Munir, N.; Daniyal, M.; Nasir, S.; Mehboob, H. Antiviral potential of medicinal plants against HIV, HSV, influenza, hepatitis, and coxsackievirus: A systematic review. Phytother. Res. 2018, 32, 811–822. [Google Scholar] [CrossRef]
- Mohan, S.; Elhassan Taha, M.M.; Makeen, H.A.; Alhazmi, H.A.; Al Bratty, M.; Sultana, S.; Ahsan, W.; Najmi, A.; Khalid, A. Bioactive natural antivirals: An updated review of the available plants and isolated molecules. Molecules 2020, 25, 4878. [Google Scholar] [CrossRef]
- Geng, C.A.; Chen, J.J. The progress of anti-HBV constituents from medicinal plants in China. Nat. Prod. Bioprospect. 2018, 8, 227–244. [Google Scholar] [CrossRef] [Green Version]
- Saleh, M.S.M.; Kamisah, Y. Potential medicinal plants for the treatment of dengue fever and severe acute respiratory syndrome-coronavirus. Biomolecules 2020, 11, 42. [Google Scholar] [CrossRef]
- Frederico, É.H.F.F.; Cardoso, A.L.B.D.; Moreira-Marconi, E.; de Sá-Caputo, D.D.C.; Guimarães, C.A.S.; Dionello, C.D.F.; Morel, D.S.; Paineiras-Domingos, L.L.; de Souza, P.L.; Brandão-Sobrinho-Neto, S.; et al. Anti-viral effects of medicinal plants in the management of dengue: A systematic review. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Chan, Y.S.; Khoo, K.S.; Sit, N.W. Investigation of twenty selected medicinal plants from Malaysia for anti-Chikungunya virus activity. Int. Microbiol. 2016, 19, 175–182. [Google Scholar] [CrossRef]
- Ledoux, A.; Cao, M.; Jansen, O.; Mamede, L.; Campos, P.E.; Payet, B.; Clerc, P.; Grondin, I.; Girard-Valenciennes, E.; Hermann, T.; et al. Antiplasmodial, anti-chikungunya virus and antioxidant activities of 64 endemic plants from the Mascarene Islands. Int. J. Antimicrob. Agents 2018, 52, 622–628. [Google Scholar] [CrossRef]
- Weber, C.; Sliva, K.; von Rhein, C.; Kümmerer, B.M.; Schnierle, B.S. The green tea catechin, epigallocatechin gallate inhibits chikungunya virus infection. Antivir. Res. 2015, 113, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Mounce, B.C.; Cesaro, T.; Carrau, L.; Vallet, T.; Vignuzzi, M. Curcumin inhibits Zika and chikungunya virus infection by inhibiting cell binding. Antivir. Res. 2017, 142, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Thiruchelvan, M.; Lee, R.C.; Chen, H.; Chen, K.C.; Ng, M.L.; Chu, J.J. Inhibition of chikungunya virus replication by harringtonine, a novel antiviral that suppresses viral protein expression. Antimicrob. Agents Chemother. 2013, 57, 155–167. [Google Scholar] [CrossRef] [Green Version]
- Maria John, K.M.; Harnly, J.; Luthria, D. Influence of direct and sequential extraction methodology on metabolic profiling. J. Chromatogr. B 2018, 1073, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thongnest, S.; Boonsombat, J.; Prawat, H.; Mahidol, C.; Ruchirawat, S. Ailanthusins A-G and nor-lupane triterpenoids from Ailanthus triphysa. Phytochemistry 2017, 134, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Shukri, R.; Mohamed, S.; Mustapha, N.M.; Hamid, A.A. Evaluating the toxic and beneficial effects of jering beans (Archidendron jiringa) in normal and diabetic rats. J. Sci. Food Agric. 2011, 91, 2697–2706. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, W.; Du, Y.; Su, P.; Qiu, Y.; Ning, J.; Liu, M. Phytochemistry and pharmacological activities of Arundina graminifolia (D.Don) Hochr. and other common Orchidaceae medicinal plants. J. Ethnopharmacol. 2021, 276, 114143. [Google Scholar] [CrossRef]
