Possible Overestimation of Seed Transmission in the Spread of Pospiviroids in Commercial Pepper and Tomato Crops Based on Large-Scale Grow-Out Trials and Systematic Literature Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Grow-Out Trials
2.1.1. Selection of Seed Lots
2.1.2. Confirmation of the Identity and Viability of the Viroids in the Selected Seed Lots
2.1.3. Conditions Grow-Out Trials
2.1.4. Detection of Pospiviroids in Leaf Samples from Seedlings
2.2. Review of Published Data on Seed Transmission and Outbreaks
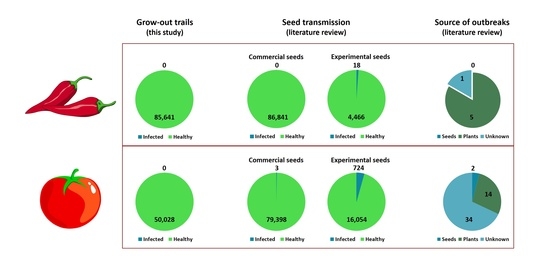
3. Results
3.1. Grow-Out Trials
3.1.1. Confirmation of the Identity and Viability of the Viroids in the Selected Seed Lots
3.1.2. Detection of Pospiviroids in Leaf Samples from Seedlings
3.2. Review of Published Data on Seed Transmission and Data from This Study
3.2.1. Pepper
| CLVd | PCFVd | PSTVd | TASVd | TCDVd | TPMVd | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 179 a | 11 | 59 b | 0 | 25 d | 0 | 217 a | 0 | 1105 e | 0 | 46 c |
| 0 | 337 1a | 7 | 2230 2e | ||||||||
| 0 | 46 c | 0 | 222 a | ||||||||
| CLVd | PCFVd | PSTVd | TASVd | ||||
|---|---|---|---|---|---|---|---|
| 0 | 27,703 a | 0 | 55,438 1a | 0 | 2500 a | 0 | 1200 b |
3.2.2. Tomato
| CLVd | CEVd | PCFVd | PSTVd | TASVd | TCDVd | TPMVd | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1599 1,a | 0 | 1849 1,a | 3 | 941 d | 0 | 22 a | 24 | 30 k | 0 | 4000 l | 0 | 425 n |
| 0 | 200 b | 3 | 60 e | 0 | 1232 1,a | 0 | 251c | 13 | 1039 d | ||||
| 46 | 793 2c | 0 | 25 f | 0 | 1343 3,c | 0 | ? m | ||||||
| 111 | 285 4,c | 209 | 280 j | ||||||||||
| 0 | 92 5,g | ||||||||||||
| 178 | 350 h | ||||||||||||
| 107 | 1192 i | ||||||||||||
| 30 | 46 j | ||||||||||||
| CLVd | PCFVd | PSTVd | TCDVd | ||||
|---|---|---|---|---|---|---|---|
| 0 | 25,500 a | 0 | 47,528 b | 1 | 370 c | 2–20 1 | 2500 e |
| 0 | 1000 d | ||||||
| 0 | 2500 b | ||||||
3.3. Review of Published Data on Outbreaks
3.3.1. Pepper
3.3.2. Tomato
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Candresse, T.; Verhoeven, J.T.J.; Stancanelli, G.; Hammond, R.W.; Winter, S. Other pospiviroids infecting solanaceous plants. In Viroids and Satellites; Hadidi, A., Flores, R., Palukaitis, P., Randles, J., Eds.; Elsevier Academic Press: London, UK, 2017; pp. 159–168. [Google Scholar]
- Hammond, R.W. Economic significance of viroids in vegetable and field crops. In Viroids and Satellites; Hadidi, A., Flores, R., Palukaitis, P., Randles, J., Eds.; Elsevier Academic Press: London, UK, 2017; pp. 5–13. [Google Scholar]
- Barba, M.; James, D. Quarantine and certification for viroids and viroid diseases. In Viroids and Satellites; Hadidi, A., Flores, R., Palukaitis, P., Randles, J., Eds.; Elsevier Academic Press: London, UK, 2017; pp. 415–424. [Google Scholar]
- Owens, R.A.; Verhoeven, J.T.J. Potato spindle tuber viroid. In Viroids and Satellites; Hadidi, A., Flores, R., Palukaitis, P., Randles, J., Eds.; Elsevier Academic Press: London, UK, 2017; pp. 149–158. [Google Scholar]
