Systematic Review of Potential Anticancerous Activities of Erythrina senegalensis DC (Fabaceae)
Abstract
:1. Introduction
2. Compounds Isolated from Erythrina senegalensis
3. In Vitro Anticancerous Activities of Compounds from E. senegalensis: Mechanisms of Action
3.1. Isoflavonoids and Flavonoids
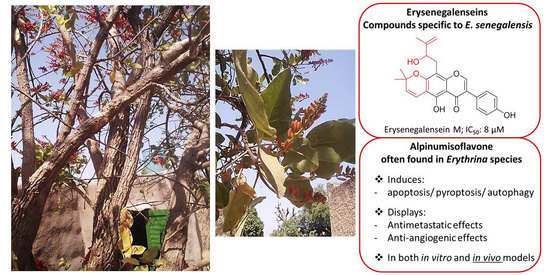
3.1.1. Alpinumisoflavone (AIF)
3.1.2. Derrone
3.1.3. Warangalone (Scandenone), Auriculatin and Auriculasin
3.1.4. Erysenegalenseins, Neobavaisoflavone and Sigmoidin H
3.2. Pterocarpans
3.3. Tripterpenes
3.3.1. Oleanolic Acid (OA)
3.3.2. Erythrodiol and Maniladiol
4. In Vivo Preclinical Studies of E. senegalensis Compounds
4.1. In Vivo Studies of Antitumor Activities of Oleanolic Acid (OA)
4.2. In Vivo Antitumor Studies of Alpinumisoflavone (AIF)
5. Discussion
5.1. Cytotoxic Alkaloids Isolated from E. senegalensis
5.2. Potential Anticancerous Isoflavonoids and Flavonoids Isolated from E. senegalensis
5.3. Potential Anticancerous Pterocarpans Isolated from E. senegalensis
5.4. Cytotoxic Pentacyclic Triterpenes Isolated from E. senegalensis
5.5. Current Status, Limitations and Perspectives
6. Materials and Methods
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the Global Cancer Incidence and Mortality in 2018: GLOBOCAN Sources and Methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tariq, A.; Sadia, S.; Pan, K.; Ullah, I.; Mussarat, S.; Sun, F.; Abiodun, O.O.; Batbaatar, A.; Li, Z.; Song, D.; et al. A Systematic Review on Ethnomedicines of Anti-Cancer Plants. Phytother. Res. PTR 2017, 31, 202–264. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from January 1981 to September 2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Pinto, D.C.G.A. Plant Secondary Metabolites as Anticancer Agents: Successes in Clinical Trials and Therapeutic Application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutt, R.; Garg, V.; Khatri, N.; Madan, A.K. Phytochemicals in Anticancer Drug Development. Anticancer Agents Med. Chem. 2019, 19, 172–183. [Google Scholar] [CrossRef]
- de Araújo-Júnior, J.; Oliveira, M.; Aquino, P.; Alexandre-Moreira, M.; Sant’Ana, A. A Phytochemical and Ethnopharmacological Review of the Genus Erythrina; InTech: London, UK, 2012; ISBN 978-953-51-0296-0. [Google Scholar]
- Nacoulma, O.G. Plantes Médicinales et Pratiques Médicales Traditionnelles Au Burkina Faso: Cas Du Plateau Central. Ph.D. Thesis, Faculty of Science and Technology, University of Ouagadougou, Ouagadougou, Burkina Faso, 1996; 320p. [Google Scholar]
- Togola, A.; Austarheim, I.; Theïs, A.; Diallo, D.; Paulsen, B.S. Ethnopharmacological Uses of Erythrina senegalensis: A Comparison of Three Areas in Mali, and a Link between Traditional Knowledge and Modern Biological Science. J. Ethnobiol. Ethnomed. 2008, 4, 6. [Google Scholar] [CrossRef] [Green Version]
- Iwu, M.M. African Medicinal Plants; CRC Press: Baltimore, MD, USA, 1993. [Google Scholar]
- Wandji, J.; Awanchiri, S.; Fomum, Z.T.; Tillequin, F.; Libot, F. Isoflavones and Alkaloids from the Stem Bark and Seeds of Erythrina senegalensis. Phytochemistry 1995, 39, 677–681. [Google Scholar] [CrossRef]
- Wandji, J.; Fomum, Z.T.; Tillequin, F.; Baudouin, G.; Koch, M. Epoxyisoflavones from Erythrina senegalensis. Phytochemistry 1994, 35, 1573–1577. [Google Scholar] [CrossRef]
- Fomum, Z.T.; Ayafor, J.F.; Wandji, J.; Fomban, W.G.; Nkengfack, A.E. Erythrinasinate, an Ester from Three Erythrina Species. Phytochemistry 1986, 25, 757–759. [Google Scholar] [CrossRef]
- Togola, A.; Hedding, B.; Theis, A.; Wangensteen, H.; Rise, F.; Smestad Paulsen, B.; Diallo, D.; Egil Malterud, K. 15-Lipoxygenase Inhibitory Effects of Prenylated Flavonoids from Erythrina senegalensis. Planta Med. 2009, 75, 1168–1170. [Google Scholar] [CrossRef]
- Kuete, V.; Sandjo, L.P.; Kwamou, G.M.N.; Wiench, B.; Nkengfack, A.E.; Efferth, T. Activity of Three Cytotoxic Isoflavonoids from Erythrina excelsa and Erythrina senegalensis (Neobavaisoflavone, Sigmoidin H and Isoneorautenol) toward Multi-Factorial Drug Resistant Cancer Cells. Phytomed. Int. J. Phytother. Phytopharm. 2014, 21, 682–688. [Google Scholar] [CrossRef]
- Wandji, J.; Fomum, Z.T.; Tillequin, F.; Libot, F.; Koch, M. Erysenegalenseins B and C, Two New Prenylated Isoflavanones from Erythrina senegalensis. J. Nat. Prod. 1995, 58, 105–108. [Google Scholar] [CrossRef]
- Oh, W.K.; Lee, H.S.; Ahn, S.C.; Ahn, J.S.; Mbafor, J.T.; Wandji, J.; Fomum, Z.T.; Chang, H.K.; Kim, Y.H. Prenylated Isoflavonoids from Erythrina senegalensis. Phytochemistry 1999, 51, 1147–1150. [Google Scholar] [CrossRef]
- Wandji, J.; Fomum, Z.T.; Tillequin, F.; Seguin, E.; Koch, M. Two Isoflavones from Erythrina senegalensis. Phytochemistry 1993, 35, 245–248. [Google Scholar] [CrossRef]
- Wandji, J.; Fomum, Z.T.; Tillequin, F.; Skaltsounis, A.L.; Koch, M. Erysenegalenseins H and I: Two New Isoflavones from Erythrina senegalensis 1. Planta Med. 1994, 60, 178–180. [Google Scholar] [CrossRef]
- Taylor, R.B.; Corley, D.G.; Tempesta, M.S.; Fomum, Z.T.; Ayafor, J.F.; Wandji, J.; Ifeadike, P.N. 2,3-Dihydroauriculatin, a New Prenylated Isoflavanone from Erythrina senegalensis. Application of the Selective Inept Technique. J. Nat. Prod. 1986, 49, 670–673. [Google Scholar] [CrossRef] [PubMed]
- Fomum, Z.T.; Ayafor, J.F.; Wandji, J. Erythrisenegalone, a Prenylated-Flavanone from Erythrina senegalensis. Phytochemistry 1985, 24, 3075–3076. [Google Scholar]
- Fomum, Z.T.; Ayafor, J.F.; Wandji, J. Senegalensein, a Novel Prenylated Flavanone from Erythrina senegalensis. J. Nat. Prod. 1987, 50, 921–922. [Google Scholar] [CrossRef]
- Wandji, J.; Awanchiri, S.S.; Fomum, Z.T.; Tillequin, F.; Michel-Daniwicz, S. Prenylated Isoflavonoids from Erythrina Sensegalensis. Phytochemistry 1995, 38, 1309–1313. [Google Scholar] [CrossRef]
- Wandji, J.; Nkengfack, A.E.; Fomum, Z.T.; Ubillas, R.; Killday, K.B.; Tempesta, M.S. A New Prenylated Isoflavone and Long Chain Esters from Two Erythrina Species. J. Nat. Prod. 1990, 53, 1425–1429. [Google Scholar] [CrossRef] [PubMed]