- Saleem, S.; Muhammad, G.; Hussain, M.A.; Bukhari, S.N.A. A comprehensive review of phytochemical profile, bioactives for pharmaceuticals, and pharmacological attributes of Azadirachta indica. Phytother. Res. 2018, 32, 1241–1272. [Google Scholar] [CrossRef]
- Deshmukh, S.A.; Gaikwad, D.K. A review of the taxonomy, ethnobotany, phytochemistry and pharmacology of Basella alba (Basellaceae). J. Appl. Pharm. Sci. 2014, 4, 153–165. [Google Scholar] [CrossRef]
- Hamedi, S.; Honarvar, M. Beta vulgaris—A mini review of traditional uses in Iran, phytochemistry and pharmacology. Curr. Drug Discov. Technol. 2019, 16, 74–81. [Google Scholar] [CrossRef]
- Kamarudin, M.N.A.; Sarker, M.M.R.; Kadir, H.A.; Ming, L.C. Ethnopharmacological uses, phytochemistry, biological activities, and therapeutic applications of Clinacanthus nutans (Burm. f.) Lindau: A comprehensive review. J. Ethnopharmacol. 2017, 206, 245–266. [Google Scholar] [CrossRef] [PubMed]
- Ayati, Z.; Ramezani, M.; Amiri, M.S.; Moghadam, A.T.; Rahimi, H.; Abdollahzade, A.; Sahebkar, A.; Emami, S.A. Ethnobotany, phytochemistry and traditional uses of Curcuma spp. and pharmacological profile of two important species (C. longa and C. zedoaria): A review. Curr. Pharm. Des. 2019, 25, 871–935. [Google Scholar] [CrossRef]
- Akah, P.A.; Orisakwe, O.E.; Gamaniel, K.S.; Shittu, A. Evaluation of Nigerian traditional medicines: II. Effects of some Nigerian folk remedies on peptic ulcer. J. Ethnopharmacol. 1998, 62, 123–127. [Google Scholar] [CrossRef]
- Abe, R.; Ohtani, K. An ethnobotanical study of medicinal plants and traditional therapies on Batan Island, the Philippines. J. Ethnopharmacol. 2013, 145, 554–565. [Google Scholar] [CrossRef]
- Bunawan, H.; Amin, N.M.; Bunawan, S.N.; Baharum, S.N.; Mohd Noor, N. Ficus deltoidea Jack: A review on its phytochemical and pharmacological importance. Evid. Based Complement. Alternat. Med. 2014, 2014, 902734. [Google Scholar] [CrossRef] [Green Version]
- Teoh, W.Y.; Sim, K.S.; Moses Richardson, J.S.; Abdul Wahab, N.; Hoe, S.Z. Antioxidant capacity, cytotoxicity, and acute oral toxicity of Gynura bicolor. Evid. Based Complement. Altern. Med. 2013, 2013, 958407. [Google Scholar] [CrossRef] [Green Version]
- Siriwatanametanon, N.; Fiebich, B.L.; Efferth, T.; Prieto, J.M.; Heinrich Michael, M. Traditionally used Thai medicinal plants: In vitro anti-inflammatory, anticancer and antioxidant activities. J. Ethnopharmacol. 2010, 130, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Hazarika, I.; Mukundan, G.K.; Sundari, P.S.; Laloo, D. Journey of Hydrocotyle sibthorpioides Lam.: From traditional utilization to modern therapeutics—A review. Phytother. Res. 2021, 35, 1847–1871. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.K. Edible Medicinal and Non-Medicinal Plants: Fruits; Springer Science + Business Media: Dordrecht, The Netherlands, 2013; Volume 6, pp. 110–118. [Google Scholar]