- Di Serio, F.; Li, S.-F.; Pallás, V.; Owens, R.A.; Randles, J.W.; Sano, T.; Verhoeven, J.T.J.; Vidalakis, G.; Flores, R. Viroid taxonomy. In Viroids and Satellites; Hadidi, A., Flores, R., Palukaitis, P., Randles, J., Eds.; Elsevier Academic Press: London, UK, 2017; pp. 135–146. [Google Scholar]
- Di Serio, F.; Owens, R.A.; Li, S.-F.; Matoušek, J.; Pallás, V.; Randles, J.W.; Sano, T.; Verhoeven, J.T.J.; Vidalakis, G.; Flores, R. ICTV Virus Taxonomy Profile: Pospiviroidae. J. Gen. Virol. 2020, 102, 001543. [Google Scholar]
- Verhoeven, J.T.J.; Hammond, R.W.; Stancanelli, G. Economic significance of viroids in ornamental crops. In Viroids and Satellites; Hadidi, A., Flores, R., Palukaitis, P., Randles, J., Eds.; Elsevier Academic Press: London, UK, 2017; pp. 27–38. [Google Scholar]
- Manzer, F.E.; Merriam, D. Field transmission of the potato spindle tuber virus and virus X by cultivating and hilling equipment. Am. Potato J. 1961, 38, 346–352. [Google Scholar] [CrossRef]
- Verhoeven, J.T.J.; Huner, L.; Virscek Marn, M.; Mavric Plesko, I.; Roenhorst, J.W. Mechanical transmission of Potato spindle tuber viroid between plants of Brugmansia suaveolens, Solanum jasminoides and potatoes and tomatoes. Eur. J. Plant Pathol. 2010, 128, 417–421. [Google Scholar] [CrossRef]
- Mackie, A.E.; McKirdy, S.J.; Rodoni, B.; Kumar, S. Potato spindle tuber viroid eradicated in Western Australia. Australas. Plant Pathol. 2002, 31, 311–312. [Google Scholar] [CrossRef]
- Matsushita, Y.; Kanda, A.; Usugi, T.; Tsuda, S. First report of Tomato chlorotic dwarf viroid disease on tomato plants in Japan. J. Gen. Plant Pathol. 2008, 74, 182–184. [Google Scholar] [CrossRef]
- Verhoeven, J.T.J.; Jansen, C.C.C.; Willemen, T.M.; Kox, L.F.F.; Owens, R.A.; Roenhorst, J.W. Natural infections of tomato by Citrus exocortis viroid, Columnea latent viroid, Potato spindle tuber viroid and Tomato chlorotic dwarf viroid. Eur. J. Plant Pathol. 2004, 110, 823–831. [Google Scholar] [CrossRef]
- Navarro, B.; Silletti, M.R.; Trisciuzzi, V.N.; Di Serio, F. Identification and characterization of Potato spindle tuber viroid infecting tomato in Italy. J. Plant Pathol. 2009, 91, 723–726. [Google Scholar]
- Verhoeven, J.T.J.; Jansen, C.C.C.; Botermans, M.; Roenhorst, J.W. Epidemiological evidence that vegetatively propagated, solanaceous plant species act as sources of Potato spindle tuber viroid inoculum for tomato. Plant Pathol. 2010, 59, 3–12. [Google Scholar] [CrossRef]
- Querci, M.; Owens, R.A.; Bartoli, I.; Lazarte, V.; Salazar, L.F. Evidence for heterologous encapsidation of potato spindle tuber viroid in particles of potato leafroll virus. J. Gen. Virol. 1997, 78, 1207–1211. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kurz, J. RT-PCR analysis of PSTVd aphid transmission in association with PLRV. Can. J. Plant Pathol. 1997, 19, 418–424. [Google Scholar] [CrossRef]
- Vo, T.T.; Dehne, H.W.; Hamacher, J. Transmission of Tomato chlorotic dwarf viroid by Myzus persicae assisted by Potato leafroll virus. J. Plant Dis. Prot. 2018, 125, 259–266. [Google Scholar] [CrossRef]