- Sheu, Y.W.; Chiang, L.C.; Chen, I.S.; Chen, Y.C.; Tsai, I.L. Cytotoxic Flavonoids and New Chromenes from Ficus Formosana f. Formosana. Planta Med. 2005, 71, 1167–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wätjen, W.; Kulawik, A.; Suckow-Schnitker, A.K.; Chovolou, Y.; Rohrig, R.; Ruhl, S.; Kampkötter, A.; Addae-Kyereme, J.; Wright, C.W.; Passreiter, C.M. Pterocarpans Phaseollin and Neorautenol Isolated from Erythrina addisoniae Induce Apoptotic Cell Death Accompanied by Inhibition of ERK Phosphorylation. Toxicology 2007, 242, 71–79. [Google Scholar] [CrossRef]
- Iranshahi, M.; Vu, H.; Pham, N.; Zencak, D.; Forster, P.; Quinn, R.J. Cytotoxic Evaluation of Alkaloids and Isoflavonoids from the Australian Tree Erythrina Vespertilio. Planta Med. 2012, 78, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Rukachaisirikul, T.; Saekee, A.; Tharibun, C.; Watkuolham, S.; Suksamrarn, A. Biological Activities of the Chemical Constituents of Erythrina Stricta and Erythrina Subumbrans. Arch. Pharm. Res. 2007, 30, 1398–1403. [Google Scholar] [CrossRef]
- Min, H.-Y.; Jung, Y.; Park, K.H.; Oh, W.K.; Lee, H.-Y. Erybraedin A Is a Potential Src Inhibitor That Blocks the Adhesion and Viability of Non-Small-Cell Lung Cancer Cells. Biochem. Biophys. Res. Commun. 2018, 502, 145–151. [Google Scholar] [CrossRef]
- Tchokouaha, R.F.; Alexi, X.; Chosson, E.; Besson, T.; Skaltsounis, A.-L.; Seguin, E.; Alexis, M.N.; Wandji, J. Erymildbraedin A and B, Two Novel Cytotoxic Dimethylpyrano-Isoflavones from the Stem Bark of Erythrina mildbraedii: Evaluation of Their Activity toward Endocrine Cancer Cells. J. Enzyme Inhib. Med. Chem. 2010, 25, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Maurich, T.; Iorio, M.; Chimenti, D.; Turchi, G. Erybraedin C and Bitucarpin A, Two Structurally Related Pterocarpans Purified from Bituminaria bituminosa, Induced Apoptosis in Human Colon Adenocarcinoma Cell Lines MMR- and P53-Proficient and -Deficient in a Dose-, Time-, and Structure-Dependent Fashion. Chem. Biol. Interact. 2006, 159, 104–116. [Google Scholar] [CrossRef]
- Cottiglia, F.; Casu, L.; Bonsignore, L.; Casu, M.; Floris, C.; Leonti, M.; Gertsch, J.; Heilmann, J. New Cytotoxic Prenylated Isoflavonoids from Bituminaria morisiana. Planta Med. 2005, 71, 254–260. [Google Scholar] [CrossRef]
- Ukiya, M.; Akihisa, T.; Tokuda, H.; Suzuki, H.; Mukainaka, T.; Ichiishi, E.; Yasukawa, K.; Kasahara, Y.; Nishino, H. Constituents of Compositae Plants III. Anti-Tumor Promoting Effects and Cytotoxic Activity against Human Cancer Cell Lines of Triterpene Diols and Triols from Edible Chrysanthemum Flowers. Cancer Lett. 2002, 177, 7–12. [Google Scholar] [CrossRef]
- Ito, A.; Chai, H.-B.; Kardono, L.B.S.; Setowati, F.M.; Afriastini, J.J.; Riswan, S.; Farnsworth, N.R.; Cordell, G.A.; Pezzuto, J.M.; Swanson, S.M.; et al. Saponins from the Bark of Nephelium maingayi. J. Nat. Prod. 2004, 67, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Desta, Z.Y.; Sewald, N.; Majinda, R.R. Cytotoxic Flavonoids from Erythrina Caffra Thunb. Bull. Chem. Soc. Ethiop. 2016, 30, 427–435. [Google Scholar] [CrossRef] [Green Version]
- Ito, C.; Murata, T.; Itoigawa, M.; Nakao, K.; Kumagai, M.; Kaneda, N.; Furukawa, H. Induction of Apoptosis by Isoflavonoids from the Leaves of Millettia Taiwaniana in Human Leukemia HL-60 Cells. Planta Med. 2006, 72, 424–429. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Jiang, Y.; Chu, L.; Wu, T.; You, J. Alpinumisoflavone Suppresses Tumour Growth and Metastasis of Clear-Cell Renal Cell Carcinoma. Am. J. Cancer Res. 2017, 7, 999–1015. [Google Scholar]
- Nkengfack, A.E.; Azebaze, A.G.; Waffo, A.K.; Fomum, Z.T.; Meyer, M.; van Heerden, F.R. Cytotoxic Isoflavones from Erythrina Indica. Phytochemistry 2001, 58, 1113–1120. [Google Scholar] [CrossRef]
- Kuete, V.; Mbaveng, A.T.; Nono, E.C.N.; Simo, C.C.; Zeino, M.; Nkengfack, A.E.; Efferth, T. Cytotoxicity of Seven Naturally Occurring Phenolic Compounds towards Multi-Factorial Drug-Resistant Cancer Cells. Phytomed. Int. J. Phytother. Phytopharm. 2016, 23, 856–863. [Google Scholar] [CrossRef]
- Liu, Y.; Veena, C.K.; Morgan, J.B.; Mohammed, K.A.; Jekabsons, M.B.; Nagle, D.G.; Zhou, Y.-D. Methylalpinumisoflavone Inhibits Hypoxia-Inducible Factor-1 (HIF-1) Activation by Simultaneously Targeting Multiple Pathways. J. Biol. Chem. 2009, 284, 5859–5868. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Li, X.; Li, G.; Meng, Y.; Jin, Y.; Shang, S.; Li, Y. Alpinumisoflavone Causes DNA Damage in Colorectal Cancer Cells via Blocking DNA Repair Mediated by RAD51. Life Sci. 2019, 216, 259–270. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.; Sun, M.; He, T.; Liu, Y.; Yang, X.; Shi, X.; Liu, X. Alpinumisoflavone Suppresses Hepatocellular Carcinoma Cell Growth and Metastasis via NLRP3 Inflammasome-Mediated Pyroptosis. Pharmacol. Rep. 2020, 72, 1370–1382. [Google Scholar] [CrossRef]
- Kumar, S.; Pathania, A.S.; Saxena, A.K.; Vishwakarma, R.A.; Ali, A.; Bhushan, S. The Anticancer Potential of Flavonoids Isolated from the Stem Bark of Erythrina Suberosa through Induction of Apoptosis and Inhibition of STAT Signaling Pathway in Human Leukemia HL-60 Cells. Chem. Biol. Interact. 2013, 205, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Tjahjandarie, T.S.; Tanjung, M. Phenolic Compounds from the Stem Bark of Erythrina Orientalis and Their Cytotoxic and Antioxidant Activities. Pharma Chem. 2015, 7, 206–211. [Google Scholar]
- Namkoong, S.; Kim, T.-J.; Jang, I.-S.; Kang, K.-W.; Oh, W.-K.; Park, J. Alpinumisoflavone Induces Apoptosis and Suppresses Extracellular Signal-Regulated Kinases/Mitogen Activated Protein Kinase and Nuclear Factor-ΚB Pathways in Lung Tumor Cells. Biol. Pharm. Bull. 2011, 34, 203–208. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Fan, X.; Wang, Z.; Zhu, W.; Li, J. Alpinumisoflavone Radiosensitizes Esophageal Squamous Cell Carcinoma through Inducing Apoptosis and Cell Cycle Arrest. Biomed. Pharmacother. 2017, 95, 199–206. [Google Scholar] [CrossRef]