- Zengin, G.; Ferrante, C.; Gnapi, D.E.; Sinan, K.I.; Orlando, G.; Recinella, L.; Diuzheva, A.; Jekő, J.; Cziáky, Z.; Chiavaroli, A.; et al. Comprehensive approaches on the chemical constituents and pharmacological properties of flowers and leaves of American basil (Ocimum americanum L.). Food Res. Int. 2019, 125, 108610. [Google Scholar] [CrossRef]
- Saleh, M.S.M.; Jalil, J.; Zainalabidin, S.; Asmadi, A.Y.; Mustafa, N.H.; Kamisah, Y. Genus Parkia: Phytochemical, medicinal uses, and pharmacological properties. Int. J. Mol. Sci. 2021, 22, 618. [Google Scholar] [CrossRef] [PubMed]
- Farzaei, M.H.; Abbasabadi, Z.; Ardekani, M.R.S.; Rahimi, R.; Farzaei, F. Parsley: A review of ethnopharmacology, phytochemistry and biological activities. J. Tradit. Chin. Med. 2013, 33, 815–826. [Google Scholar] [CrossRef] [Green Version]
- Saleh, M.S.M.; Siddiqui, M.J.; Mediani, A.; Ismail, N.H.; Ahmed, Q.U.; So’ad, S.Z.M.; Saidi-Besbes, S. Salacca zalacca: A short review of the palm botany, pharmacological uses and phytochemistry. Asian Pac. J. Trop. Med. 2018, 11, 645–652. [Google Scholar] [CrossRef]
- Vieira, E.F.; Pinho, O.; Ferreira, I.M.P.L.V.O.; Delerue-Matos, C. Chayote (Sechium edule): A review of nutritional composition, bioactivities and potential applications. Food Chem. 2019, 275, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, S.M.; Amri, C.N.A.C.; Shahari, R. Ethnobotany and traditional knowledge of Acanthaceae in Peninsular Malaysia: A review. Pharmacogn. J. 2020, 12, 1482–1488. [Google Scholar] [CrossRef]
- Bernard, E.; Solignat, M.; Gay, B.; Chazal, N.; Higgs, S.; Devaux, C.; Briant, L. Endocytosis of chikungunya virus into mammalian cells: Role of clathrin and early endosomal compartments. PLoS ONE 2010, 5, e11479. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.; Santhosh, S.R.; Tiwari, M.; Lakshmana Rao, P.V.; Parida, M. Assessment of in vitro prophylactic and therapeutic efficacy of chloroquine against Chikungunya virus in Vero cells. J. Med. Virol. 2010, 82, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Bourjot, M.; Delang, L.; Nguyen, V.H.; Neyts, J.; Guéritte, F.; Leyssen, P.; Litaudon, M. Prostratin and 12-O-tetradecanoylphorbol 13-acetate are potent and selective inhibitors of Chikungunya virus replication. J. Nat. Prod. 2012, 75, 2183–2187. [Google Scholar] [CrossRef]
- Scholte, F.E.; Tas, A.; Martina, B.E.; Cordioli, P.; Narayanan, K.; Makino, S.; Snijder, E.J.; van Hemert, M.J. Characterization of synthetic Chikungunya viruses based on the consensus sequence of recent E1-226V isolates. PLoS ONE 2013, 8, e71047. [Google Scholar] [CrossRef] [Green Version]
