Identification and First Report of Fusarium andiyazi Causing Sheath Rot of Zizania latifolia in China
Abstract
:1. Introduction
2. Results
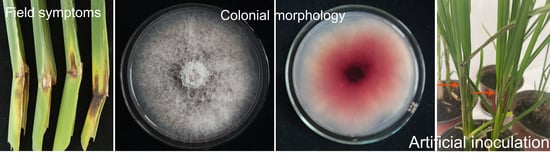
2.1. Occurrence of Sheath Rot in Zizania latifolia
2.2. Fungal Isolation and Morphological Identification
2.3. Molecular Identification and Phylogenetic Analysis
2.4. Pathogenicity Assays
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Isolation
4.2. Morphological and Cultural Characterization
4.3. DNA Extraction, PCR Amplification, and Sequencing
4.4. Phylogenetic Analysis
4.5. Pathogenicity Tests
4.6. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, X.; Walters, C.; Antolin, M.F.; Alexander, M.L.; Lutz, S.; Ge, S.; Wen, J. Phylogeny and biogeography of the eastern Asian–North American disjunct wild-rice genus (Zizania L., Poaceae). Mol. Phylogenet. Evol. 2010, 55, 1008–1017. [Google Scholar] [CrossRef]
- Zhang, J.-Z.; Chu, F.-Q.; Guo, D.-P.; Hyde, K.D.; Xie, G.-L. Cytology and ultrastructure of interactions between Ustilago esculenta and Zizania latifolia. Mycol. Prog. 2012, 11, 499–508. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, P.; Zhao, S.; Nie, C.; Wang, N.; Du, X.; Zhou, Y. Characterization, antioxidant activity and immunomodulatory activity of polysaccharides from the swollen culms of Zizania latifolia. Int. J. Biol. Macromol. 2017, 95, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.B.; Li, S.M.; Peng, J.; Ke, W.D. Zizania latifolia Turcz. cultivated in China. Genet. Resour. Crop. Evol. 2007, 54, 1211–1217. [Google Scholar] [CrossRef]
- Xiao, Z.-L.; Hyde, K.D.; Zhang, J.-Z. Synonymy of two species of Bipolaris from aquatic crops of Poaceae. Mycotaxon 2015, 130, 131–143. [Google Scholar] [CrossRef]
- Cho, W.D.; Shin, H.D. List of Plant Diseases in Korea; The Korean Society of Plant Pathology: Suwon, Korea, 2004; pp. 168–169. [Google Scholar]
- Huang, J.-H.; Chen, C.-Y.; Lin, Y.-S.; Ann, P.-J.; Huang, H.-C.; Chung, W.-H. Six new species of Pythiogeton in Taiwan, with an account of the molecular phylogeny of this genus. Mycoscience 2013, 54, 130–147. [Google Scholar] [CrossRef]
- Ann, P.J.; Huang, J.H.; Wang, I.T.; Ko, W.H. Pythiogeton zizaniae, a new species causing basal stalk rot of water bamboo in Taiwan. Mycologia 2006, 98, 116–120. [Google Scholar] [CrossRef]
- Li, P.P.; Cao, Z.Y.; Wang, K.; Zhai, H.; Jia, H.; Liu, N.; Li, S.H.; Hao, Z.M.; Gu, S.Q.; Dong, J.G. First report of Fusarium equiseti causing a sheath rot of corn in China. Plant Dis. 2014, 98, 998. [Google Scholar] [CrossRef]
- Lan, H.; Xiude, X.; Yu, J. Study on biological characteristics of corn sheath rot causal agent. J. Maize Sci. 2008, 16, 131–134. [Google Scholar]
- Yang, Q.; Balint-Kurti, P.; Xu, M. Quantitative Disease Resistance: Dissection and Adoption in Maize. Mol. Plant 2017, 10, 402–413. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Wang, B.; Sun, X.; Qi, X.; Zhao, C.; Chang, X.; Khaskheli, M.I.; Gong, G. Symptoms and pathogens diversity of Corn Fusarium sheath rot in Sichuan Province, China. Sci. Rep. 2021, 11, 2835. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. (Eds.) The Fusarium Laboratory Manual; Blackwell: Ames, IO, USA, 2006. [Google Scholar]
- Geiser, D.M.; Jiménez-Gasco, M.D.M.; Kang, S.; Makalowska, I.; Veeraraghavan, N.; Ward, T.J.; Zhang, N.; Kuldau, G.A.; O’Donnell, K. FUSARIUM-ID v. 1.0: A DNA Sequence Database for Identifying Fusarium. Eur. J. Plant Pathol. 2004, 110, 473–479. [Google Scholar] [CrossRef]
