Use of Conventional and Innovative Technologies for the Production of Food Grade Hop Extracts: Focus on Bioactive Compounds and Antioxidant Activity
Abstract
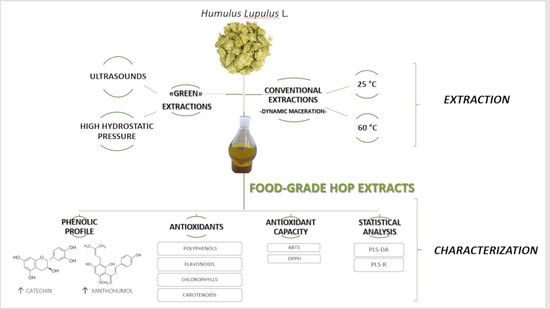
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals
2.3. Preliminary Operations
2.4. Preliminary Experiments
2.5. Conventional Extraction by Dynamic Maceration
2.6. Innovative Extractions
2.6.1. UAE Extraction
2.6.2. HHP Extraction
2.7. Total Phenolic Content and Antioxidant Capacity
2.8. Total Flavonoid Content
2.9. Content of Chlorophyll α, Chlorophyll β and Total Carotenoid
2.10. Content of Phenolic Compounds and Xanthohumol by HPLC Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Phenolic Profile of Hop Extracts
3.2. Hop Extracts Antioxidants and Antioxidant Capacity
3.3. Supervised Multivariate Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shah, M.A.; Bosco, S.J.D.; Mir, S.A. Plant extracts as natural antioxidants in meat and meat products. Meat Sci. 2014, 98, 21–33. [Google Scholar] [CrossRef]
- Flamminii, F.; Di Mattia, C.D.; Difonzo, G.; Neri, L.; Faieta, M.; Caponio, F.; Pittia, P. From by-product to food ingredient: Evaluation of compositional and technological properties of olive-leaf phenolic extracts. J. Sci. Food Agric. 2019, 99, 6620–6627. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, V.; Neri, L.; Moscetti, R.; Di Mattia, C.D.; Sacchetti, G.; Massantini, R.; Pittia, P. Combined Use of Blanching and Vacuum Impregnation with Trehalose and Green Tea Extract as Pre-treatment to Improve the Quality and Stability of Frozen Carrots. Food Bioprocess Technol. 2021, 14, 1326–1340. [Google Scholar] [CrossRef]
- Di Mattia, C.D.; Sacchetti, G.; Mastrocola, D.; Serafini, M. From Cocoa to Chocolate: The Impact of Processing on In Vitro Antioxidant Activity and the Effects of Chocolate on Antioxidant Markers In Vivo. Front. Immunol. 2017, 8, 1207. [Google Scholar] [CrossRef] [Green Version]
- Sanz, V.; Torres, M.D.; Vilariño, J.M.L.; Domínguez, H. What is new on the hop extraction? Trends Food Sci. Technol. 2019, 93, 12–22. [Google Scholar] [CrossRef]
- Zanoli, P.; Zavatti, M. Pharmacognostic and pharmacological profile of Humulus lupulus L. J. Ethnopharmacol. 2008, 116, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, D.; Świeca, M.; Cichocka, J.; Gawlik-Dziki, U. The phenolic content and antioxidant activity of the aqueous and hydroalcoholic extracts of hops and their pellets. J. Inst. Brew. 2013, 119, 103–110. [Google Scholar] [CrossRef]
- Inui, T.; Okumura, K.; Matsui, H.; Hosoya, T.; Kumazawa, S. Effect of harvest time on some in vitro functional properties of hop polyphenols. Food Chem. 2017, 225, 69–76. [Google Scholar] [CrossRef]