- Antignus, Y.; Lachman, O.; Pearlsman, M. Spread of Tomato apical stunt viroid (TASVd) in greenhouse tomato crops is associated with seed transmission and bumble bee activity. Plant Dis. 2007, 91, 47–50. [Google Scholar] [CrossRef] [Green Version]
- Matsuura, S.; Matsushita, Y.; Kozuka, R.; Shimizu, S.; Tsuda, S. Transmission of Tomato chlorotic dwarf viroid by bumblebees (Bombus ignitus) in tomato plants. Eur. J. Plant Pathol. 2010, 126, 111–115. [Google Scholar] [CrossRef]
- Nielsen, S.L.; Enkegaard, A.; Nicolaisen, M.; Kryger, P.; Virscek Marn, M.; Mavric Plesko, I.; Kahrer, A.; Gottsberger, R.A. No transmission of Potato spindle tuber viroid shown in experiments with thrips (Frankliniella occidentalis, Thrips tabaci), honey bees (Apis mellifera) and bumblebees (Bombus terrestris). Eur. J. Plant Pathol. 2012, 133, 505–509. [Google Scholar] [CrossRef]
- Faggioli, F.; Luigi, M.; Sveikauskas, V.; Olivier, T.; Virscek Marn, M.; Mavric Plesko, I.; De Jonghe, K.; Van Bogaert, N.; Grausgruber-Gröger, S. An assessment of the transmission rate of four pospiviroid species through tomato seeds. Eur. J. Plant Pathol. 2015, 143, 613–617. [Google Scholar] [CrossRef]
- Simmons, H.E.; Ruchti, T.B.; Munkvold, G.P. Frequencies of seed infection and transmission to seedlings by potato spindle tuber viroid (a Pospiviroid) in tomato. J. Plant Pathol. Microbiol. 2015, 6, 1000275. [Google Scholar]
- Candresse, T.; Marais, A.; Tassus, X.; Suhard, P.; Renaudin, I.; Leguay, A.; Poliakoff, F.; Blancard, D. First report of Tomato chlorotic dwarf viroid in tomato in France. Plant Dis. 2010, 94, 633. [Google Scholar] [CrossRef]
- Van Brunschot, S.L.; Verhoeven, J.T.J.; Persley, D.M.; Geering, A.D.W.; Drenth, A.; Thomas, J.E. An outbreak of Potato spindle tuber viroid in tomato is linked to imported seed. Eur. J. Plant Pathol. 2014, 139, 1–7. [Google Scholar] [CrossRef]
- EPPO. PM7/138 Detection and identification of Pospiviroids. Bull. OEPP 2021, 51, 144–177. [Google Scholar]
- Spieker, R.L. A viroid from Brunfelsia undulata closely related to the Columnea latent viroid. Arch. Virol. 1996, 141, 1823–1832. [Google Scholar] [CrossRef]
- Verhoeven, J.T.J.; Jansen, C.C.C.; Roenhorst, J.W.; Flores, R.; De la Peña, M. Pepper chat fruit viroid: Biological and molecular properties of a proposed new species of the genus Pospiviroid. Virus Res. 2009, 144, 209–214. [Google Scholar] [CrossRef]
- Verhoeven, J.T.J.; Koenraadt, H.M.S.; Westenberg, M.; Roenhorst, J.W. Characterization of tomato apical stunt viroid isolated from a 24-year old seed lot of Capsicum annuum. Arch. Virol. 2017, 162, 1741–1744. [Google Scholar] [CrossRef] [PubMed]
- Van de Vossenberg, B.T.L.H.; Van der Straten, M.J. Development and validation of real-time PCR tests for the identification of four Spodoptera species: Spodoptera eridania, Spodoptera frugiperda, Spodoptera littoralis, and Spodoptera litura (Lepidoptera: Noctuidae). J. Econ. Entomol. 2014, 107, 1643–1654. [Google Scholar] [CrossRef] [PubMed]