- Raghavendhar, S.; Tripati, P.K.; Ray, P.; Patel, A.K. Evaluation of medicinal herbs for Anti-CHIKV activity. Virology 2019, 533, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Sakdarat, S.; Shuyprom, A.; Pientong, C.; Ekalaksananan, T.; Thongchai, S. Bioactive constituents from the leaves of Clinacanthus nutans Lindau. Bioorg. Med. Chem. 2009, 17, 1857–1860. [Google Scholar] [CrossRef]
- Kunsorn, P.; Ruangrungsi, N.; Lipipun, V.; Khanboon, A.; Rungsihirunrat, K. The identities and anti-herpes simplex virus activity of Clinacanthus nutans and Clinacanthus siamensis. Asian Pac. J. Trop. Biomed. 2013, 3, 284–290. [Google Scholar] [CrossRef] [Green Version]
- Sookmai, W.; Ekalaksananan, T.; Pientong, C.; Sakdarat, S.; Kongyingyoes, B. The anti-papillomavirus infectivity of Clinacanthus nutans compounds. Srinagarind Med. J. 2011, 26, 240–243. [Google Scholar]
- Tu, S.-F.; Liu, R.H.; Cheng, Y.-B.; Hsu, Y.-M.; Du, Y.-C.; El-Shazly, M.; Wu, Y.-C.; Chang, F.-R. Chemical constituents and bioactivities of Clinacanthus nutans aerial parts. Molecules 2014, 19, 20382–20390. [Google Scholar] [CrossRef]
- Husin, F.; Chan, Y.Y.; Gan, S.H.; Sulaiman, S.A.; Shueb, R.H. The effect of Hydrocotyle sibthorpioides Lam. extracts on in vitro dengue replication. Evid. Based Complement. Altern. Med. 2015, 2015, 596109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Q.; Zhang, S.; Huang, R.; Wei, L.; Chen, Y.; Lv, S.; Liang, C.; Tan, S.; Liang, S.; Zhuo, L.; et al. Isolation and identification of an anti-hepatitis B virus compound from Hydrocotyle sibthorpioides Lam. J. Ethnopharmacol. 2013, 150, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Yucharoen, R.; Anuchapreeda, S.; Tragoolpua, Y. Anti-herpes simplex virus activity of extracts from the culinary herbs Ocimum sanctum L., Ocimum basilicum L. and Ocimum americanum L. Afr. J. Biotechnol. 2011, 10, 860–866. [Google Scholar]
- Hanisa, H.; Mohd Azmi, M.L.; Suhaila, M.; Somchit, M.N. In vitro anti-viral activity of Centella asiatica L., Curcuma longa L. and Strobilanthes crispus L. against herpes virus. Int. J. Pharma Bio Sci. 2014, 5, B42–B52. [Google Scholar]
- Mouhajir, F.; Hudson, J.B.; Rejdali, M.; Towers, G.H.N. Multiple antiviral activities of endemic medicinal plants used by Berber peoples of Morocco. Pharm. Biol. 2001, 39, 364–374. [Google Scholar] [CrossRef]
- Chan, Y.S.; Ong, C.W.; Chuah, B.L.; Khoo, K.S.; Chye, F.Y.; Sit, N.W. Antimicrobial, antiviral and cytotoxic activities of selected marine organisms collected from the coastal areas of Malaysia. J. Mar. Sci. Technol. 2018, 26, 128–136. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Chan, S.M.; Khoo, K.S.; Sit, N.W. Interactions between plant extracts and cell viability indicators during cytotoxicity testing: Implications for ethnopharmacological studies. Trop. J. Pharm. Res. 2015, 14, 1991–1998. [Google Scholar] [CrossRef] [Green Version]
- Ali, U.H.; Vasan, S.S.; Thayan, R.; Angamuthu, C.; Lim, L.H.; Sekaran, S.D. Development and evaluation of a one-step SYBR-Green I-based realtime RT-PCR assay for the detection and quantification of Chikungunya virus in human, monkey and mosquito samples. Trop. Biomed. 2010, 27, 611–623. [Google Scholar]