- Olšovská, J.; Boštíková, V.; Dusek, M.; Jandovská, V.; Bogdanová, K.; Čermák, P.; Bostik, P.; Mikyska, A.; Kolář, M. Humulus lupulus L.(hops)—A valuable source of compounds with bioactive effects for future therapiess. Mil. Med. Sci. Lett. 2016, 85, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.-H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, B.K. Ultrasound: A clean, green extraction technology. TrAC Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Corrales, M.; Toepfl, S.; Butz, P.; Knorr, D.; Tauscher, B. Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: A comparison. Innov. Food Sci. Emerg. Technol. 2008, 9, 85–91. [Google Scholar] [CrossRef]
- Scepankova, H.; Martins, M.; Estevinho, L.; Delgadillo, I.; Saraiva, J.A. Enhancement of Bioactivity of Natural Extracts by Non-Thermal High Hydrostatic Pressure Extraction. Plant Foods Hum. Nutr. 2018, 73, 253–267. [Google Scholar] [CrossRef] [Green Version]
- Santarelli, V.; Neri, L.; Sacchetti, G.; Di Mattia, C.D.; Mastrocola, D.; Pittia, P. Response of organic and conventional apples to freezing and freezing pre-treatments: Focus on polyphenols content and antioxidant activity. Food Chem. 2020, 308, 125570. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; González, R.L.; Ricci, A.; Fontecha, J.; Fernández-López, J.; Pérez-Alvarez, J.A.; Viuda-Martos, M. Chemical, fatty acid, polyphenolic profile, techno-functional and antioxidant properties of flours obtained from quinoa (Chenopodium quinoa Willd) seeds. Ind. Crop. Prod. 2018, 111, 38–46. [Google Scholar] [CrossRef]
- Kobus-Cisowska, J.; Szymanowska-Powałowska, D.; Szczepaniak, O.; Kmiecik, D.; Przeor, M.; Gramza-Michałowska, A.; Cielecka-Piontek, J.; Smuga-Kogut, M.; Szulc, P. Composition and In Vitro Effects of Cultivars of Humulus lupulus L. Hops on Cholinesterase Activity and Microbial Growth. Nutrients 2019, 11, 1377. [Google Scholar] [CrossRef] [Green Version]
- Carbone, K.; Macchioni, V.; Petrella, G.; Cicero, D.O. Exploring the potential of microwaves and ultrasounds in the green extraction of bioactive compounds from Humulus lupulus for the food and pharmaceutical industry. Ind. Crop. Prod. 2020, 156, 112888. [Google Scholar] [CrossRef]
- Perva-Uzunalić, A.; Škerget, M.; Knez, Ž.; Weinreich, B.; Otto, F.; Grüner, S. Extraction of active ingredients from green tea (Camellia sinensis): Extraction efficiency of major catechins and caffeine. Food Chem. 2006, 96, 597–605. [Google Scholar] [CrossRef]
- Nicoli, M.C.; Calligaris, S.; Manzocco, L. Effect of Enzymatic and Chemical Oxidation on the Antioxidant Capacity of Catechin Model Systems and Apple Derivatives. J. Agric. Food Chem. 2000, 48, 4576–4580. [Google Scholar] [CrossRef]