- Botermans, M.; Van de Vossenberg, B.T.L.H.; Verhoeven, J.T.J.; Roenhorst, J.W.; Hooftman, M.; Dekter, R.; Meekes, E.T.M. Development and validation of a real-time RT-PCR assay for generic detection of pospiviroids. J. Virol. Meth. 2013, 187, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, J.T.J.; Koenraadt, H.M.S.; Jodlowska, A.; Huner, L.; Roenhorst, J.W. Pospiviroids infections in Capsicum annuum: Disease symptoms and lack of seed transmission. Eur. J. Plant Pathol. 2020, 156, 21–29. [Google Scholar] [CrossRef]
- Yanagisawa, H.; Matsushita, Y. Host ranges and seed transmission of Tomato planta macho viroid and Pepper chat fruit viroid. Eur. J. Plant Pathol. 2017, 149, 211–217. [Google Scholar] [CrossRef]
- Matsushita, Y.; Tsuda, S. Seed transmission of potato spindle tuber viroid, tomato chlorotic dwarf viroid, tomato apical stunt viroid, and Columnea latent viroid in horticultural plants. Eur. J. Plant Pathol. 2016, 145, 1007–1011. [Google Scholar] [CrossRef]
- Lebas, B.S.M.; Clover, G.R.G.; Ochoa-Corona, F.M.; Elliott, D.R.; Tang, Z.; Alexander, B.J.R. Distribution of Potato spindle tuber viroid in New Zealand glasshouse crops of capsicum and tomato. Australas. Plant Pathol. 2005, 34, 129–133. [Google Scholar] [CrossRef]
- Semancik, J.S. Citrus exocortis viroid. Descr. Plant Viruses 1980, 226. Available online: https://www.dpvweb.net/dpv/showdpv/?dpvno=226 (accessed on 23 May 2021).
- Fox, A.; Monger, W. Detection and Elimination of Solanaceous Viroids in Tomato Seeds and Seedlings; Final Report of the Project PC294, December 2010; Horticultural Development Company: Kenilworth, UK, 2011; 24p, Available online: https://ahdb.org.uk/pc-294-detection-and-elimination-of-solanaceous-viroids-in-tomato-seeds-and-seedlings (accessed on 23 May 2021).
- Khoury, J.; Singh, R.P.; Boucher, A.; Coombs, D.H. Concentration and distribution of mild and severe strains of potato spindle tuber viroid in cross-protected tomato plants. Phytopathology 1988, 78, 1331–1336. [Google Scholar] [CrossRef]
- Singh, R.P.; Dilworth, A.D. Tomato chlorotic dwarf viroid in the ornamental plant Vinca minor and its transmission through tomato seed. Eur. J. Plant Pathol. 2009, 123, 111–116. [Google Scholar] [CrossRef]
- Benson, A.P.; Singh, R.P. Seed transmission of Potato Spindle Tuber Virus in tomato. Am. Potato J. 1964, 41, 294. [Google Scholar]
- Kryczynski, S.; Paduch-Cichal, E.; Skrzeczkowsk, L.R. Transmission of three viroids through seed and pollen of tomato plants. J. Phytopathol. 1988, 121, 51–57. [Google Scholar] [CrossRef]
- Menzel, W.; Winter, S. Investigations on seed- and aphid-transmissibility of Potato spindle tuber viroid. Julius-Kühn-Archiv 2010, 428, 392–393. [Google Scholar]
- Batuman, O.; Çiftçi, O.C.; Osei, M.K.; Miller, S.A.; Rojas, M.R.; Gilbertson, R.L. Rasta disease of tomato in Ghana is caused by the pospiviroids potato spindle tuber viroid and tomato apical stunt viroid. Plant Dis. 2019, 103, 1525–1535. [Google Scholar] [CrossRef] [Green Version]
- McClean, A.P.D. Bunchy-top disease of the tomato: Additional host plants, and the transmission of the virus through seed of infected plants. Union S. Afr. Dept. Agric. Sci. Bull. 1948, 256, 1–28. [Google Scholar]