- Madania, A.; Altawil, M.; Naffaa, W.; Volker, P.H.; Hawat, M. Morphological and Molecular Characterization of Fusarium Isolated from Maize in Syria. J. Phytopathol. 2013, 161, 452–458. [Google Scholar] [CrossRef]
- Prà, M.D.; Tonti, S.; Pancaldi, D.; Nipoti, P.; Alberti, I. First Report of Fusarium andiyazi Associated with Rice Bakanae in Italy. Plant Dis. 2010, 94, 1070. [Google Scholar] [CrossRef]
- Qiu, J.; Lu, Y.; He, D.; Lee, Y.W.; Ji, F.; Xu, J.; Shi, J. Fusarium fujikuroi species complex associated with rice, maize, and soybean from Jiangsu Province, China: Phylogenetic, pathogenic, and toxigenic analysis. Plant Dis. 2020, 104, 2193–2201. [Google Scholar] [CrossRef] [PubMed]
- Wulff, E.G.; Sørensen, J.L.; Lübeck, M.; Nielsen, K.F.; Thrane, U.; Torp, J. Fusariumspp. associated with rice Bakanae: Ecology, genetic diversity, pathogenicity and toxigenicity. Environ. Microbiol. 2010, 12, 649–657. [Google Scholar] [CrossRef]
- Marasas, W.F.O.; Rheeder, J.P.; Lamprecht, S.C.; Zeller, K.A.; Leslie, J.F. Fusarium andiyazi sp. nov., a new species from sorghum. Mycologia 2001, 93, 1203–1210. [Google Scholar] [CrossRef]
- Leslie, J.F.; Zeller, K.A.; Lamprecht, S.C.; Rheeder, J.P.; Marasas, W.F.O. Toxicity, Pathogenicity, and Genetic Differentiation of Five Species of Fusarium from Sorghum and Millet. Phytopathology 2005, 95, 275–283. [Google Scholar] [CrossRef] [Green Version]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Kelly, L.A.; Tan, Y.P.; Ryley, M.J.; Aitken, E. Fusarium species associated with stalk rot and head blight of grain sorghum in Queensland and New South Wales, Australia. Plant Pathol. 2017, 66, 1413–1423. [Google Scholar] [CrossRef]
- Govender, P.; McFarlane, S.A.; Rutherford, R.S. Fusarium species causing pokkah boeng and their effect on Eldana saccharina walker (lepidoptera pyralidae). Proc. S. Afr. Sug. Technol. Assess. 2010, 83, 267–270. [Google Scholar]
- Costa, M.M.; Melo, M.P.; Guimarães, E.A.; Veiga, C.M.O.; Carmo Sandin, F.; Moreira, G.M.; Costa, S.S.; Pfenning, L.H. Iden-tification and pathogenicity of Fusarium species associated with pokkah boeng of sugarcane in Brazil. Plant. Pathol. 2019, 68, 1350–1360. [Google Scholar] [CrossRef]
- Bao, Y.; Huang, Z.; Li, T.; Yao, Z.; Powell, C.A.; Zhang, M. First Report of Fusarium andiyazi Causing Sugarcane Pokkah Boeng Disease in China. Plant Dis. 2020, 104, 286. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, W.; Pan, Y.; Xu, J.S.; Chen, W.Q.; Feng, J. First Report of Fusarium Ear Rot of Maize Caused by Fusarium andiyazi in China. Plant Dis. 2014, 98, 1428. [Google Scholar] [CrossRef]
- Venturini, G.; Toffolatti, S.L.; Quaglino, F.; Casati, P. First Report of Fusarium andiyazi Causing Ear Rot on Maize in Italy. Plant Dis. 2017, 101, 839. [Google Scholar] [CrossRef]
- Seo, Y.; Kim, Y.H. Potential Reasons for Prevalence of Fusarium Wilt in Oriental Melon in Korea. Plant Pathol. J. 2017, 33, 249–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isaac, M.R.; Leyva-Mir, S.G.; Sahagun-Castellanos, J.; Camara-Correia, K.; Tovar-Pedraza, J.M.; Rodriguez-Perez, J.E. Occurrence, identification, and pathogenicity of Fusarium spp. associated with tomato wilt in Mexico. Not. Bot. Horti. Agrobo. 2018, 46, 484–493. [Google Scholar] [CrossRef] [Green Version]
