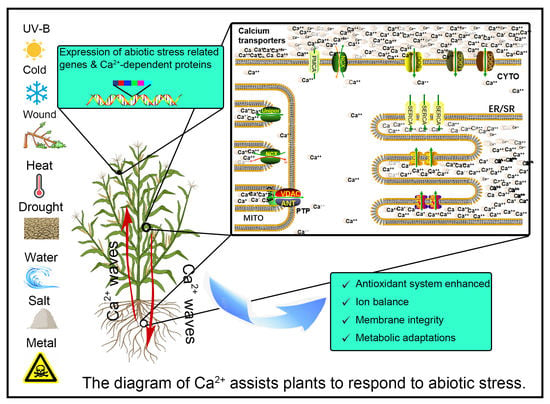
Crosstalk between Ca2+ and Other Regulators Assists Plants in Responding to Abiotic Stress
Abstract
:1. Introduction
2. Molecular Mechanisms of Crosstalk between Ca2+ and Other Regulators in Response to Abiotic Stresses in Plants
2.1. Drought Stress
2.2. Salt Stress
2.3. Extreme Temperature Stress
2.3.1. Low-Temperature Stress
2.3.2. High-Temperature Stress
2.4. Heavy-Metal Stress
2.5. Wound Stress
2.6. Waterlogging Stress
2.7. UV-B Radiation Stress
3. Calcium Ion Downstream Signaling Response
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| [Ca2+]cyt | Cytosolic Ca2+ concentration |
| ABA | Abscisic acid |
| ABPs | Actin-binding proteins |
| ACAs | Ca2+-ATPases |
| ACS | 1-aminocyclopropane-1-carboxylic acid synthases |
| ADH | Alcohol dehydrogenases |
| APX | Ascorbate peroxidase |
| AtBTs | Arabidopsis thaliana BTB and TAZ domain proteins |
| AtTPC1 | Arabidopsis thaliana Two pore channel 1 |
| BIK1 | Botrytis-induced kinase 1 |
| CA | Citric acid |
| cADPR | Cyclic ADP-ribose |
| CaMs | Calmodulins |
| CAX | Ca2+ ex-changers |
| CBLs | Calcineurin-B like proteins |
| CDPKs | Ca2+-dependent protein kinases |
| cGMP | Cyclic guanosine 3′,5′-monophosphate |
| CIPKs | CBL-interacting protein kinases |
| CMLs | Calmodulin-like-proteins |
| CNGCs | Cyclic nucleotide-gated channels |
| CPK | Calcium-dependent protein kinase |
| ECAs | ER-type Ca2+ -ATPases |
| EGTA | Ethylene glycol diethyl ether diamine tetraacetic acid |
| ET | Evapotranspiration |
| GA | Gibberellin |
| GABA | Gamma-aminobutyric acid |
| glR3.3/3.6 | Glutamate receptor-like 3.3/3.6 |
| GLRs | Glutamate receptor-like channels |
| GTL | GT-2like 1 |
| H2O2 | Hydrogen peroxide |
| H2S | Hydrogen sulfide |
| HACCs | Hyperpolarization-activated calcium channels |
| HMA1 | P1-ATPases |
| HsfA1 | Heat stress transcription factor A1 |
| HSFs | Heat shock transcription factors |
| HSPs | Heat stress transcription factors |
| IP3 | Inositol 1,4,5-trisphosphate |
| JA | Jasmonic acid |
| JAV1 | Jasmonate-associated VQ domain protein 1 |
| JAZ8 | JASMONATE ZIM domain protein 8 |
| JJW | JAV1-JAZ8-WRKY51 |
| KORC | K+ permeable outwardly rectifying conductance |
| LOX2 | Lipoxygenase 2 |
| MAPKs | Mitogen-activated protein kinases |
| MCAs | Mid1-complementing activity channels |
| MCUC | Mitochondrial calcium uniporter complex |
| MeJA | Methyl jasmonate |
| MPK | Mitogen-activated protein kinase |
| MscS | Mechanosensitive channels of small |
| MSLs | Conductance-like channels |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NO | Nitric oxide |
| NSCCs | Nonselective cation channels |
| O2− | Superoxide anion radicals |
| OH | Hydroxyl radical |
| OSCAs | Hyperosmolality-induced Ca2+ increase channels |
| PCA1 | Ca2+-ATPase |
| PLC | Phopholipase C |
| PLD | Phopholipase D |
| POD | Peroxidase |
| ROS | Reactive oxygen species |
| SA | Salicylic acid |
| SOD | Superoxide dismutase |
| SOS | Salt overly sensitive |
| TPC | Total phenolic contents |
| UV-B | Ultraviolet-B radiation stress |
| VP | Variation potential |
References
- Poovaiah, B.W.; Reddy, A.S.N.; Leopold, A.C. Calcium messenger system in plants. CRC Crit. Rev. Plant Sci. 1987, 6, 47–103. [Google Scholar] [CrossRef] [PubMed]