- Allouche, Y.; Warleta, F.; Campos, M.; Sánchez-Quesada, C.; Uceda, M.; Beltrán, G.; Gaforio, J.J. Antioxidant, Antiproliferative, and pro-Apoptotic Capacities of Pentacyclic Triterpenes Found in the Skin of Olives on MCF-7 Human Breast Cancer Cells and Their Effects on DNA Damage. J. Agric. Food Chem. 2011, 59, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Juan, M.E.; Wenzel, U.; Daniel, H.; Planas, J.M. Erythrodiol, a Natural Triterpenoid from Olives, Has Antiproliferative and Apoptotic Activity in HT-29 Human Adenocarcinoma Cells. Mol. Nutr. Food Res. 2008, 52, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.M.D.; Ibrahim, N.A.; Awad, N.E.; Matloub, A.A.; Mohamed-Ali, A.G.; Barakat, E.E.; Mohamed, A.E.; Colla, P.L. Anti-HIV-1 and Cytotoxicity of the Alkaloids of Erythrina Abyssinica Lam. Growing in Sudan. Nat. Prod. Res. 2012, 26, 1565–1575. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-J.; Kim, S.-Y.; Kwon, E.-B.; Jo, Y.H.; Lee, M.K.; Lee, H.-S.; Moon, D.-O.; Kim, M.-O. Derrone Induces Autophagic Cell Death through Induction of ROS and ERK in A549 Cells. PLoS ONE 2019, 14, e0218659. [Google Scholar] [CrossRef] [PubMed]
- Hoang, N.T.M.; Phuong, T.T.; Nguyen, T.T.N.; Tran, Y.T.H.; Nguyen, A.T.N.; Nguyen, T.L.; Bui, K.T.V. In Vitro Characterization of Derrone as an Aurora Kinase Inhibitor. Biol. Pharm. Bull. 2016, 39, 935–945. [Google Scholar] [CrossRef] [Green Version]
- Adem, F.A.; Mbaveng, A.T.; Kuete, V.; Heydenreich, M.; Ndakala, A.; Irungu, B.; Yenesew, A.; Efferth, T. Cytotoxicity of Isoflavones and Biflavonoids from Ormocarpum kirkii towards Multi-Factorial Drug Resistant Cancer. Phytomed. Int. J. Phytother. Phytopharm. 2019, 58, 152853. [Google Scholar] [CrossRef]
- Shan, J.; Xuan, Y.; Ruan, S.; Sun, M. Proliferation-Inhibiting and Apoptosis-Inducing Effects of Ursolic Acid and Oleanolic Acid on Multi-Drug Resistance Cancer Cells in Vitro. Chin. J. Integr. Med. 2011, 17, 607–611. [Google Scholar] [CrossRef]
- Kim, G.-J.; Jo, H.-J.; Lee, K.-J.; Choi, J.W.; An, J.H. Oleanolic Acid Induces P53-Dependent Apoptosis via the ERK/JNK/AKT Pathway in Cancer Cell Lines in Prostatic Cancer Xenografts in Mice. Oncotarget 2018, 9, 26370–26386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Song, Y.; Zhang, P.; Zhu, H.; Chen, L.; Xiao, Y.; Xing, Y. Oleanolic Acid Inhibits Cell Survival and Proliferation of Prostate Cancer Cells in Vitro and in Vivo through the PI3K/Akt Pathway. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2016, 37, 7599–7613. [Google Scholar] [CrossRef]
- Duan, L.; Yang, Z.; Jiang, X.; Zhang, J.; Guo, X. Oleanolic Acid Inhibits Cell Proliferation Migration and Invasion and Induces SW579 Thyroid Cancer Cell Line Apoptosis by Targeting Forkhead Transcription Factor A. Anticancer Drugs 2019, 30, 812–820. [Google Scholar] [CrossRef]
- Mu, D.-W.; Guo, H.-Q.; Zhou, G.-B.; Li, J.-Y.; Su, B. Oleanolic Acid Suppresses the Proliferation of Human Bladder Cancer by Akt/MTOR/S6K and ERK1/2 Signaling. Int. J. Clin. Exp. Pathol. 2015, 8, 13864–13870. [Google Scholar]
- Song, Y.; Zhang, P.; Sun, Y.; Li, X.; Chen, L.; Xiao, Y.; Xing, Y. AMPK Activation-Dependent Autophagy Compromises Oleanolic Acid-Induced Cytotoxicity in Human Bladder Cancer Cells. Oncotarget 2017, 8, 67942–67954. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhong, W.; Zhao, J.; Zhang, H.; Zhang, Q.; Liang, Y.; Chen, S.; Liu, H.; Zong, S.; Tian, Y.; et al. Oleanolic Acid Inhibits Epithelial-Mesenchymal Transition of Hepatocellular Carcinoma by Promoting INOS Dimerization. Mol. Cancer Ther. 2019, 18, 62–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Song, Q.; Hu, D.; Zhuang, X.; Yu, S.; Teng, D. Oleanolic Acid Induced Autophagic Cell Death in Hepatocellular Carcinoma Cells via PI3K/Akt/MTOR and ROS-Dependent Pathway. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2016, 20, 237–243. [Google Scholar] [CrossRef] [Green Version]
- Liese, J.; Abhari, B.A.; Fulda, S. Smac Mimetic and Oleanolic Acid Synergize to Induce Cell Death in Human Hepatocellular Carcinoma Cells. Cancer Lett. 2015, 365, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zeng, X.; Wu, X. Effect of Oleanolic Acid on Apoptosis and Autophagy of SMMC-7721 Hepatoma Cells. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e921606. [Google Scholar] [CrossRef]
- Sasikumar, K.; Dubey, V.; Ghosh, A.R. Oleanolic Acid from Black Raisins, Vitis Vinifera with Antioxidant and Antiproliferative Potentials on HCT 116 Colon Cancer Cell Line. Braz. J. Pharm. Sci. 2020, 56. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Lin, J.; Sun, G.; Wei, L.; Shen, A.; Zhang, M.; Peng, J. Oleanolic Acid Inhibits Colorectal Cancer Angiogenesis in Vivo and in Vitro via Suppression of STAT3 and Hedgehog Pathways. Mol. Med. Rep. 2016, 13, 5276–5282. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, Q.; Yang, W.; Wu, T.; Lu, X. Oleanolic Acid Reduces Aerobic Glycolysis-Associated Proliferation by Inhibiting Yes-Associated Protein in Gastric Cancer Cells. Gene 2019, 712, 143956. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Wang, Y.; Qin, Y.; Gong, X.-G. Oleanolic Acid Induces Autophagic Death in Human Gastric Cancer Cells In Vitro and In Vivo. Cell Biol. Int. 2016, 40, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Liu, M.; Liu, H.; Wang, H.; Wang, F.; Zhang, Y.; Han, L.; Lin, X. Oleanolic Acid Arrests Cell Cycle and Induces Apoptosis via ROS-Mediated Mitochondrial Depolarization and Lysosomal Membrane Permeabilization in Human Pancreatic Cancer Cells. J. Appl. Toxicol. JAT 2013, 33, 756–765. [Google Scholar] [CrossRef]
- Zhang, P.; Li, H.; Chen, D.; Ni, J.; Kang, Y.; Wang, S. Oleanolic Acid Induces Apoptosis in Human Leukemia Cells through Caspase Activation and Poly(ADP-Ribose) Polymerase Cleavage. Acta Biochim. Biophys. Sin. 2007, 39, 803–809. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Hua, H.; Pei, Y.; Chen, D.; Jing, Y. Triterpenoids from the Resin of Styrax Tonkinensis and Their Antiproliferative and Differentiation Effects in Human Leukemia HL-60 Cells. J. Nat. Prod. 2006, 69, 807–810. [Google Scholar] [CrossRef]
- Xu, Y.; Shu, B.; Tian, Y.; Wang, G.; Wang, Y.; Wang, J.; Dong, Y. Oleanolic Acid Induces Osteosarcoma Cell Apoptosis by Inhibition of Notch Signaling. Mol. Carcinog. 2018, 57, 896–902. [Google Scholar] [CrossRef]