- Hsuan, H.M.; Salleh, B.; Zakaria, L. Molecular Identification of Fusarium Species in Gibberella fujikuroi Species Complex from Rice, Sugarcane and Maize from Peninsular Malaysia. Int. J. Mol. Sci. 2011, 12, 6722–6732. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.W.; Hong, S.K.; Lee, Y.K.; Kim, W.G.; Chun, S. Taxonomy of Fusarium fujikuroi species complex associated with ba-kanae on rice in Korea. Australas. Plant. Path. 2018, 47, 23–34. [Google Scholar] [CrossRef]
- Taha, E.M. Morphological, phylogenetic, and pathogenic analyses of Fusarium andiyazi associated with sugar beet root diseases. Arch. Phytopathol. Plant Prot. 2020, 54, 319–333. [Google Scholar] [CrossRef]
- O’Donnell, K.; Ward, T.J.; Robert, V.A.R.G.; Crous, P.W.; Geiser, D.M.; Kang, S. DNA sequence-based identification of Fusarium: Current status and future directions. Phytoparasitica 2015, 43, 583–595. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Qiu, J.; Han, Z.; Ye, Z.; Chen, C.; Liu, C.; Xin, X.; Ye, C.-Y.; Wang, Y.-Y.; Xie, H.; et al. A host plant genome (Zizania latifolia) after a century-long endophyte infection. Plant J. 2015, 83, 600–609. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.C.; Zhao, S.W.; Zhu, P.L.; Nie, C.; Ma, S.P.; Wang, N.F.; Du, X.F.; Zhou, Y.B. Purification, characterization and im-munomodulatory activity of water extractable polysaccharides from the swollen culms of Zizania latifolia. Int. J. Biol. Macro Mol. 2018, 107, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Pathak, V.N. Essentials of Plant Pathology; Prakesh Publishers: Jaipur, India, 1972; p. 448. [Google Scholar]
- Burgess, L.W.; Summerell, B.A.; Nelson, P.E. An Evaluation of Several Media for Use in Identification of Some Fusarium Species. Australas. Plant Pathol. 1991, 20, 86–88. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S. Amplification and direct seqencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. 1990, 18, 315–322. [Google Scholar]
- O’Donnell, K.; Cigelnik, E.; Nirenberg, H.I. Molecular Systematics and Phylogeography of the Gibberella fujikuroi Species Complex. Mycologia 1998, 90, 465. [Google Scholar] [CrossRef]
- O’Donnell, K.; Sutton, D.A.; Rinaldi, M.G.; Sarver, B.A.J.; Balajee, S.A.; Schroers, H.-J.; Summerbell, R.C.; Robert, V.A.R.G.; Crous, P.W.; Zhang, N.; et al. Internet-Accessible DNA Sequence Database for Identifying Fusaria from Human and Animal Infections. J. Clin. Microbiol. 2010, 48, 3708–3718. [Google Scholar] [CrossRef] [Green Version]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence align-ment aided by quality analysis tools. Nucleic. Acids. Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Stecher, G.; Peterson, D. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [Green Version]



| Isolate | Conidial Size (μm) a | Mean ± SD b (μm) | ||||
|---|---|---|---|---|---|---|
| Microconidia | Macroconidia | Microconidia | Macroconidia | |||
| Length | Width | Length | Width | |||
| JB-2 | 7.28–24.81 a | 1.32–4.30 a | 32.48–80.47 a | 1.60–4.63 a | 14.50 ± 0.69 × 2.68 ± 0.12 | 44.37 ± 1.48 × 3.35 ± 0.12 |
| JB-3 | 7.13–24.56 a | 1.23–4.02 a | 31.95–78.16 a | 1.69–4.37 a | 14.56 ± 0.60 × 2.69 ± 0.10 | 43.97 ± 1.72 × 3.19 ± 0.11 |
| JB-4 | 6.85–25.29 a | 1.38–4.14 a | 31.36–81.35 a | 1.54–4.47 a | 14.55 ± 0.61 × 2.53 ± 0.09 | 43.96 ± 1.69 × 3.25 ± 0.10 |
| JB-5 | 7.43–24.67 a | 1.17–4.55 a | 31.64–79.51 a | 1.82–4.55 a | 14.74 ± 0.58 × 2.7 ± 0.11 | 44.31 ± 1.62 × 3.03 ± 0.09 |