- Krieg, P.A. Improved synthesis of full-length RNA probe at reduced incubation temperatures. Nucleic Acids Res. 1990, 18, 6463. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Plant Name | Family | Vernacular Name | Part Used | Medicinal or Folkloric Uses | Voucher Number |
|---|---|---|---|---|---|
| Ailanthus triphysa (Dennst.) Alston | Simaroubaceae | White siris | Leaf | Hypertension, bronchitis, dysentery [32] | UTAR/FSC/11/004 |
| Archidendron jiringa (Jack) I.C.Nielsen | Leguminosae | Djengkol bean | Seed | Bladder stones, hypertension, diabetes [33] | Nil |
| Arundina graminifolia (D.Don) Hochr. | Orchidaceae | Grass orchid | Leaf | Snake bites, rheumatism, stomachache [34] | UTAR/FSC/10/011 |
| Azadirachta indica A.Juss. | Meliaceae | Neem | Leaf | Leprosy, skin ulcers, biliousness, epistaxis, eye problem, anorexia, intestinal worms [35] | UTAR/FSC/11/001 |
| Basella alba L. | Basellaceae | Ceylon spinach, Malabar spinach | Leaf | Constipation, liver and urinary diseases, catarrh, gonorrhea, boils, sore throat, hypertension, burns [36] | UTAR/FSC/10/014 |
| Beta vulgaris L. | Amaranthaceae | Beetroot | Root | Dandruff, decreased libido, constipation, joint pain [37] | Nil |
| Clinacanthus nutans (Burm.f.) Lindau | Acanthaceae | Sabah snake grass | Leaf | Diabetes, dysentery, eye diseases, skin rashes, allergic responses, insect and snake bites [38] | UTAR/FSC/11/003 |
| Curcuma longa L. | Zingiberaceae | Turmeric | Rhizome | Stomachic and intestinal diseases, arthritis, gall stones, emmenagogue, bruise, as a tonic [39] | Nil |
| Diodella sarmentosa (Sw.) Bacigalupo & Cabral ex Borhidi | Rubiaceae | Tropical buttonweed | Leaf and stem | Ulcers, snake bite, rheumatic inflammatory disorders, venereal diseases [40] | UTAR/FSC/10/018 |
| Diplazium esculentum (Retz.) Sw. | Athyriaceae | Vegetable fern | Leaf and stem | Constipation, hypertension [41] | UTAR/FSC/10/023 |
| Ficus deltoidea Jack | Moraceae | Mistletoe fig | Leaf | Wounds, rheumatism, sores, as an after-birth tonic [42] | UTAR/FSC/10/021 |
| Gynura bicolor (Roxb. ex Willd.) DC. | Compositae | Okinawa spinach | Leaf | Blood circulation improvement, diabetes, dysmenorrhea, hemoptysis, post-labor recovery [43] | UTAR/FSC/11/005 |
| Homalocladium platycladum (F.Muell.) L.H.Bailey. | Polygonaceae | Centipede plant | Stem | Skin swelling, sores, insect and snake bites, fracture injuries, fever [44] | UTAR/FSC/10/017 |
| Hydrocotyle sibthorpioides Lam. | Araliaceae | Lawn marsh pennywort | Whole plant | Cough, cold, fever, zoster, eczema, hepatitis, jaundice [45] | UTAR/FSC/10/019 |
| Manilkara zapota (L.) P.Royen | Sapotaceae | Sapodilla, Ciku | Fruit | Diarrhea, pulmonary complaints [46] | Nil |
| Ocimum americanum L. | Lamiaceae | Hoary basil | Leaf | Fever, colds, dysentery, toothache, migraine [47] | UTAR/FSC/10/013 |
| Parkia speciosa Hassk. | Leguminosae | Stink bean | Seed and pod | Urinary infections, diabetes, loss of appetite [48] | UTAR/FSC/10/015 |