- Lee, J.; Scagel, C.F. Chicoric acid: Chemistry, distribution, and production. Front. Chem. 2013, 1, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.-Q.; Ye, X.-Q.; Fang, Z.-X.; Chen, J.-C.; Xu, G.-H.; Liu, D.-H. Phenolic Compounds and Antioxidant Activity of Extracts from Ultrasonic Treatment of Satsuma Mandarin (Citrus unshiu Marc.) Peels. J. Agric. Food Chem. 2008, 56, 5682–5690. [Google Scholar] [CrossRef]
- Jun, X. Caffeine extraction from green tea leaves assisted by high pressure processing. J. Food Eng. 2009, 94, 105–109. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, X.-H.; Tang, J.; Liu, K. Antioxidant Activities of Hops (Humulus Lupulus) and Their Products. J. Am. Soc. Brew. Chem. 2007, 65, 116–121. [Google Scholar] [CrossRef]
- Wu, C.-N.; Sun, L.-C.; Chu, Y.-L.; Yu, R.-C.; Hsieh, C.-W.; Hsu, H.-Y.; Hsu, F.-C.; Cheng, K.-C. Bioactive compounds with anti-oxidative and anti-inflammatory activities of hop extracts. Food Chem. 2020, 330, 127244. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Mafakheri, M.; Hamidoghli, Y.; Mafakhery, M.; Oghli, Y. Effect of different extraction solvents on phenolic compounds and antioxidant capacity of hop flowers (Humulus lupulus L.). Acta Hortic. 2019, 1, 1–6. [Google Scholar] [CrossRef]
- Assefa, A.D.; Keum, Y.-S.; Saini, R.K. A comprehensive study of polyphenols contents and antioxidant potential of 39 widely used spices and food condiments. J. Food Meas. Charact. 2018, 12, 1548–1555. [Google Scholar] [CrossRef]
- Hidalgo, G.-I.; Almajano, M.P. Red Fruits: Extraction of Antioxidants, Phenolic Content, and Radical Scavenging Determination: A Review. Antioxidants 2017, 6, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellegrini, N.; Serafini, M.; Salvatore, S.; Del Rio, D.; Bianchi, M.; Brighenti, F. Total antioxidant capacity of spices, dried fruits, nuts, pulses, cereals and sweets consumed in Italy assessed by three different in vitro assays. Mol. Nutr. Food Res. 2006, 50, 1030–1038. [Google Scholar] [CrossRef]
- Thoo, Y.Y.; Ho, S.K.; Liang, J.Y.; Ho, C.W.; Tan, C.P. Effects of binary solvent extraction system, extraction time and extraction temperature on phenolic antioxidants and antioxidant capacity from mengkudu (Morinda citrifolia). Food Chem. 2010, 120, 290–295. [Google Scholar] [CrossRef]
- Neri, L.; Faieta, M.; Di Mattia, C.; Sacchetti, G.; Mastrocola, D.; Pittia, P. Antioxidant Activity in Frozen Plant Foods: Effect of Cryoprotectants, Freezing Process and Frozen Storage. Foods 2020, 9, 1886. [Google Scholar] [CrossRef] [PubMed]
- Khanam, U.K.S.; Oba, S.; Yanase, E.; Murakami, Y. Phenolic acids, flavonoids and total antioxidant capacity of selected leafy vegetables. J. Funct. Foods 2012, 4, 979–987. [Google Scholar] [CrossRef]
- Arts, M.J.; Haenen, G.; Voss, H.-P.; Bast, A. Antioxidant capacity of reaction products limits the applicability of the Trolox Equivalent Antioxidant Capacity (TEAC) assay. Food Chem. Toxicol. 2004, 42, 45–49. [Google Scholar] [CrossRef]