| JB-6 | 7.55–22.92 a | 1.51–3.91 a | 32.55–80.76 a | 1.96–4.60 a | 14.18 ± 0.45 × 2.58 ± 0.09 | 44.83 ± 1.53 × 3.30 ± 0.11 |
| JB-8 | 7.97–23.45 a | 1.15–4.60 a | 31.82–81.09 a | 2.02–4.32 a | 14.44 ± 0.62 × 2.70 ± 0.13 | 44.16 ± 1.74 × 3.01 ± 0.10 |
| JB-11 | 7.21–23.78 a | 1.37–4.16 a | 30.99–80.95 a | 1.88–4.09 a | 14.63 ± 0.56 × 2.74 ± 0.11 | 43.81 ± 1.60 × 2.99 ± 0.09 |
| JB-12 | 7.89–24.35 a | 1.25–4.68 a | 32.03–80.28 a | 2.27–4.14 a | 14.75 ± 0.55 × 2.71 ± 0.12 | 44.32 ± 1.39 × 3.19 ± 0.07 |
| JB-25 | 7.76–24.03 a | 1.68–3.97 a | 31.74–80.19 a | 2.11–4.26 a | 14.38 ± 0.56 × 2.67 ± 0.08 | 44.04 ± 1.31 × 3.02 ± 0.08 |
| JB-27 | 7.46–23.86 a | 1.43–4.09 a | 31.58–79.93 a | 2.05–4.21 a | 14.46 ± 0.58 × 2.57 ± 0.10 | 44.56 ± 1.62 × 3.21 ± 0.09 |
| Species | Isolate/Strain | GenBank Accession Number | |
|---|---|---|---|
| TEF1 | RPB2 | ||
| Fusarium andiyazi | NRRL 31727 | MN193854.1 | MN193882.1 |
| Fusarium andiyazi | CBS 119856 | MN533989.1 | MN534286.1 |
| Fusarium andiyazi | CBS 134430 | KC954401.1 | LR792614.1 |
| Fusarium andiyazi | JB-2 | MZ396373 | MZ396383 |
| Fusarium andiyazi | JB-3 | MZ396374 | MZ396384 |
| Fusarium andiyazi | JB-4 | MZ396375 | MZ396385 |
| Fusarium andiyazi | JB-5 | MZ396376 | MZ396386 |
| Fusarium andiyazi | JB-6 | MZ396377 | MZ396387 |
| Fusarium andiyazi | JB-8 | MZ396378 | MZ396388 |
| Fusarium andiyazi | JB-11 | MZ396379 | MZ396389 |
| Fusarium andiyazi | JB-12 | MZ396380 | MZ396390 |
| Fusarium andiyazi | JB-25 | MZ396381 | MZ396391 |
| Fusarium andiyazi | JB-27 | MZ396381 | MZ396392 |
| Fusarium solani | MRC 2565 | MH582420.1 | MH582410.1 |
| Fusarium solani | NRRL 32810 | DQ247118.1 | EU329624.1 |
| Fusarium ensiforme | CPC 27190 | LT746199.1 | LT746312.1 |
| Fusarium ensiforme | CPC 27191 | LT746200.1 | LT746313.1 |
| Fusarium ficicrescens | CBS 125177 | KP662898.1 | KT154001.1 |
| Fusarium ficicrescens | CBS 125181 | KP662900.1 | KT154003.1 |
| Fusarium fujikuroi | CBS 130402 | KU604446.1 | KU604261.1 |
| Fusarium fujikuroi | CBS 121864 | KU604442.1 | KU604258.1 |
| Fusarium thapsinum | CBS 733.97 | KU604463.1 | KU604299.1 |
| Fusarium thapsinum | CBS 776.96 | KU604462.1 | KU604294.1 |
| Fusarium verticillioides | CBS 102699 | KU604385.1 | KU604218.1 |
| Fusarium verticillioides | CBS 579.78 | KU604390.1 | KU604223.1 |
| Fusarium verticillioides | CBS 116665 | KU604388.1 | KU604221.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.-M.; Zhu, J.-Z.; Li, X.-G.; Wang, L.-L.; Zhong, J. Identification and First Report of Fusarium andiyazi Causing Sheath Rot of Zizania latifolia in China. Plants 2021, 10, 1844. https://doi.org/10.3390/plants10091844
Ma Y-M, Zhu J-Z, Li X-G, Wang L-L, Zhong J. Identification and First Report of Fusarium andiyazi Causing Sheath Rot of Zizania latifolia in China. Plants. 2021; 10(9):1844. https://doi.org/10.3390/plants10091844
Chicago/Turabian StyleMa, Ya-Min, Jun-Zi Zhu, Xiao-Gang Li, Lai-Liang Wang, and Jie Zhong. 2021. "Identification and First Report of Fusarium andiyazi Causing Sheath Rot of Zizania latifolia in China" Plants 10, no. 9: 1844. https://doi.org/10.3390/plants10091844
APA StyleMa, Y. -M., Zhu, J. -Z., Li, X. -G., Wang, L. -L., & Zhong, J. (2021). Identification and First Report of Fusarium andiyazi Causing Sheath Rot of Zizania latifolia in China. Plants, 10(9), 1844. https://doi.org/10.3390/plants10091844