| Petroselinum crispum (Mill.) Fuss | Apiaceae | Parsley | Leaf and stem | Skin diseases, eczema, hypertension, diabetes, nosebleed, constipation pain, baldness [49] | UTAR/FSC/10/024 |
| Salacca zalacca (Gaertn.) Voss | Arecaceae | Salak | Fruit | Diabetes [50] | Nil |
| Sechium edule (Jacq.) Sw. | Cucurbitaceae | Chayote | Leaf and stem | Kidney stones, hypertension [51] | UTAR/FSC/10/022 |
| Strobilanthes crispus (L.) Blume | Acanthaceae | Yellow strobilanthus, “kejibeling” | Leaf | Kidney stones, enhance immune system [52] | UTAR/FSC/10/020 |
| Plant | Part | Concurrent Mode | Non-Concurrent Mode | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Extract # | HX | CF | EA | EN | MN | WT | HX | CF | EA | EN | MN | WT | |
| Ailanthus triphysa | Leaf | W | W | W | W | W | W | W | W | W | W | W | W |
| Archidendron jiringa | Seed | W | I | W | W | W | W | W | I | W | W | W | W |
| Arundina graminifolia | Leaf | W | W | W | W | W | W | W | I | I | W | W | W |
| Azadirachta indica | Leaf | I | S | S | S | S | W | W | W | W | W | W | W |
| Basella alba | Leaf | W | I | W | W | W | W | I | I | I | W | W | W |
| Beta vulgaris | Root | W | W | W | W | W | W | W | W | W | W | W | W |
| Clinacanthus nutans | Leaf | W | S | S | S | S | W | W | W | W | W | I | W |
| Curcuma longa | Rhizome | W | I | W | W | W | I | W | W | W | W | W | W |
| Diodella sarmentosa | Leaf and stem | W | S | S | S | W | W | W | I | W | I | W | W |
| Diplazium esculentum | Leaf and stem | W | S | S | S | S | W | W | W | W | I | W | W |
| Ficus deltoidea | Leaf | W | S | I | S | W | W | W | I | S | S | S | W |
| Gynura bicolor | Leaf | W | S | S | S | S | S | W | W | I | W | W | W |
| Homalocladium platycladum | Stem | W | S | S | S | I | S | W | W | I | W | W | W |
| Hydrocotyle sibthorpioides | Whole plant | W | S | I | W | S | W | W | W | W | W | W | W |
| Manilkara zapota | Fruit | W | W | W | W | W | W | W | W | W | W | W | W |
| Ocimum americanum | Leaf | I | S | S | S | S | S | W | W | W | W | W | W |
| Parkia speciosa | Pod | W | I | I | W | W | W | W | W | W | W | W | W |
| Parkia speciosa | Seed | W | W | W | W | W | W | W | W | W | W | W | W |
| Petroselinum crispum | Leaf and stem | W | S | S | S | W | W | W | W | W | I | W | W |
| Salacca zalacca | Fruit | W | W | W | W | W | W | W | W | W | W | W | W |
| Sechium edule | Leaf and stem | W | S | S | S | W | W | W | W | I | I | W | W |
| Strobilanthes crispus | Leaf | W | S | I | S | S | W | W | W | W | W | W | W |
| Plant | Extract | Half-Maximal Cytotoxic Concentration, CC50 (µg/mL) | Mode ^ | Half-Maximal Effective Concentration, EC50 (µg/mL) | Selectivity Index (= CC50/EC50) | Viral RNA Copy Number (Molecules/µL) | Log Reduction # |
|---|---|---|---|---|---|---|---|
| Clinacanthus nutans | Chloroform | 602.67 ± 9.29 | C | 120.67 ± 4.62 | 4.99 | 7.75 × 109 ± 1.69 × 109 * | 0.89 |
| Ethyl acetate | 133.00 ± 9.17 | C | 9.93 ± 0.91 | 13.39 | 1.68 × 1010 ± 0.51 × 1010 * | 0.55 | |
| Ethanol | >640 | C | 31.30 ± 0.95 | >20.45 | 8.72 × 109 ± 1.25 × 109 * | 0.83 | |