- Mokrani, A.; Madani, K. Effect of solvent, time and temperature on the extraction of phenolic compounds and antioxidant capacity of peach (Prunus persica L.) fruit. Sep. Purif. Technol. 2016, 162, 68–76. [Google Scholar] [CrossRef]
- D’Alessandro, L.G.; Kriaa, K.; Nikov, I.; Dimitrov, K. Ultrasound assisted extraction of polyphenols from black chokeberry. Sep. Purif. Technol. 2012, 93, 42–47. [Google Scholar] [CrossRef]
- Okur, I.; Baltacıoğlu, C.; Ağçam, E.; Baltacıoğlu, H.; Alpas, H. Evaluation of the Effect of Different Extraction Techniques on Sour Cherry Pomace Phenolic Content and Antioxidant Activity and Determination of Phenolic Compounds by FTIR and HPLC. Waste Biomass Valorization 2019, 10, 3545–3555. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, S.; Chun, J.; Lee, H.; Lee, J. Influence of heat treatment on the antioxidant activities and polyphenolic compounds of Shiitake (Lentinus edodes) mushroom. Food Chem. 2006, 99, 381–387. [Google Scholar] [CrossRef]
- Toma, M.; Vinatoru, M.; Paniwnyk, L.; Mason, T.J. Investigation of the effects of ultrasound on vegetal tissues during solvent extraction. Ultrason. Sonochemistry 2001, 8, 137–142. [Google Scholar] [CrossRef]
- González-Centeno, M.; Comas-Serra, F.; Femenia, A.; Rosselló, C.; Simal, S. Effect of power ultrasound application on aqueous extraction of phenolic compounds and antioxidant capacity from grape pomace (Vitis vinifera L.): Experimental kinetics and modeling. Ultrason. Sonochemistry 2015, 22, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Herzi, N.; Bouajila, J.; Camy, S.; Romdhane, M.; Condoret, J.-S. Comparison of different methods for extraction from Tetraclinis articulata: Yield, chemical composition and antioxidant activity. Food Chem. 2013, 141, 3537–3545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, K.N.; Yang, B.; Shi, J.; Yu, C.; Zhao, M.; Xue, S.; Jiang, Y. Enhanced antioxidant and antityrosinase activities of longan fruit pericarp by ultra-high-pressure-assisted extraction. J. Pharm. Biomed. Anal. 2010, 51, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Shen, D.; Li, Y.; Zhang, R. Ultrahigh pressure extraction as a tool to improve the antioxidant activities of green tea extracts. Food Res. Int. 2011, 44, 2783–2787. [Google Scholar] [CrossRef]




| mg g−1 | |||||||||
| Extraction Method | Time (min) | GA | pOH-B | SyrA | EllA | ProCA | Cat | EGC | |
| CONV 25 °C | 15 | n.d. | n.d. | 0.320 ± 0.008 i | 0.262 ± 0.008 g | 0.433 ± 0.048 ef | 2.38 ± 0.07 de | 0.095 ± 0.006 ef | |
| 30 | n.d. | 0.452 ± 0.005 e | 0.374 ± 0.013 h | 0.290 ± 0.003 fg | 0.519 ± 0.009 bc | 2.19 ± 0.05 f | 0.087 ± 0.001 fgh | ||