- Aldon, D.; Mbengue, M.; Mazars, C.; Galaud, J.-P. Calcium signalling in plant biotic interactions. Int. J. Mol. Sci. 2018, 19, 665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.L.; Zhang, G.Z. A calcium sensor calcineurin B-like 9 negatively regulates cold tolerance via calcium signaling in Arabidopsis thaliana. Plant Signal. Behav. 2019, 14, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Pan, Z.; Zhang, Y.; Qu, X.L.; Zhang, Y.G.; Yang, Y.Q.; Jiang, X.N.; Huang, S.J.; Yuan, M.; Schumaker, K.S.; et al. The actin-related protein2/3 complex regulates mitochondrial-associated calcium signaling during salt stress in Arabidopsis. Plant Cell 2013, 25, 4544–4559. [Google Scholar] [CrossRef] [Green Version]
- Jing, X.; Cai, C.J.; Fan, S.H.; Wang, L.J.; Zeng, X.L. Spatial and temporal calcium signaling and Its physiological effects in Moso Bamboo under drought stress. Forests 2019, 10, 224. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, Z.; Iqbal, M.S.; Singh, S.P.; Buaboocha, T. Ca2+/Calmodulin complex triggers CAMTA transcriptional machinery under stress in plants: Signaling cascade and molecular regulation. Front. Plant Sci. 2020, 11, 16. [Google Scholar] [CrossRef]
- Liang, C.J.; Zhang, Y.Q.; Ren, X.Q. Calcium regulates antioxidative isozyme activity for enhancing rice adaption to acid rain stress. Plant Sci. 2021, 306, 10. [Google Scholar] [CrossRef]
- Demidchik, V.; Shabala, S.; Isayenkov, S.; Cuin, T.A.; Pottosin, I. Calcium transport across plant membranes: Mechanisms and functions. New Phytol. 2018, 220, 49–69. [Google Scholar] [CrossRef] [Green Version]
- Zeng, H.; Zhao, B.; Wu, H.; Zhu, Y.; Chen, H. Comprehensive in silico characterization and expression profiling of nine gene families associated with calcium transport in soybean. Agronomy 2020, 10, 1539. [Google Scholar] [CrossRef]
- Ranty, B.; Aldon, D.; Cotelle, V.; Galaud, J.P.; Thuleau, P.; Mazars, C. Calcium sensors as key hubs in plant responses to biotic and abiotic stresses. Front. Plant Sci. 2016, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Michal Johnson, J.; Reichelt, M.; Vadassery, J.; Gershenzon, J.; Oelmüller, R. An Arabidopsis mutant impaired in intracellular calcium elevation is sensitive to biotic and abiotic stress. BMC Plant Biol. 2014, 14, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthus, E.; Wilkins, K.A.; Swarbreck, S.M.; Doddrell, N.H.; Doccula, F.G.; Costa, A.; Davies, J.M. Phosphate starvation alters abiotic-stress-induced cytosolic free calcium increases in roots. Plant Physiol. 2019, 179, 1754–1767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barkla, B.J.; Hirschi, K.D.; Pittman, J.K. Exchangers man the pumps. Plant Signal. Behav. 2008, 3, 354–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bose, J.; Pottosin, I.I.; Shabala, S.S.; Palmgren, M.G.; Shabala, S. Calcium efflux systems in stress signaling and adaptation in plants. Front. Plant Sci. 2011, 2, 17. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.P.; Guo, Y.; Sun, Y.; Sun, D.Y.; Wang, X.J. Influx of extracellular Ca2+ involved in jasmonic-acid-induced elevation of Ca2+ (cyt) and JR1 expression in Arabidopsis thaliana. J. Plant Res. 2006, 119, 343–350. [Google Scholar] [CrossRef]
- Bouche, N.; Yellin, A.; Snedden, W.A.; Fromm, H. Plant-specific calmodulin-binding proteins. Annu. Rev. Plant Biol. 2005, 56, 435–466. [Google Scholar] [CrossRef]
- Ma, X.; Li, Q.H.; Yu, Y.N.; Qiao, Y.M.; Haq, S.U.; Gong, Z.H. The CBL-CIPK pathway in plant response to stress signals. Int. J. Mol. Sci. 2020, 21, 5668. [Google Scholar] [CrossRef]
- Demidchik, V.; Shabala, S. Mechanisms of cytosolic calcium elevation in plants: The role of ion channels, calcium extrusion systems and NADPH oxidase-mediated ‘ROS-Ca2+ Hub’. Funct. Plant Biol. 2018, 45, 9–27. [Google Scholar] [CrossRef]