- Singh, R.P.; Nie, X.; Singh, M. Tomato chlorotic dwarf viroid: An evolutionary link in the origin of pospiviroids. J. Gen. Virol. 1999, 80, 2823–2828. [Google Scholar] [CrossRef]
- Koenraadt, H.; Jodlowska, A.; Van Vliet, A.; Verhoeven, K. Detection of TCDVd and PSTVd in seeds of tomato. Phytopathology 2009, 99, S66. [Google Scholar]
- Belalcazar, C.S.; Galindo, A.J. Estudio sobre el virus de la “planta macho” de; jitomate (Lycopersicon sculentum Mill). Agrocienc. Urug. 1974, 18, 79–88. [Google Scholar]
- Singh, R.P. Seed transmission of Potato spindle tuber virus in Tomato and Potato. Am. Potato J. 1970, 47, 225–227. [Google Scholar] [CrossRef]
- Verhoeven, J.T.J.; Roenhorst, J.W.; Van Vliet, A.C.A.; Ebskamp, M.J.M.; Koenraadt, H.M.S. A potato spindle tuber viroid-positive tested seed lot of tomato may produce a viroid-free crop. In Proceedings of the Abstracts of the 5th Conference of the International Working Group of Legume and Vegetable Viruses, Haarlem, The Netherlands, 30 August–3 September 2015; p. 95. [Google Scholar]
- Elliott, D.R.; Alexander, B.J.R.; Smales, T.E.; Tang, Z.; Clover, G.R.G. First report of Potato spindle tuber viroid in tomato in New Zealand. Plant Dis. 2001, 85, 1027. [Google Scholar] [CrossRef]
- Ward, L.I.; Tang, J.; Veerakone, S.; Quinn, B.D.; Harper, S.J.; Delmiglio, C.; Clover, G.R.G. First Report of Potato spindle tuber viroid in cape gooseberry (Physalis peruviana) in New Zealand. Plant Dis. 2010, 94, 479. [Google Scholar] [CrossRef]
- Mackie, A.E.; Rodoni, B.C.; Barbetti, M.J.; McKirdy, S.J.; Jones, R.A.C. Potato spindle tuber viroid: Alternative host reservoirs and strain found in a remote subtropical irrigation area. Eur. J. Plant Pathol. 2016, 145, 433–446. [Google Scholar] [CrossRef]
- EPPO. First confirmed report of Potato spindle tuber viroid in Switzerland. EPPO Report. Serv. 2016, 4, 084. [Google Scholar]
- Verhoeven, J.T.J.; Voogd, J.G.B.; Strik, N.; Roenhorst, J.W. First report of Potato spindle tuber viroid in vegetatively propagated plants of Capsicum annuum in The Netherlands. N. Dis. Rep. 2016, 34, 12. [Google Scholar] [CrossRef] [Green Version]
- Verhoeven, J.T.J.; Botermans, M.; Janse, C.C.C.; Roenhorst, J.W. First report of Pepper chat fruit viroid in capsicum pepper in Canada. N. Dis. Rep. 2011, 23, 15. [Google Scholar] [CrossRef] [Green Version]
- Ling, K.-S.; Zhang, W. First report of a natural infection Mexican papita viroid and Tomato chlorotic dwarf viroid on greenhouse tomatoes in Mexico. Plant Dis. 2009, 93, 1216. [Google Scholar] [CrossRef]
- Matsushita, Y.; Usugi, T.; Tsuda, S. Development of a multiplex RT-PCR detection and identification system for Potato spindle tuber viroid and Tomato chlorotic dwarf viroid. Eur. J. Plant Pathol. 2010, 128, 165–170. [Google Scholar] [CrossRef]
- Batuman, O.; Gilbertson, R.L. First Report of Columnea latent viroid (CLVd) in Tomato in Mali. Plant Dis. 2013, 97, 692. [Google Scholar] [CrossRef]