- Hua, Y.; Zhang, Z.; Li, J.; Li, Q.; Hu, S.; Li, J.; Sun, M.; Cai, Z. Oleanolic Acid Derivative Dex-OA Has Potent Anti-Tumor and Anti-Metastatic Activity on Osteosarcoma Cells In Vitro and In Vivo. Investig. New Drugs 2011, 29, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, X.; Pang, T.; Li, W.; Pan, X. MiR-370 Targeted FoxM1 Functions as a Tumor Suppressor in Laryngeal Squamous Cell Carcinoma (LSCC). Biomed. Pharmacother. 2014, 68, 149–154. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, J.-Y.; Zhang, X.-P.; Lv, Z.-W.; Fu, D.; Lu, Y.-C.; Hu, G.-H.; Luo, C.; Chen, J.-X. RLIP76 Is Overexpressed in Human Glioblastomas and Is Required for Proliferation, Tumorigenesis and Suppression of Apoptosis. Carcinogenesis 2013, 34, 916–926. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Yang, X.; Zhao, N.; Peng, J.; Gao, H.; Qiu, X. Alpinumisoflavone Induces Apoptosis in Esophageal Squamous Cell Carcinoma by Modulating MiR-370/PIM1 Signaling. Am. J. Cancer Res. 2016, 6, 2755–2771. [Google Scholar]
- Jorgensen, I.; Miao, E.A. Pyroptotic Cell Death Defends against Intracellular Pathogens. Immunol. Rev. 2015, 265, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Defining the Role of Hypoxia-Inducible Factor 1 in Cancer Biology and Therapeutics. Oncogene 2010, 29, 625–634. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; Chang, Y.; Wang, X.; Ban, C.; Zhang, F. Reduction of COX-2 through Modulating MiR-124/SPHK1 Axis Contributes to the Antimetastatic Effect of Alpinumisoflavone in Melanoma. Am. J. Transl. Res. 2017, 9, 986–998. [Google Scholar] [PubMed]
- Tang, A.; Gao, K.; Chu, L.; Zhang, R.; Yang, J.; Zheng, J. Aurora Kinases: Novel Therapy Targets in Cancers. Oncotarget 2017, 8, 23937–23954. [Google Scholar] [CrossRef] [Green Version]
- Galetta, D.; Cortes-Dericks, L. Promising Therapy in Lung Cancer: Spotlight on Aurora Kinases. Cancers 2020, 12, 3371. [Google Scholar] [CrossRef]
- Wen, L.; Zhou, T.; Jiang, Y.; Chang, S.K.; Yang, B. Prenylated Flavonoids in Foods and Their Applications on Cancer Prevention. Crit. Rev. Food Sci. Nutr. 2021, 1–14. [Google Scholar] [CrossRef]
- Cho, H.-D.; Lee, J.-H.; Moon, K.-D.; Park, K.-H.; Lee, M.-K.; Seo, K.-I. Auriculasin-Induced ROS Causes Prostate Cancer Cell Death via Induction of Apoptosis. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018, 111, 660–669. [Google Scholar] [CrossRef]
- Cho, H.-D.; Moon, K.-D.; Park, K.-H.; Lee, Y.-S.; Seo, K.-I. Effects of Auriculasin on Vascular Endothelial Growth Factor (VEGF)-Induced Angiogenesis via Regulation of VEGF Receptor 2 Signaling Pathways in Vitro and in Vivo. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018, 121, 612–621. [Google Scholar] [CrossRef]
- Cho, H.-D.; Gu, I.-A.; Won, Y.-S.; Moon, K.-D.; Park, K.-H.; Seo, K.-I. Auriculasin Sensitizes Primary Prostate Cancer Cells to TRAIL-Mediated Apoptosis through up-Regulation of the DR5-Dependent Pathway. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2019, 126, 223–232. [Google Scholar] [CrossRef]
- Yeatman, T.J. A Renaissance for SRC. Nat. Rev. Cancer 2004, 4, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Elfiky, A.; Han, M.; Chen, C.; Saif, M.W. The Role of Src in Colon Cancer and Its Therapeutic Implications. Clin. Colorectal Cancer 2014, 13, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Castrejón-Jiménez, N.S.; Leyva-Paredes, K.; Baltierra-Uribe, S.L.; Castillo-Cruz, J.; Campillo-Navarro, M.; Hernández-Pérez, A.D.; Luna-Angulo, A.B.; Chacón-Salinas, R.; Coral-Vázquez, R.M.; Estrada-García, I.; et al. Ursolic and Oleanolic Acids Induce Mitophagy in A549 Human Lung Cancer Cells. Molecules 2019, 24, 3444. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Liu, M.; Li, D. Oleanolic Acid Suppresses the Proliferation of Lung Carcinoma Cells by MiR-122/Cyclin G1/MEF2D Axis. Mol. Cell. Biochem. 2015, 400, 1–7. [Google Scholar] [CrossRef]
- Ma, D.; Jia, H.; Qin, M.; Dai, W.; Wang, T.; Liang, E.; Dong, G.; Wang, Z.; Zhang, Z.; Feng, F. MiR-122 Induces Radiosensitization in Non-Small Cell Lung Cancer Cell Line. Int. J. Mol. Sci. 2015, 16, 22137–22150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shyu, M.-H.; Kao, T.-C.; Yen, G.-C. Oleanolic Acid and Ursolic Acid Induce Apoptosis in HuH7 Human Hepatocellular Carcinoma Cells through a Mitochondrial-Dependent Pathway and Downregulation of XIAP. J. Agric. Food Chem. 2010, 58, 6110–6118. [Google Scholar] [CrossRef]
- Amara, S.; Zheng, M.; Tiriveedhi, V. Oleanolic Acid Inhibits High Salt-Induced Exaggeration of Warburg-like Metabolism in Breast Cancer Cells. Cell Biochem. Biophys. 2016, 74, 427–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, C.; Li, X.; Yu, S.; Liang, L. Inhibition of Cancer Cell Growth by Oleanolic Acid in Multidrug Resistant Liver Carcinoma Is Mediated via Suppression of Cancer Cell Migration and Invasion, Mitochondrial Apoptosis, G2/M Cell Cycle Arrest and Deactivation of JNK/P38 Signalling Pathway. J. BUON Off. J. Balk. Union Oncol. 2019, 24, 1964–1969. [Google Scholar]
- Hosny, S.; Sahyon, H.; Youssef, M.; Negm, A. Oleanolic Acid Suppressed DMBA-Induced Liver Carcinogenesis through Induction of Mitochondrial-Mediated Apoptosis and Autophagy. Nutr. Cancer 2021, 73, 968–982. [Google Scholar] [CrossRef]
- Xiaofei, J.; Mingqing, S.; Miao, S.; Yizhen, Y.; Shuang, Z.; Qinhua, X.; Kai, Z. Oleanolic Acid Inhibits Cervical Cancer Hela Cell Proliferation through Modulation of the ACSL4 Ferroptosis Signaling Pathway. Biochem. Biophys. Res. Commun. 2021, 545, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Moerke, C.; Bleibaum, F.; Kunzendorf, U.; Krautwald, S. Combined Knockout of RIPK3 and MLKL Reveals Unexpected Outcome in Tissue Injury and Inflammation. Front. Cell Dev. Biol. 2019, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; Xu, H.; Bao, X.; Zhang, C.; Guan, X.; Liu, H.; Lv, L.; Deng, S.; Gao, D.; Wang, C.; et al. Oleanolic Acid-Loaded PLGA-TPGS Nanoparticles Combined with Heparin Sodium-Loaded PLGA-TPGS Nanoparticles for Enhancing Chemotherapy to Liver Cancer. Life Sci. 2016, 165, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Viallard, C.; Larrivée, B. Tumor Angiogenesis and Vascular Normalization: Alternative Therapeutic Targets. Angiogenesis 2017, 20, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Sun, L.; Pei, Y.; Wang, D. Oleanolic Acid Inhibits Colorectal Cancer Angiogenesis by Blocking the VEGFR2 Signaling Pathway. Anticancer Agents Med. Chem. 2018, 18, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Ganta, V.C.; Choi, M.H.; Kutateladze, A.; Fox, T.E.; Farber, C.R.; Annex, B.H. A MicroRNA93-Interferon Regulatory Factor-9-Immunoresponsive Gene-1-Itaconic Acid Pathway Modulates M2-Like Macrophage Polarization to Revascularize Ischemic Muscle. Circulation 2017, 135, 2403–2425. [Google Scholar] [CrossRef]