| 60 | n.d. | 0.519 ± 0.005 d | 0.380 ± 0.001 gh | 0.485 ± 0.068 c | 0.441 ± 0.005 def | 1.22 ± 0.02 l | 0.086 ± 0.004 fgh | ||
| 120 | n.d. | 0.531 ± 0.002 d | 0.398 ± 0.010 fgh | 0.441 ± 0.014 d | 0.462 ± 0.001 cdef | 1.34 ± 0.02 il | 0.083 ± 0.004 fgh | ||
| CONV 60 °C | 15 | n.d. | n.d. | 0.416 ± 0.001 efgh | 0.315 ± 0.001 f | 0.555 ± 0.030 ab | 2.74 ± 0.09 a | 0.105 ± 0.009 de | |
| 30 | n.d. | 0.529 ± 0.042 d | 0.515 ± 0.042 bc | 0.361 ± 0.005 e | 0.418 ± 0.006 f | 2.50 ± 0.05 cd | 0.146 ± 0.003 a | ||
| 60 | n.d. | 0.614 ± 0.005 bc | 0.482 ± 0.006 cd | 0.628 ± 0.025 a | 0.525 ± 0.001 bc | 1.44 ± 0.05 hi | 0.124 ± 0.008 b | ||
| 120 | n.d. | 0.634 ± 0.002 ab | 0.544 ± 0.009 ab | 0.570 ± 0.023 b | 0.558 ± 0.001 ab | 1.61 ± 0.04 g | 0.147 ± 0.005 a | ||
| HPUS | 15 | 0.079 ± 0.001 d | 0.474 ± 0.001 e | 0.411 ± 0.026 efgh | 0.300 ± 0.013 fg | 0.506 ± 0.024 bcde | 2.68 ± 0.09 ab | 0.094 ± 0.002 ef | |
| 30 | 0.087 ± 0.001 bc | 0.466 ± 0.009 e | 0.419 ± 0.047 efgh | 0.292 ± 0.001 fg | 0.566 ± 0.050 ab | 2.72 ± 0.13 ab | 0.117 ± 0.001 bc | ||
| 60 | 0.086 ± 0.001 c | 0.589 ± 0.032 c | 0.419 ± 0.014 efg | 0.490 ± 0.011 c | 0.580 ± 0.104 ab | 1.26 ± 0.03 l | 0.079 ± 0.002 h | ||
| 120 | n.d. | 0.589 ± 0.015 c | 0.481 ± 0.024 cd | 0.494 ± 0.025 c | 0.515 ± 0.020 bcd | 1.52 ± 0.07 gh | 0.108 ± 0.009 cd | ||
| US | 15 | 0.085 ± 0.003 c | 0.529 ± 0.010 d | 0.453 ± 0.021 de | 0.297 ± 0.001 fg | 0.548 ± 0.041 ab | 2.37 ± 0.00 e | 0.088 ± 0.002 fgh | |
| 30 | 0.099 ± 0.002 a | 0.591 ± 0.010 c | 0.575 ± 0.007 a | 0.379 ± 0.007 e | 0.616 ± 0.010 a | 2.60 ± 0.05 bc | 0.141 ± 0.005 a | ||
| 60 | 0.091 ± 0.003 b | 0.640 ± 0.007 ab | 0.439 ± 0.024 ef | 0.521 ± 0.001 c | 0.509 ± 0.012 bcd | 1.33 ± 0.06 il | 0.086 ± 0.003 fgh | ||
| 120 | n.d. | 0.654 ± 0.002 a | 0.515 ± 0.002 bc | 0.522 ± 0.001 c | 0.513 ± 0.049 bcd | 1.60 ± 0.00 g | 0.122 ± 0.001 b | ||
| HHP | 5 | n.d. | 0.472 ± 0.021 e | 0.401 ± 0.001 fgh | 0.295 ± 0.005 fg | 0.544 ± 0.005 ab | 2.34 ± 0.05 e | 0.091 ± 0.004 fg | |
| on | mg g−1 | ||||||||
| Extraction Method | Time (min) | ChlA | ChicA | CafA | FerA | pCuA | Rut | XAN | TOT |
| CONV 25 °C | 15 | 0.466 ± 0.001 m | n.d. | n.d. | n.d. | n.d. | 0.653 ± 0.024 i | 1.92 ± 0.09 d | 6.53 ± 0.26 ilm |
| 30 | 0.484 ± 0.004 lm | n.d. | 0.012 ± 0.002 de | 0.012 ± 0.005 ef | 0.029 ± 0.004 hi | 0.656 ± 0.029 i | 1.73 ± 0.16 ef | 6.83 ± 0.27 ghi | |
| 60 | 0.503 ± 0.006 il | n.d. | 0.008 ± 0.005 e | 0.022 ± 0.019 def | 0.014 ± 0.010 i | 0.612 ± 0.002 l | 1.88 ± 0.01 de | 6.17 ± 0.12 n | |
| 120 | 0.520 ± 0.001 hi | n.d. | 0.020 ± 0.001 cd | 0.015 ± 0.013 ef | 0.014 ± 0.007 i | 0.770 ± 0.010 g | 1.86 ± 0.08 de | 6.46 ± 0.01 lmn | |
| CONV 60 °C | 15 | 0.525 ± 0.013 hi | n.d. | 0.010 ± 0.004 de | 0.021 ± 0.006 def | 0.114 ± 0.001 de | 0.727 ± 0.005 h | 2.34 ± 0.10 bc | 7.87 ± 0.05 cd |