- EPPO. Situation of Potato spindle tuber viroid in Austria in 2008. EPPO Report. Serv. 2008, 9, 177. [Google Scholar]
- Hailstones, D.L.; Tesoriero, L.A.; Terras, M.A.; Dephoff, C. Detection and eradication of Potato spindle tuber viroid in tomatoes in commercial production in New South Wales, Australia. Australas. Plant Pathol. 2003, 32, 317–318. [Google Scholar] [CrossRef]
- Nixon, T.; Glover, R.; Mathews-Berry, S.; Daly, M.; Hobden, E.; Lanbourne, C.; Harju, V.; Skelton, A. Columnea latent viroid (CLVd) in tomato: The first report in the United Kingdom. N. Dis. Rep. 2009, 19, 30. [Google Scholar] [CrossRef]
- Antignus, Y.; Lachman, O.; Pearlsmand, M.; Gofman, R.; Bar-Joseph, M. A new disease of greenhouse tomatoes in Israel caused by a distinct strain to Tomato apical stunt viroid (TASVd). Phytoparasitica 2002, 30, 502–510. [Google Scholar] [CrossRef]
- Fagoaga, C.; Duran-Vila, N. Naturally occurring variants of citrus exocortis viroid in vegetable crops. Plant Pathol. 1996, 45, 45–53. [Google Scholar] [CrossRef]
- EPPO. Occurrence of Potato spindle tuber pospiviroid (Pstvd) in tomato plants in Germany. EPPO Report. Serv. 2004, 1, 006. [Google Scholar]
- Verhoeven, J.T.J.; Botermans, M.; Meekes, E.T.M.; Roenhorst, J.W. Tomato apical stunt viroid in the Netherlands: Most prevalent pospiviroid in ornamentals and first outbreak in tomatoes. Eur. J. Plant Pathol. 2012, 133, 803–810. [Google Scholar] [CrossRef]
- Fox, A.; Daly, M.; Nixon, T.; Brurberg, M.B.; Blystad, D.R.; Harju, V.; Skelton, A.; Adams, I.P. First report of Tomato chlorotic dwarf viroid (TCDVd) in tomato in Norway and subsequent eradication. N. Dis. Rep. 2013, 27, 8. [Google Scholar] [CrossRef] [Green Version]
- Parrella, G.; Numitone, G. First report of Tomato apical stunt viroid in tomato in Italy. Plant Dis. 2014, 98, 1164. [Google Scholar] [CrossRef] [PubMed]
- Ling, K.-S.; Sfetcu, D. First report of natural infection of greenhouse tomatoes by Potato spindle tuber viroid in the United States. Plant Dis. 2010, 94, 1376. [Google Scholar] [CrossRef]
- Behjatnia, S.A.A.; Dry, I.B.; Krake, L.R.; Condé, B.D.; Connelly, M.I.; Randles, J.W.; Rezaian, M.A. New potato spindle tuber viroid and tomato leaf curl geminivirus strains from a wild Solanum sp. Phytopathology 1996, 86, 880–886. [Google Scholar] [CrossRef]
- Shiraishi, T.; Maejima, K.; Komatsu, K.; Hashimoto, M.; Okano, Y.; Kitazawa, Y.; Yamaji, Y.; Namba, S. First report of tomato chlorotic dwarf viroid isolated from symptomless petunia plants (Petunia spp.) in Japan. J. Gen. Plant Pathol. 2013, 79, 214–216. [Google Scholar] [CrossRef]
- Verhoeven, J.T.J.; Roenhorst, J.W. High stability of original predominant pospiviroid genotypes upon mechanical inoculation from ornamentals to potato and tomato. Arch. Virol. 2010, 155, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Parrella, G.; Crescenzi, A.; Pacella, R. First record of Columnea latent viroid (CLVd) in tomato in Italy. Acta Hortic. 2011, 914, 149–152. [Google Scholar] [CrossRef]