- Salybekov, A.A.; Kawaguchi, A.T.; Masuda, H.; Vorateera, K.; Okada, C.; Asahara, T. Regeneration-Associated Cells Improve Recovery from Myocardial Infarction through Enhanced Vasculogenesis, Anti-Inflammation, and Cardiomyogenesis. PLoS ONE 2018, 13, e0203244. [Google Scholar] [CrossRef] [Green Version]
- Martín, R.; Ibeas, E.; Carvalho-Tavares, J.; Hernández, M.; Ruiz-Gutierrez, V.; Nieto, M.L. Natural Triterpenic Diols Promote Apoptosis in Astrocytoma Cells through ROS-Mediated Mitochondrial Depolarization and JNK Activation. PLoS ONE 2009, 4, e5975. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Bai, H.; Zhang, X.; Liu, J.; Cao, P.; Liao, N.; Zhang, W.; Wang, Z.; Hai, C. Inhibitory Effect of Oleanolic Acid on Hepatocellular Carcinoma via ERK-P53-Mediated Cell Cycle Arrest and Mitochondrial-Dependent Apoptosis. Carcinogenesis 2013, 34, 1323–1330. [Google Scholar] [CrossRef]
- Woo, J.-S.; Yoo, E.-S.; Kim, S.-H.; Lee, J.-H.; Han, S.-H.; Jung, S.-H.; Jung, G.-H.; Jung, J.-Y. Anticancer Effects of Oleanolic Acid on Human Melanoma Cells. Chem. Biol. Interact. 2021, 347, 109619. [Google Scholar] [CrossRef]
- Li, L.; Wei, L.; Shen, A.; Chu, J.; Lin, J.; Peng, J. Oleanolic Acid Modulates Multiple Intracellular Targets to Inhibit Colorectal Cancer Growth. Int. J. Oncol. 2015, 47, 2247–2254. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.S.; Yuan, Y.; Song, G.; Lin, S.Q. Inhibitory Effect of Ursolic Acid and Oleanolic Acid from Eriobotrya Fragrans on A549 Cell Viability In Vivo. Genet. Mol. Res. GMR 2016, 15, 1–8. [Google Scholar] [CrossRef]
- Li, H.-F.; Wang, X.-A.; Xiang, S.-S.; Hu, Y.-P.; Jiang, L.; Shu, Y.-J.; Li, M.-L.; Wu, X.-S.; Zhang, F.; Ye, Y.-Y.; et al. Oleanolic Acid Induces Mitochondrial-Dependent Apoptosis and G0/G1 Phase Arrest in Gallbladder Cancer Cells. Drug Des. Dev. Ther. 2015, 9, 3017–3030. [Google Scholar] [CrossRef] [Green Version]
- Hsu, H.Y.; Yang, J.J.; Lin, C.C. Effects of Oleanolic Acid and Ursolic Acid on Inhibiting Tumor Growth and Enhancing the Recovery of Hematopoietic System Postirradiation in Mice. Cancer Lett. 1997, 111, 7–13. [Google Scholar] [CrossRef]
- Lúcio, K.A.; Rocha, G.d.G.; Monção-Ribeiro, L.C.; Fernandes, J.; Takiya, C.M.; Gattass, C.R. Oleanolic Acid Initiates Apoptosis in Non-Small Cell Lung Cancer Cell Lines and Reduces Metastasis of a B16F10 Melanoma Model in Vivo. PLoS ONE 2011, 6, e28596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fofana, S.; Kouama, B.; Da, O.; Yerbanga, S.; Ilboudo, S.; Ouédraogo, N.; Ouédraogo, M.; Guissou, I.P. Acute and Subchronic Toxicity Assessment of the Dichloromethane/Methanol Stem Bark Extract from Erythrina senegalensis DC (Fabaceae) in Wistar Rats. Issues Biol. Sci. Pharm. Res. 2021, 9, 19–26. [Google Scholar]
- Fofana, S.; Gnoula, C.; Ouedraogo Moussa, M.; Palé, E.; Nebié, R.H.; Nikiema, J.-B.; Guissou, I.P.; Simporé, J. DPPH Radical Scavenging and Lipoxygenase Inhibitory Effects in Extracts from Erythrina senegalensis (Fabaceae) DC. Afr. J. Pharm. Pharmacol. 2016, 10, 185–191. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Xie, N.; Yi, S.; Liu, C.; Jiang, H.; Ma, Z.; Feng, J.; Yan, H.; Zhang, X. Bioassay-Guided Isolation of Potent Aphicidal Erythrina Alkaloids against Aphis Gossypii from the Seed of Erythrina Crista-Galli L. Pest Manag. Sci. 2018, 74, 210–218. [Google Scholar] [CrossRef]
- Koniuszy, F.; Wiley, P.F.; Folkers, K. Erythrina Alkaloids; Studies on the Constitution of Erysodine Erysovine and Erysopine. J. Am. Chem. Soc. 1949, 71, 875–878. [Google Scholar] [CrossRef]
- Fahmy, N.M.; Al-Sayed, E.; El-Shazly, M.; Nasser Singab, A. Alkaloids of Genus Erythrina: An Updated Review. Nat. Prod. Res. 2020, 34, 1891–1912. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Etoh, T.; Hayashi, M.; Komiyama, K.; Kishida, A.; Ohsaki, A. TRAIL-Enhancing Activity of Erythrinan Alkaloids from Erythrina Velutina. Bioorg. Med. Chem. Lett. 2009, 19, 234–236. [Google Scholar] [CrossRef]
- Zarev, Y.; Foubert, K.; Cos, P.; Maes, L.; Elgorashi, E.; Apers, S.; Ionkova, I.; Pieters, L. HPLC-DAD-SPE-NMR Isolation of Tetracyclic Spiro-Alkaloids with Antiplasmodial Activity from the Seeds of Erythrina Latissima. Nat. Prod. Res. 2020, 34, 1037–1040. [Google Scholar] [CrossRef]
- Sun, H.; Zhu, X.; Cai, W.; Qiu, L. Hypaphorine Attenuates Lipopolysaccharide-Induced Endothelial Inflammation via Regulation of TLR4 and PPAR-γ Dependent on PI3K/Akt/MTOR Signal Pathway. Int. J. Mol. Sci. 2017, 18, 844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, F.; Hou, B.; Zhu, X.; Liu, Y.; Shi, X.; Li, S.; Li, Z.; Cai, W.; Zhou, Y.; Qiu, L. Vaccaria N-Butanol Extract Lower the Production of Proinflammatory Cytokines and the Infection Risk of T. Spiralis In Vivo. Acta Parasitol. 2019, 64, 520–527. [Google Scholar] [CrossRef]
- Chen, H.; Guo, T.; Wang, D.; Qin, R. Vaccaria Hypaphorine Impairs RANKL-Induced Osteoclastogenesis by Inhibition of ERK, P38, JNK and NF-ΚB Pathway and Prevents Inflammatory Bone Loss in Mice. Biomed. Pharmacother. 2018, 97, 1155–1163. [Google Scholar] [CrossRef]
- Luan, G.; Tie, F.; Yuan, Z.; Li, G.; He, J.; Wang, Z.; Wang, H. Hypaphorine, an Indole Alkaloid Isolated from Caragana Korshinskii Kom., Inhibites 3T3-L1 Adipocyte Differentiation and Improves Insulin Sensitivity in Vitro. Chem. Biodivers. 2017, 14, e1700038. [Google Scholar] [CrossRef]