| Diodella sarmentosa | Ethyl acetate | 203.33 ± 6.11 | C | 8.33 ± 0.57 | 24.40 | 1.83 × 105 ± 1.07 × 105 * † | 5.51 |
| Diplazium esculentum | Chloroform | 99.00 ± 3.61 | C | 6.80 ± 0.26 | 14.56 | 4.23 × 104 ± 0.59 × 104 * † | 6.15 |
| Ethyl acetate | 184.33 ± 9.24 | C | 14.07 ± 0.06 | 13.10 | 1.38 × 104 ± 0.62 × 104 * † | 6.63 | |
| Ethanol | 220.67 ± 1.53 | C | 14.30 ± 0.20 | 15.43 | 3.40 × 104 ± 1.02 × 104 * † | 6.24 | |
| Methanol | 461.00 ± 1.73 | C | 29.70 ± 0.60 | 15.52 | 1.12 × 109 ± 0.11 × 109 * † | 1.73 | |
| Ficus deltoidea | Ethanol | >640 | NC | 15.20 ± 0.20 | >42.10 | 4.42 × 106 ± 2.71 × 106 † | 4.13 |
| Gynura bicolor | Chloroform | 117.67 ± 9.50 | C | 3.65 ± 0.06 | 32.21 | 3.50 × 109 ± 1.18 × 109 * † | 1.23 |
| Ethyl acetate | 31.33 ± 4.16 | C | 1.91 ± 0.03 | 16.43 | 3.71 × 108 ± 2.90 × 108 † | 2.21 | |
| Ethanol | 55.00 ± 3.46 | C | 3.62 ± 0.10 | 15.18 | 2.33 × 105 ± 0.58 × 105 * † | 5.41 | |
| Water | > 640 | C | 244.67 ± 4.73 | >2.62 | 3.29 × 105 ± 1.78 × 105 † | 5.26 | |
| Hydrocotyle sibthorpioides | Ethanol | 610.33 ± 9.50 | C | 95.33 ± 2.47 | 6.40 | 4.01 × 1010 ± 1.54 × 1010 * | 0.17 |
| Water | - | C | 394.00 ± 6.93 | - | 4.39 × 105 ± 2.74 × 105 † | 5.13 | |
| Ocimum americanum | Chloroform | 86.33 ± 4.73 | C | 3.61 ± 0.11 | 23.92 | 5.50 × 105 ± 0.75 × 105 † | 5.03 |
| Ethyl acetate | 60.83 ± 2.02 | C | 1.37 ± 0.06 | 4.45 | 3.57 × 105 ± 0.26 × 105 † | 5.22 | |
| Ethanol | 226.33 ± 9.87 | C | 1.33 ± 0.10 | 170.18 | 1.71 × 1010 ± 0.48 × 1010 * | 0.54 | |
| Methanol | >640 | C | 21.93 ± 0.84 | >29.18 | 7.81 × 109 ± 2.32 × 109 * | 0.88 | |
| Sechium edule | Ethyl acetate | 100.67 ± 9.29 | C | 2.71 ± 0.25 | 37.10 | 7.43 × 109 ± 2.79 × 109 * | 0.90 |
| Ethanol | - | C | 90.33 ± 0.28 | - | 1.14 × 1010 ± 0.16 × 1010 * | 0.72 | |
| Chloroquine | - | 16.33 ± 0.76 | NC | 9.05 ± 0.05 | 2.89 | 1.68 × 106 ± 0.49 × 106 * † | 4.55 |
| C | 1.92 ± 0.13 ** | 13.65 | 3.94 × 105 ± 0.70 × 105 † | 5.18 | |||
| Virus inoculum | - | - | - | - | - | 6.25 × 105 ± 2.09 × 105 | - |
| Virus control | - | - | - | - | - | 5.96 × 1010 ± 3.33 × 1010 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, S.M.; Khoo, K.S.; Sekaran, S.D.; Sit, N.W. Mode-Dependent Antiviral Activity of Medicinal Plant Extracts against the Mosquito-Borne Chikungunya Virus. Plants 2021, 10, 1658. https://doi.org/10.3390/plants10081658
Chan SM, Khoo KS, Sekaran SD, Sit NW. Mode-Dependent Antiviral Activity of Medicinal Plant Extracts against the Mosquito-Borne Chikungunya Virus. Plants. 2021; 10(8):1658. https://doi.org/10.3390/plants10081658
Chicago/Turabian StyleChan, Sze Mun, Kong Soo Khoo, Shamala Devi Sekaran, and Nam Weng Sit. 2021. "Mode-Dependent Antiviral Activity of Medicinal Plant Extracts against the Mosquito-Borne Chikungunya Virus" Plants 10, no. 8: 1658. https://doi.org/10.3390/plants10081658
APA StyleChan, S. M., Khoo, K. S., Sekaran, S. D., & Sit, N. W. (2021). Mode-Dependent Antiviral Activity of Medicinal Plant Extracts against the Mosquito-Borne Chikungunya Virus. Plants, 10(8), 1658. https://doi.org/10.3390/plants10081658