- Candresse, T.; Smith, D.; Diener, T.O. Nucleotide sequence of a full-length infectious clone of the Indonesian strain of tomato apical stunt viroid (TASV). Nucleic Acids Res. 1987, 15, 10597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Candresse, T.; Marais, A.; Ollivier, F.; Verdin, E.; Blancard, D. First report of the presence of Tomato apical stunt viroid on tomato in Sénégal. Plant Dis. 2007, 91, 330. [Google Scholar] [CrossRef] [PubMed]
- EPPO. Outbreak of Potato spindle tuber viroid in tomato in the United Kingdom. EPPO Report. Serv. 2011, 9, 202. [Google Scholar]
- EPPO. Incidental finding of Potato spindle tuber viroid in tomatoes in the Netherlands. EPPO Report. Serv. 2013, 7, 148. [Google Scholar]
- EPPO. First report of Tomato apical stunt viroid in France. EPPO Report. Serv. 2013, 11, 236. [Google Scholar]
- Ling, K.-S.; Bledsoe, M.E. First report of Mexican papita viroid infecting greenhouse tomato in Canada. Plant Dis. 2009, 93, 839. [Google Scholar] [CrossRef]
- Ling, K.-S.; Verhoeven, J.T.J.; Singh, R.P.; Brown, J.K. First report of Tomato chlorotic dwarf viroid in greenhouse tomatoes in Arizona. Plant Dis. 2009, 93, 1075. [Google Scholar] [CrossRef] [PubMed]
- Ling, K.-S.; Li, R.; Panthee, D.R.; Gardner, R.G. First report of Potato spindle tuber viroid naturally infecting greenhouse tomatoes in North Carolina. Plant Dis. 2013, 97, 148. [Google Scholar] [CrossRef] [PubMed]
- Ling, K.-S.; Li, R.; Groth-Helms, D.; Assis-Filho, F.M. First report of Potato spindle tuber viroid naturally infecting field tomatoes in the Dominican Republic. Plant Dis. 2014, 98, 701. [Google Scholar] [CrossRef]
- Mishra, M.D.; Hammond, R.W.; Owens, R.A.; Smith, D.R.; Diener, T.O. Indian bunchy top disease of tomato plants is caused by a distinct strain of citrus exocortis viroid. J. Gen. Virol. 1991, 72, 1781–1785. [Google Scholar] [CrossRef]
- Mumford, R.A.; Jarvis, B.; Skelton, A. The first report of Potato spindle tuber viroid (PSTVd) in commercial tomatoes in the UK. Plant Pathol. 2004, 53, 242. [Google Scholar] [CrossRef]
- Puchta, H.; Herold, T.; Verhoeven, K.; Roenhorst, A.; Ramm, K.; Schmidt-Puchta, W.; Sänger, H.L. A new strain of potato spindle tuber viroid (PSTVd-N) exhibits major sequence differences as compared to all other PSTVd strains sequenced so far. Plant Mol. Biol. 1990, 15, 509–511. [Google Scholar] [CrossRef]
- Reanwarakorn, K.; Klinkong, S.; Porsoongnurn, J. First report of natural infection of Pepper chat fruit viroid in tomato plants in Thailand. N. Dis. Rep. 2011, 24, 6. [Google Scholar] [CrossRef] [Green Version]
- Steyer, S.; Olivier, T.; Skelton, A.; Nixon, T.; Hobden, E. Columnea latent viroid (CLVd): First report in tomato in France. Plant Pathol. 2009, 59, 794. [Google Scholar] [CrossRef]
- Verhoeven, J.T.J.; Janse, C.C.C.; Roenhors, J.W. First report of Tomato apical stunt viroid in tomato in Tunisia. Plant Dis. 2006, 90, 528. [Google Scholar] [CrossRef]
- Verhoeven, J.T.J.; Janse, C.C.C.; Roenhorst, J.W.; Steyer, S.; Michelante, D. First report of Potato spindle tuber viroid in tomato in Belgium. Plant Dis. 2007, 91, 1055. [Google Scholar] [CrossRef]