- Wang, J.-F.; Liu, S.-S.; Song, Z.-Q.; Xu, T.-C.; Liu, C.-S.; Hou, Y.-G.; Huang, R.; Wu, S.-H. Naturally Occurring Flavonoids and Isoflavonoids and Their Microbial Transformation: A Review. Molecules 2020, 25, 5112. [Google Scholar] [CrossRef]
- Gupta, C.; Prakash, D. Phytonutrients as Therapeutic Agents. J. Complement. Integr. Med. 2014, 11, 151–169. [Google Scholar] [CrossRef]
- Lee, J.; Oh, W.K.; Ahn, J.S.; Kim, Y.H.; Mbafor, J.T.; Wandji, J.; Fomum, Z.T. Prenylisoflavonoids from Erythrina senegalensis as Novel HIV-1 Protease Inhibitors. Planta Med. 2009, 75, 268–270. [Google Scholar] [CrossRef] [PubMed]
- Amen, Y.M.; Marzouk, A.M.; Zaghloul, M.G.; Afifi, M.S. Bioactive Compounds from Tipuana Tipu Growing in Egypt. J. Am. Sci. 2013, 9, 334–339. [Google Scholar]
- Dai, X.; Cheng, H.; Bai, Z.; Li, J. Breast Cancer Cell Line Classification and Its Relevance with Breast Tumor Subtyping. J. Cancer 2017, 8, 3131–3141. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.-S.; Wang, Y.; Wang, P.; Chen, Z.-D. Expression of Oestrogen Receptor-Alpha and Oestrogen Receptor-Beta in Prostate Cancer. Chin. Med. J. 2007, 120, 1611–1615. [Google Scholar] [CrossRef]
- Magne Nde, C.B.; Njamen, D.; Tanee Fomum, S.; Wandji, J.; Simpson, E.; Clyne, C.; Vollmer, G. In Vitro Estrogenic Activity of Two Major Compounds from the Stem Bark of Erythrina lysistemon (Fabaceae). Eur. J. Pharmacol. 2012, 674, 87–94. [Google Scholar] [CrossRef]
- Xin, D.; Wang, H.; Yang, J.; Su, Y.-F.; Fan, G.-W.; Wang, Y.-F.; Zhu, Y.; Gao, X.-M. Phytoestrogens from Psoralea corylifolia Reveal Estrogen Receptor-Subtype Selectivity. Phytomed. Int. J. Phytother. Phytopharm. 2010, 17, 126–131. [Google Scholar] [CrossRef]
- Nana, F.; Sandjo, L.P.; Keumedjio, F.; Ambassa, P.; Malik, R.; Kuete, V.; Rincheval, V.; Choudhary, M.I.; Ngadjui, B.T. Ceramides and Cytotoxic Constituents from Ficus glumosa Del.(Moraceae). J. Braz. Chem. Soc. 2012, 23, 482–487. [Google Scholar] [CrossRef] [Green Version]
- Militão, G.C.G.; Dantas, I.N.F.; Pessoa, C.; Falcão, M.J.C.; Silveira, E.R.; Lima, M.A.S.; Curi, R.; Lima, T.; Moraes, M.O.; Costa-Lotufo, L.V. Induction of Apoptosis by Pterocarpans from Platymiscium Floribundum in HL-60 Human Leukemia Cells. Life Sci. 2006, 78, 2409–2417. [Google Scholar] [CrossRef] [PubMed]
- Tesauro, C.; Fiorani, P.; D’Annessa, I.; Chillemi, G.; Turchi, G.; Desideri, A. Erybraedin C, a Natural Compound from the Plant Bituminaria bituminosa, Inhibits Both the Cleavage and Religation Activities of Human Topoisomerase I. Biochem. J. 2010, 425, 531–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohan Rao, K.S.R.M.; Rukmani Iyer, C.S.; Iyer, P.R. Synthesis of Alpinum Isoflavone, Derrone and Related Pyranoisoflavones. Tetrahedron 1987, 43, 3015–3019. [Google Scholar] [CrossRef]
- Li, J.; Jiang, Z. Synthesis of Alpinumisoflavone with Molluscicidal Activity. Chin. J. Hyg. Insectic. Equip. 2007, 2, 124–125. [Google Scholar]
- Fernández, J.; Silván, B.; Entrialgo-Cadierno, R.; Villar, C.J.; Capasso, R.; Uranga, J.A.; Lombó, F.; Abalo, R. Antiproliferative and Palliative Activity of Flavonoids in Colorectal Cancer. Biomed. Pharmacother. Biomed. Pharmacother. 2021, 143, 112241. [Google Scholar] [CrossRef]
- Liu, K.; Qin, Y.-H.; Yu, J.-Y.; Ma, H.; Song, X.-L. 3-β-Εrythrodiol Isolated from Conyza Canadensis Inhibits MKN-45 Human Gastric Cancer Cell Proliferation by Inducing Apoptosis, Cell Cycle Arrest, DNA Fragmentation, ROS Generation and Reduces Tumor Weight and Volume in Mouse Xenograft Model. Oncol. Rep. 2016, 35, 2328–2338. [Google Scholar] [CrossRef] [Green Version]
- Bao, X.; Gao, M.; Xu, H.; Liu, K.-X.; Zhang, C.-H.; Jiang, N.; Chu, Q.-C.; Guan, X.; Tian, Y. A Novel Oleanolic Acid-Loaded PLGA-TPGS Nanoparticle for Liver Cancer Treatment. Drug Dev. Ind. Pharm. 2015, 41, 1193–1203. [Google Scholar] [CrossRef]
- Sarfraz, M.; Afzal, A.; Raza, S.M.; Bashir, S.; Madni, A.; Khan, M.W.; Ma, X.; Xiang, G. Liposomal Co-Delivered Oleanolic Acid Attenuates Doxorubicin-Induced Multi-Organ Toxicity in Hepatocellular Carcinoma. Oncotarget 2017, 8, 47136–47153. [Google Scholar] [CrossRef] [Green Version]
- Shukla, R.P.; Urandur, S.; Banala, V.T.; Marwaha, D.; Gautam, S.; Rai, N.; Singh, N.; Tiwari, P.; Shukla, P.; Mishra, P.R. Development of Putrescine Anchored Nano-Crystalsomes Bearing Doxorubicin and Oleanolic Acid: Deciphering Their Role in Inhibiting Metastatic Breast Cancer. Biomater. Sci. 2021, 9, 1779–1794. [Google Scholar] [CrossRef] [PubMed]
- Oprean, C.; Borcan, F.; Pavel, I.; Dema, A.; Danciu, C.; Soica, C.; Dehelean, C.; Nicu, A.; Ardelean, A.; Cristea, M.; et al. In Vivo Biological Evaluation of Polyurethane Nanostructures with Ursolic and Oleanolic Acids on Chemically-Induced Skin Carcinogenesis. In Vivo 2016, 30, 633–638. [Google Scholar]
- Caunii, A.; Oprean, C.; Cristea, M.; Ivan, A.; Danciu, C.; Tatu, C.; Paunescu, V.; Marti, D.; Tzanakakis, G.; Spandidos, D.A.; et al. Effects of Ursolic and Oleanolic on SK-MEL-2 Melanoma Cells: In Vitro and in Vivo Assays. Int. J. Oncol. 2017, 51, 1651–1660. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Zhong, Q.; Zhong, R.; Huang, H.; Xia, Z.; Ke, Z.; Zhang, Z.; Song, J.; Jia, X. Preparation and Antitumor Evaluation of Self-Assembling Oleanolic Acid-Loaded Pluronic P105/d-α-Tocopheryl Polyethylene Glycol Succinate Mixed Micelles for Non-Small-Cell Lung Cancer Treatment. Int. J. Nanomed. 2016, 11, 6337–6352. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Luo, X.; Xu, X.; Gao, N.; Liu, X. Preparation, Characterization and in Vivo Pharmacokinetic Study of PVP-Modified Oleanolic Acid Liposomes. Int. J. Pharm. 2017, 517, 1–7. [Google Scholar] [CrossRef]
- Bao, Y.; Zhang, S.; Chen, Z.; Chen, A.T.; Ma, J.; Deng, G.; Xu, W.; Zhou, J.; Yu, Z.-Q.; Yao, G.; et al. Synergistic Chemotherapy for Breast Cancer and Breast Cancer Brain Metastases via Paclitaxel-Loaded Oleanolic Acid Nanoparticles. Mol. Pharm. 2020, 17, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Soica, C.; Oprean, C.; Borcan, F.; Danciu, C.; Trandafirescu, C.; Coricovac, D.; Crăiniceanu, Z.; Dehelean, C.A.; Munteanu, M. The Synergistic Biologic Activity of Oleanolic and Ursolic Acids in Complex with Hydroxypropyl-γ-Cyclodextrin. Molecules 2014, 19, 4924–4940. [Google Scholar] [CrossRef] [Green Version]