| 30 | 0.532 ± 0.007 ghi | n.d. | 0.026 ± 0.003 bc | 0.029 ± 0.010 cde | 0.159 ± 0.020 b | 0.681 ± 0.011 i | 2.67 ± 0.01 a | 8.57 ± 0.01 a | |
| 60 | 0.902 ± 0.003 a | n.d. | 0.034 ± 0.003 b | 0.054 ± 0.005 bc | 0.046 ± 0.001 h | 0.739 ± 0.005 gh | 2.19 ± 0.02 c | 7.77 ± 0.13 cd | |
| 120 | 0.580 ± 0.008 cd | n.d. | 0.055 ± 0.002 a | 0.097 ± 0.021 a | 0.104 ± 0.009 ef | 0.859 ± 0.009 ab | 2.49 ± 0.15 b | 8.25 ± 0.02 ab | |
| HPUS | 15 | 0.531 ± 0.023 hi | n.d. | 0.018 ± 0.004 cd | 0.009 ± 0.02 ef | 0.109 ± 0.009 de | 0.767 ± 0.032 fg | 1.66 ± 0.15 fg | 7.64 ± 0.04 de |
| 30 | 0.563 ± 0.024 defg | n.d. | 0.032 ± 0.001 b | 0.023 ± 0.001 def | 0.150 ± 0.005 b | 0.814 ± 0.020 cd | 1.41 ± 0.02 hi | 7.66 ± 0.29 d | |
| 60 | 0.547 ± 0.026 efgh | n.d. | 0.033 ± 0.003 b | 0.033 ± 0.023 def | 0.030 ± 0.007 hi | 0.785 ± 0.001 def | 1.44 ± 0.01 h | 6.36 ± 0.10 nm | |
| 120 | 0.789 ± 0.001 b | n.d. | 0.057 ± 0.008 a | 0.089 ± 0.019 a | 0.078 ± 0.019 g | 0.789 ± 0.005 def | 1.65 ± 0.04 fg | 7.15 ± 0.14 g | |
| US | 15 | 0.577 ± 0.024 cde | n.d. | n.d. | 0.017 ± 0.005 def | 0.142 ± 0.015 bc | 0.736 ± 0.001 h | 1.49 ± 0.02 gh | 7.33 ± 0.02 ef |
| 30 | 0.565 ± 0.026 cdef | 0.162 ± 0.002 a | 0.049 ± 0.012 a | 0.078 ± 0.002 ab | 0.199 ± 0.001 a | 0.783 ± 0.003 def | 1.23 ± 0.13 l | 8.08 ± 0.24 bc | |
| 60 | 0.567 ± 0.001 cdef | 0.011 ± 0.001 c | 0.033 ± 0.001 b | 0.042 ± 0.006 cd | 0.126 ± 0.006 cd | 0.831 ± 0.001 bc | 1.24 ± 0.00 il | 6.45 ± 0.11 mn | |
| 120 | 0.597 ± 0.007 c | 0.117 ± 0.005 b | 0.057 ± 0.005 a | 0.094 ± 0.005 a | 0.087 ± 0.001 fg | 0.877 ± 0.009 a | 1.23 ± 0.01 l | 6.98 ± 0.02 gh | |
| HHP | 5 | 0.538 ± 0.015 fgh | n.d. | 0.026 ± 0.007 bc | 0.014 ± 0.007 ef | 0.119 ± 0.012 de | 0.801 ± 0.021 cde | 1.09 ± 0.05 l | 6.73 ± 0.09 hil |
| Extraction Method | Temperature (°C) | Time (min) | TPC | sd | TFC | sd | TEAC | sd | IC50 | sd | Chl α | sd | Chl β | sd | TCC | sd |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg GAE g−1 dm) | (mg QE g−1 dm) | (µmol g−1 dm) | (mg mL−1) | (mg g−1dm) | ||||||||||||
| CONV | 25 | 15 | 35.8 gh | 0.11 | 18.4 def | 0.02 | 173 il | 4 | 0.070 b | 0.004 | 0.369 l | 0.017 | 0.307 f | 0.067 | 1.14 h | 0.01 |
| 30 | 34.8 gh | 0.11 | 19.3 d | 0.03 | 197 gh | 6 | 0.056 c | 0.001 | 0.491 hi | 0.005 | 0.448 ef | 0.042 | 1.26 gh | 0.04 | ||
| 60 | 34.2 h | 1.56 | 18.9 de | 0.02 | 232 cd | 2 | 0.056 c | 0.001 | 0.468 il | 0.012 | 0.427 ef | 0.024 | 1.25 gh | 0.01 | ||
| 120 | 35.4 gh | 0.12 | 21.1 c | 0.13 | 230 cde | 2 | 0.057 c | 0.002 | 0.609 fg | 0.002 | 0.486 ef | 0.030 | 1.32 g | 0.02 | ||
| CONV | 60 | 15 | 41.2 f | 1.56 | 28.2 a | 0.02 | 224 def | 0 | 0.050 cde | 0.001 | 0.981 c | 0.051 | 1.043 c | 0.084 | 1.84 cd | 0.04 |