- Walter, B.; Thouvenal, J.C.; Fauque, C. Les viruses de la tomate en Cote d’Ivore. Ann. Phytopathol. 1980, 12, 259–275. [Google Scholar]
- Constable, F.; Chambers, G.; Penrose, L.; Daly, A.; Mackie, J.; Davis, K.; Rodoni, B.; Gibbs, M. Viroid-infected tomato and capsicum seed shipments to Australia. Viruses 2019, 11, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Lot Number | Crop 1 | Origin | Viroid(s) Identified | Genbank Accession Number | Estimated Infestation Rate 2 | Number of Plants Raised |
|---|---|---|---|---|---|---|
| 1 | pepper | Asia | PCFVd | MW422288 | 13% (CL95 0–52%) | 27,735 |
| 2 | pepper | Asia | CLVd | MW422289 | 9% (CL95 3–22%) | 27,703 |
| PCFVd | MW422290 | 13% (CL95 4–29%) | ||||
| 3 | pepper | Africa | PSTVd | MW422291 | nd | 2500 |
| 4 | tomato | Asia | PCFVd | MW422292 | 63% (CL95 25–91%) | 47,528 |
| 5 | tomato | Asia | PSTVd | MW422293 | nd | 2500 |
| Location 1 | Pepper | Tomato | |||
|---|---|---|---|---|---|
| Lot 1 | Lot 2 | Lot 3 | Lot 4 | Lot 5 | |
| PCFVd | CLVd and PCFVd | PSTVd | PCFVd | PSTVd | |
| 1 | 5575 | 5500 | 8256 | ||
| 2 | 5805 | 6045 | 9985 | ||
| 3 | 6000 | 6000 | 10,000 | ||
| 4 | 5605 | 5358 | |||
| 5 | 4750 | 4800 | 10,215 | ||
| 6 | 9072 | ||||
| 7 | 2500 | 2500 | |||
| Total | 27,735 | 27,703 | 2500 | 47,528 | 2500 |
| Source of Infection | Reported in Publication | Based on Review |
|---|---|---|
| Seeds | 1 a | 0 |
| Plants 1 | 4 b | 5 |
| Unknown | 1 c | 1 |
| Source of Infection | Reported in Publication | Based on Review |
|---|---|---|
| Seeds | 11 a | 2 |
| Plants 1 | 10 b | 14 |
| Unknown | 29 c | 34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verhoeven, J.T.J.; Botermans, M.; Schoen, R.; Koenraadt, H.; Roenhorst, J.W. Possible Overestimation of Seed Transmission in the Spread of Pospiviroids in Commercial Pepper and Tomato Crops Based on Large-Scale Grow-Out Trials and Systematic Literature Review. Plants 2021, 10, 1707. https://doi.org/10.3390/plants10081707
Verhoeven JTJ, Botermans M, Schoen R, Koenraadt H, Roenhorst JW. Possible Overestimation of Seed Transmission in the Spread of Pospiviroids in Commercial Pepper and Tomato Crops Based on Large-Scale Grow-Out Trials and Systematic Literature Review. Plants. 2021; 10(8):1707. https://doi.org/10.3390/plants10081707
Chicago/Turabian StyleVerhoeven, Jacobus T. J., Marleen Botermans, Ruben Schoen, Harrie Koenraadt, and Johanna W. Roenhorst. 2021. "Possible Overestimation of Seed Transmission in the Spread of Pospiviroids in Commercial Pepper and Tomato Crops Based on Large-Scale Grow-Out Trials and Systematic Literature Review" Plants 10, no. 8: 1707. https://doi.org/10.3390/plants10081707
APA StyleVerhoeven, J. T. J., Botermans, M., Schoen, R., Koenraadt, H., & Roenhorst, J. W. (2021). Possible Overestimation of Seed Transmission in the Spread of Pospiviroids in Commercial Pepper and Tomato Crops Based on Large-Scale Grow-Out Trials and Systematic Literature Review. Plants, 10(8), 1707. https://doi.org/10.3390/plants10081707