- Nair, R.V.R.; Jayasree, D.V.; Biju, P.G.; Baby, S. Anti-Inflammatory and Anticancer Activities of Erythrodiol-3-Acetate and 2,4-Di-Tert-Butylphenol Isolated from Humboldtia Unijuga. Nat. Prod. Res. 2020, 34, 2319–2322. [Google Scholar] [CrossRef]
- Shen, P.; Wang, W.; Xu, S.; Du, Z.; Wang, W.; Yu, B.; Zhang, J. Biotransformation of Erythrodiol for New Food Supplements with Anti-Inflammatory Properties. J. Agric. Food Chem. 2020, 68, 5910–5916. [Google Scholar] [CrossRef] [PubMed]
- Kazakova, O.; Rubanik, L.; Lobov, A.; Poleshchuk, N.; Baikova, I.; Kapustina, Y.; Petrova, A.; Korzun, T.; Lopatina, T.; Fedorova, A.; et al. Synthesis of Erythrodiol C-Ring Derivatives and Their Activity against Chlamydia Trachomatis. Steroids 2021, 175, 108912. [Google Scholar] [CrossRef] [PubMed]
- Liby, K.T.; Sporn, M.B. Synthetic Oleanane Triterpenoids: Multifunctional Drugs with a Broad Range of Applications for Prevention and Treatment of Chronic Disease. Pharmacol. Rev. 2012, 64, 972–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Küpeli Akkol, E.; Genç, Y.; Karpuz, B.; Sobarzo-Sánchez, E.; Capasso, R. Coumarins and Coumarin-Related Compounds in Pharmacotherapy of Cancer. Cancers 2020, 12, 1959. [Google Scholar] [CrossRef] [PubMed]

| Compounds | Formula | Chemical Family | Part of the Plant | Solvent Used | Ref. | Anticancerous Activity |
|---|---|---|---|---|---|---|
| Erysodine | C18H21NO3 | alkaloid | Seed | MeOH re-extracted by CH2Cl2 | [10] | YES |
| Hypaphorine | C14H18N2 | alkaloid | Seed | MeOH re-extracted by CH2Cl2 | [10] | NT |
| Glucoérysodine | C24H31NO8 | Alkaloid | Seed | MeOH re-extracted by CH2Cl2 | [10] | NT |
| Erysenegalensein F | C25H24O7 | Epoxy isoflavone | Stem bark | MeOH re-extracted by CH2Cl2 | [11] | NT |
| Erysenegalensein G | C25H24O7 | Epoxy isoflavone | Stem bark | MeOH re-extracted by CH2Cl2 | [11] | NT |
| Erythrinasinate | C38H66O4 | Cinnamate | Stem bark | n-hexane re-extracted by CHCl3 | [12] | NO |
| Carpachromene | C20H1605 | Flavone | Root | CH2Cl2 | [13] | YES |
| Sigmoidin H | C21H20O5 | Isoflavanone | Stem bark | MeOH/CH2Cl2 (1:1) | [14] | YES |
| Erysenegalensein B | C25H28O7 | Isoflavanone | Stem bark | MeOH re-extracted by CH2Cl2 | [15] | NT |
| Erysenegalensein C | C25H28O7 | Isoflavanone | Stem bark | MeOH re-extracted by CH2Cl2 | [15] | NT |
| Alpinumisoflavone | C20H16O5 | Isoflavone | Stem bark | MeOH re-extracted by CH2Cl2 | [16] | YES |
| Derrone | C20H16O5 | Isoflavone | Stem bark | MeOH re-extracted by CH2Cl2 | [16] | YES |
| 8-prenylluteone | C25H26O6 | Isoflavone | Stem bark | MeOH re-extracted by CH2Cl2 | [17] | NT |
| Erysenegalensein D | C25H26O7 | Isoflavone | Stem bark | MeOH re-extracted by CH2Cl2 | [17] | NT |
| Erysenegalensein E | C25H26O6 | Isoflavone | Stem bark | MeOH re-extracted by CH2Cl2 | [17] | YES |
| Erysenegalensein H | C25H26O7 | Isoflavone | Stem bark | MeOH re-extracted by CH2Cl2 | [18] | NT |
| Erysenegalensein I | C25H26O7 | Isoflavone | Stem bark | MeOH re-extracted by CH2Cl2 | [18] | NT |
| Erysenegalensein M | C25H24O7 | Isoflavone | Stem bark | MeOH re-extracted by CH2Cl2 | [10] | YES |
| Erysenegalensein L | C25H24O7 | Isoflavone | Stem bark | MeOH re-extracted by CH2Cl2 | [10] | NT |
| Lupinifolin | C25H26O5 | Isoflavone | Stem bark | MeOH re-extracted by CH2Cl2 | [18] | YES |
| Neobavaisoflavone | C20H18O4 | Isoflavone | Stem bark | MeOH/CH2Cl2 (1:1) | [14] | YES |
| 6,8-diprenylgenistein | C25H26O5 | isoflavanone | Stem bark | n-hexane re-extracted by CHCl3 | [19] | NT |
| 2,3-dihydro-auriculatin | C25H26O6 | isoflavanone | Stem bark | n-hexane re-extracted by CHCl3 | [19] | NT |
| Auriculatin | C25H24O6 | isoflavanone | Stem bark | n-hexane re-extracted by CHCl3 | [19] | NT |
| Erythrisenegalone | C25H26O5 | Flavanone | Stem bark | n-hexane re-extracted by CHCl3 | [20] | YES |
| Senegalensein (Lonchocarpol A) | C25H28O5 | Flavanone | Stem bark | n-hexane re-extracted by CHCl3 | [21] | YES |
| Warangalone (Scandenone) | C25H24O5 | isoflavanone | Stem bark | n-hexane re-extracted by CHCl3 | [20] | YES |
| Erysenegalensein J | C25H24O7 | Isoflavanone | Stem bark | MeOH re-extracted by CH2Cl2 | [22] | NT |
| Erysenegalensein K | C22H18O6 | Isoflavanone | Stem bark | MeOH re-extracted by CH2Cl2 | [22] | NT |
| Erysenegalensein N | C25H26O7 | Isoflavone | Stem bark | MeOH re-extracted by CH2Cl2 | [16] | NT |
| Erysenegalensein O | C25H26O7 | Isoflavone | Stem bark | MeOH re-extracted by CH2Cl2 | [16] | NT |
| Senegalensin | C25H26O6 | Isoflavone | Stem bark | n-hexane re-extracted by CHCl3 | [23] | YES |
| Erybraedin A | C21H20O5 | Pterocarpan | Root | CH2Cl2 | [13] | YES |
| Erybraedin C | C21H20O5 | Pterocarpan | Root | CH2Cl2 | [13] | YES |
| Eryvarine K | C21H22O5 | Pterocarpan | Root | CH2Cl2 | [13] | NT |
| Phaseollin | C20H18O4 | Pterocarpan | Root | CH2Cl2 | [13] | YES |
| Shinpterocarpin | C20H18O4 | Pterocarpan | Root | CH2Cl2 | [13] | NT |
| Erybraedin D | C25H26O4 | Pterocarpan | Root | CH2Cl2 | [13] | NT |
| Cornulacic acid | C30H48O3 | Triterpene | Stem bark | MeOH re-extracted by CH2Cl2 | [22] | NT |
| Erythrodiol | C30H50O2 | Triterpene | Stem bark | MeOH re-extracted by CH2Cl2 | [22] | YES |
| Maniladiol | C30H50O2 | Triterpene | Stem bark | MeOH re-extracted by CH2Cl2 | [22] | YES |
| Oleanolic acid | C30H48O3 | Triterpene | Stem bark | MeOH re-extracted by CH2Cl2 | [22] | YES |
| Compound | Cancer Type | Cell Line | Specificity of the Cell Line | Time of Treatment | IC50 (µM) | Ref. |
|---|---|---|---|---|---|---|
| Carpachromene | Lymphoma | Raji | NS | 0.029 | [24] | |
| Liver cancer | HepG2 | NS | 0.024 | [24] | ||
| Liver cancer | PLC/PRF/5 | NS | 0.016 | [24] | ||
| Phaseolin | Rat hepatoma cancer | H411E ** | 24 h | 1.5 | [25] | |
| Prostate cancer | PC3 | 24 h | 10 | [26] | ||
| Erybraedin A | Breast cancer | BC | NS | 7 | [27] | |
| Lung cancer | A549 | 24 h | 1–5 | [28] | ||