| 30 | 41.7 f | 0.42 | 27.1 a | 0.15 | 182 hi | 1 | 0.049 de | 0.004 | 1.34 ab | 0.01 | 1.29 b | 0.04 | 1.95 bc | 0.02 | ||
| 60 | 44.3 cd | 0.63 | 27.6 a | 0.04 | 161 l | 3 | 0.053 cd | 0.001 | 0.758 de | 0.038 | 0.887 c | 0.092 | 1.82 d | 0.21 | ||
| 120 | 45.3 bc | 0.09 | 23.0 b | 0.16 | 245 bc | 1 | 0.044 ef | 0.001 | 1.17 ab | 0.05 | 1.46 ab | 0.05 | 2.06 ab | 0.01 | ||
| HPUS | 25 | 15 | 36.6 g | 1.72 | 19.1 d | 1.51 | 187 hi | 3 | 0.035 g | 0.004 | 0.590 gh | 0.074 | 0.589 e | 0.009 | 1.52 f | 0.12 |
| 30 | 35.8 gh | 1.96 | 17.2 fgh | 0.17 | 221 def | 14 | 0.039 fg | 0.004 | 0.713 ef | 0.084 | 0.835 cd | 0.078 | 1.59 ef | 0.02 | ||
| 60 | 41.2 f | 0.20 | 18.2 def | 0.21 | 239 c | 16 | 0.037 g | 0.004 | 0.852 d | 0.013 | 1.03 c | 0.01 | 1.74 de | 0.01 | ||
| 120 | 42.1 ef | 1.46 | 19.0 d | 1.30 | 205 g | 13 | 0.032 g | 0.001 | 0.853 d | 0.062 | 0.926 c | 0.014 | 1.76 d | 0.02 | ||
| US | 25 | 15 | 43.4 de | 1.37 | 16.0 i | 0.41 | 214 efg | 6 | 0.054 cd | 0.001 | 0.848 d | 0.052 | 1.43 ab | 0.05 | 1.73 de | 0.04 |
| 30 | 45.0 cd | 0.60 | 16.2 hi | 0.28 | 271 a | 3 | 0.052 cd | 0.002 | 1.19 a | 0.04 | 1.61 a | 0.04 | 2.20 a | 0.07 | ||
| 60 | 46.9 b | 1.80 | 17.7 efg | 0.55 | 258 ab | 8 | 0.039 fg | 0.002 | 0.998 c | 0.011 | 1.54 ab | 0.01 | 2.01 bc | 0.12 | ||
| 120 | 48.5 a | 2.94 | 16.9 ghi | 0.26 | 259 ab | 11 | 0.049 de | 0.011 | 1.08 bc | 0.14 | 1.51 ab | 0.12 | 2.06 ab | 0.07 | ||
| HHP | 25 | 5 | 36.8 g | 0.15 | 13.1 l | 1.10 | 209 fg | 1 | 0.081 a | 0.004 | 0.384 il | 0.038 | 0.604 de | 0.056 | 1.30 g | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santarelli, V.; Neri, L.; Carbone, K.; Macchioni, V.; Pittia, P. Use of Conventional and Innovative Technologies for the Production of Food Grade Hop Extracts: Focus on Bioactive Compounds and Antioxidant Activity. Plants 2022, 11, 41. https://doi.org/10.3390/plants11010041
Santarelli V, Neri L, Carbone K, Macchioni V, Pittia P. Use of Conventional and Innovative Technologies for the Production of Food Grade Hop Extracts: Focus on Bioactive Compounds and Antioxidant Activity. Plants. 2022; 11(1):41. https://doi.org/10.3390/plants11010041
Chicago/Turabian StyleSantarelli, Veronica, Lilia Neri, Katya Carbone, Valentina Macchioni, and Paola Pittia. 2022. "Use of Conventional and Innovative Technologies for the Production of Food Grade Hop Extracts: Focus on Bioactive Compounds and Antioxidant Activity" Plants 11, no. 1: 41. https://doi.org/10.3390/plants11010041
APA StyleSantarelli, V., Neri, L., Carbone, K., Macchioni, V., & Pittia, P. (2022). Use of Conventional and Innovative Technologies for the Production of Food Grade Hop Extracts: Focus on Bioactive Compounds and Antioxidant Activity. Plants, 11(1), 41. https://doi.org/10.3390/plants11010041