| H1299 | 24 h | 1–5 | [28] | |||
| H226B | 24 h | 1–5 | [28] | |||
| NCI-H187 | 24 h | 5 | [27] | |||
| Breast cancer | BC | 24 h | 7 | [27] | ||
| Cervix carcinoma | KB | NS | 13 | [27] | ||
| Erysenegalensein M | Breast cancer | MCF-7 | NS | 8 | [29] | |
| Prostate cancer | LNCaP | NS | 8 | [29] | ||
| Erybraedin C | Colon cancer | HT29 | MMR +/+ p53 −/− Bcl-2 +/+ | NS | 5 | [30] |
| LoVo | MMR −/− p53 +/+ Bcl-2 −/− | NS | 4 | [30] | ||
| Leukemia | Mono-Mac-6 | 72 h | 29 | [31] | ||
| CD4+ Jurkat T * | Bcl-2 overexpression | 72 h | 18 | [31] | ||
| CD4+ Jurkat T | 72 h | 21 | [31] | |||
| Cervix carcinoma | KB | 72 h | 24 | [31] | ||
| Maniladiol | Renal cancer | RFX393 | NS | 7 | [32] | |
| Lung cancer | Lu1 | NS | 31 | [33] | ||
| Breast cancer | MCF-7 | NS | 31 | [33] | ||
| T-47D | NS | 10 | [32] | |||
| Prostate cancer | LNCaP | NS | 26 | [33] | ||
| Warangalone | Breast cancer | MCF-7 | NS | 7 | [29] | |
| Cervix carcinoma | K-B-3-1 | NS | 73 | [34] | ||
| Endometrial cancer | Ishikawa | NS | 7 | [29] | ||
| Leukemia | HL-60 | 72 h | 30 | [35] | ||
| Prostate cancer | LNCaP | NS | 7 | [29] | ||
| Alpinumisoflavone | Kidney cancer | RCC4 | NS | 5–10 | [36] | |
| Carcinoma | KB | 72 | 12 | [37] | ||
| Cervix carcinoma | KB-3-1 | NS | 71 | [34] | ||
| Breast cancer | MDA-MB-231 | NS | 5 | [38] | ||
| MDA-MB-231-BCRP | BCRP resistant | NS | 66 | [38] | ||
| MDA-MB-231-pcDNA | NS | 43 | [38] | |||
| T47D | NS | 5 | [39] | |||
| Colon cancer | HCT 116 | p53 −/− | NS | 36 | [38] | |
| HCT 116 | p53 +/+ | NS | 42 | [38] | ||
| SW 480 | 24 h | 5–10 | [40] | |||
| SW 480 | 48 h | 5–10 | [40] | |||
| Glioma | U87MG | NS | 47 | [38] | ||
| U87MG.ΔEGFR | Multiresistant | NS | 42 | [38] | ||
| Liver carcinoma | Bel 7402 | 48 h | 27 | [41] | ||
| HepG2 | 48 h | 23 | [41] | |||
| Huh7 | 48 h | 14 | [41] | |||
| SMMC 7721 | 48 h | 18 | [41] | |||
| Leukemia | CCRF-CEM | NS | 10 | [38] | ||
| CEM/ADR5000 | Pgp overexpression | NS | 6 | [38] | ||
| HL-60 | 48 h | 19 | [42] | |||
| K-562 | 48 h | 34 | [42] | |||
| MOLT-4 | 48 h | 41 | [42] | |||
| P-388 * | NS | 13 | [43] | |||
| Lung cancer | H1299 | 24 h | 39 | [43] | ||
| H2108 | 24 h | 34 | [44] | |||
| MRC-5 | 24 h | 53 | [44] | |||
| Alpinumisoflavone | Esophagal cancer | Eca 109 | 72 h | 10–20 | [45] | |
| KYSE30 | 72 h | 10–20 | [45] | |||
| Erythrodiol | Breast cancer | MCF-7 | 120 h | 12.5–25 | [46] | |
| Colon cancer | HT29 | NS | 49 | [47] | ||
| Liver carcinoma | HepG2 | NS | 11 | [48] | ||
| Erysodine | Liver cancer | HEP-2 | NS | 66 | [48] | |
| Liver cancer | HepG2 | NS | 39 | [48] | ||
| Erysenegalensein E | Cervix carcinoma | KB-3-1 | NS | 99 | [34] | |
| Cervix carcinoma | KB | NS | 15 | [37] | ||
| Derrone | Colon cancer | HCT 116 | 24 h | 42 | [49] | |
| Prostate cancer | PC3 | 24 h | 45 | [49] | ||
| Breast cancer | KPL4 | 24 h | 46 | [50] | ||
| MCF-7 | 24 h | 24 | [50] | |||
| Cervix carcinoma | HeLa | 24 h | 31 | [50] | ||
| KB-3-1 | NS | 230 | [34] | |||
| Lung cancer | A549 | 24 h | 43 | [49] | ||
| H1299 | 24 h | 24 | [50] | |||
| H292 | 24 h | 39 | [49] | |||
| Erythrinasinate | Leukemia | CCRF-CEM | 72 h | >70 | [51] | |
| CEM/ADR5000 | 72 h | >70 | [51] | |||
| Neobavalisoflavone | Glioma | U87MG.ΔEGFR | Multiresistant GBM | NS | 78 | [14] |
| Leukemia | CCRF-CEM | NS | 51 | [14] | ||
| CEM/ADR5000 | Multiresistant leukemia | NS | 43 | [14] | ||
| Liver carcinoma | HepG2 | NS | 110 | [14] | ||
| Oleanolic acid | Breast cancer | MCF-7/wt | BCR1 expression | 72 h | 28 | [52] |
| MCF-7/ADR | BCR1 expression | 72 h | 44 | [52] | ||
| MCF-7 | 24 h | 290 | [53] | |||
| Glioma | U87MG | 24 h | 358 | [53] | ||
| Prostate cancer | PC3 | 72 h | 40 | [54] | ||
| DU145 | 72 h | 30 | [54] | |||
| LNCaP | 72 h | 25 | [54] | |||
| DU145 | 24 h | 246 | [53] | |||
| Thyroid cancer | SW 579 | NS | 42 | [55] | ||
| Gall bladder cancer | GBC-SD | 72 h | 48 | [56] | ||
| NOZ | 72 h | 60 | [56] | |||
| Pancreas cancer | Panc-28 | 24 h | 102 | [57] | ||
| Bladder cancer | T24 | 48 h | 50 | [58] | ||
| EJ | 24 h | 10–20 | [58] | |||
| T24 | 24 h | 8–16 | [59] | |||
| Liver cancer | HepG2 | NS | 30 | [60] | ||
| SMC7721 | 48 h | 25 | [61] | |||
| HepG2 | 48 h | 21 | [61] | |||
| SMMC-7721 | 24 h | 30–60 | [62] | |||
| Colon cancer | HCT 116 | 48 h | 88 | [63] | ||
| HT29 | 24 h | 25 | [64] | |||
| Gastric cancer | MKN-45 | 24 h | 20–30 | [65] | ||
| BGC-823 | 72 h | 22 | [66] | |||
| MGC-803 | 72 h | 20 | [66] | |||
| SGC-7901 | 72 h | 21 | [66] | |||
| Leukemia | HL-60 | 72 h | 55 | [67] | ||
| HL-60 | 72 h | 9 | [68] | |||
| Osteosarcoma | MG63 | 72 h | 100 | [69] | ||
| Saos-2 | 72 h | 50 | [69] | |||
| MG63 | 72 h | 75 | [70] | |||
| Saos-2 | 72 h | 60 | [70] | |||
| Sigmoidin H | Glioma | U87MG | NS | 26 | [14] | |
| Leukemia | CCRF-CEM | NS | 98 | [14] | ||
| CEM/ADR5000 | Multiresistant | NS | 100 | [14] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fofana, S.; Ouédraogo, M.; Esposito, R.C.; Ouedraogo, W.P.; Delporte, C.; Van Antwerpen, P.; Mathieu, V.; Guissou, I.P. Systematic Review of Potential Anticancerous Activities of Erythrina senegalensis DC (Fabaceae). Plants 2022, 11, 19. https://doi.org/10.3390/plants11010019
Fofana S, Ouédraogo M, Esposito RC, Ouedraogo WP, Delporte C, Van Antwerpen P, Mathieu V, Guissou IP. Systematic Review of Potential Anticancerous Activities of Erythrina senegalensis DC (Fabaceae). Plants. 2022; 11(1):19. https://doi.org/10.3390/plants11010019
Chicago/Turabian StyleFofana, Souleymane, Moussa Ouédraogo, Rafaèle Calvo Esposito, Windbedema Prisca Ouedraogo, Cédric Delporte, Pierre Van Antwerpen, Véronique Mathieu, and Innocent Pierre Guissou. 2022. "Systematic Review of Potential Anticancerous Activities of Erythrina senegalensis DC (Fabaceae)" Plants 11, no. 1: 19. https://doi.org/10.3390/plants11010019
APA StyleFofana, S., Ouédraogo, M., Esposito, R. C., Ouedraogo, W. P., Delporte, C., Van Antwerpen, P., Mathieu, V., & Guissou, I. P. (2022). Systematic Review of Potential Anticancerous Activities of Erythrina senegalensis DC (Fabaceae). Plants, 11(1), 19. https://doi.org/10.3390/plants11010019