- Yang, Y.L.; Xu, S.J.; An, L.Z.; Chen, N.L. NADPH oxidase-dependent hydrogen peroxide production, induced by salinity stress, may be involved in the regulation of total calcium in roots of wheat. J. Plant Physiol. 2007, 164, 1429–1435. [Google Scholar] [CrossRef]
- Edel, K.H.; Marchadier, E.; Brownlee, C.; Kudla, J.; Hetherington, A.M. The evolution of calcium-based signalling in plants. Curr. Biol. 2017, 27, R667–R679. [Google Scholar] [CrossRef]
- Liu, J.; Niu, Y.; Zhang, J.; Zhou, Y.; Ma, Z.; Huang, X. Ca2+ channels and Ca2+ signals involved in abiotic stress responses in plant cells: Recent advances. Plant Cell Tissue Organ Cult. 2018, 132, 413–424. [Google Scholar] [CrossRef]
- Wen, F.; Ye, F.; Xiao, Z.L.; Liao, L.; Li, T.J.; Jia, M.L.; Liu, X.S.; Wu, X.Z. Genome-wide survey and expression analysis of calcium-dependent protein kinase (CDPK) in grass Brachypodium distachyon. BMC Genom. 2020, 21, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abid, M.; Ali, S.; Qi, L.K.; Zahoor, R.; Tian, Z.W.; Jiang, D.; Snider, J.L.; Dai, T.B. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Sci. Rep. 2018, 8, 15. [Google Scholar] [CrossRef]
- de Carvalho, M.H.C. Drought stress and reactive oxygen species production, scavenging and signaling. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuromori, T.; Fujita, M.; Takahashi, F.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Inter-tissue and inter-organ signaling in drought stress response and phenotyping of drought tolerance. Plant J. 2022, 109, 342–358. [Google Scholar] [CrossRef]
- Feng, X.; Liu, W.X.; Zeng, F.R.; Chen, Z.H.; Zhang, G.P.; Wu, F.B. K+ Uptake, H+-ATPase pumping activity and Ca2+ efflux mechanism are involved in drought tolerance of barley. Environ. Exp. Bot. 2016, 129, 57–66. [Google Scholar] [CrossRef]
- Wu, S.W.; Sun, X.C.; Tan, Q.L.; Hu, C.X. Molybdenum improves water uptake via extensive root morphology, aquaporin expressions and increased ionic concentrations in wheat under drought stress. Environ. Exp. Bot. 2019, 157, 241–249. [Google Scholar] [CrossRef]
- Cousson, A. Involvement of phospholipase C-independent calcium-mediated abscisic acid signalling during Arabidopsis response to drought. Biol. Plant. 2009, 53, 53–62. [Google Scholar] [CrossRef]
- Jiao, C.F.; Yang, R.Q.; Gu, Z.X. Cyclic ADP-ribose and IP3 mediate abscisic acid-induced isoflavone accumulation in soybean sprouts. Biochem. Biophys. Res. Commun. 2016, 479, 530–536. [Google Scholar] [CrossRef]
- Zou, J.-J.; Li, X.-D.; Ratnasekera, D.; Wang, C.; Liu, W.-X.; Song, L.-F.; Zhang, W.-Z.; Wu, W.-H. Arabidopsis CALCIUM-DEPENDENT PROTEIN KINASE8 and CATALASE3 function in abscisic acid-mediated signaling and H2O2 homeostasis in stomatal guard cells under drought stress. Plant Cell 2015, 27, 1445–1460. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.R.; Li, Y.P.; Miao, W.W.; Piao, T.T.; Hao, Y.; Hao, F.S. NADK2 positively modulates abscisic acid-induced stomatal closure by affecting accumulation of H2O2, Cat(2+) and nitric oxide in Arabidopsis guard cells. Plant Sci. 2017, 262, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Deng, Y.; Liu, Z.; Liao, W. Hydrogen sulfide in plants: Crosstalk with other signal molecules in response to abiotic stresses. Int. J. Mol. Sci. 2021, 22, 12068. [Google Scholar] [CrossRef] [PubMed]
- Dubovskaya, L.V.; Bakakina, Y.S.; Kolesneva, E.V.; Sodel, D.L.; McAinsh, M.R.; Hetherington, A.M.; Volotovski, I.D. cGMP-dependent ABA-induced stomatal closure in the ABA-insensitive Arabidopsis mutantabi1-1. New Phytol. 2011, 191, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xiang, Y.; Yan, J.W.; Di, P.C.; Li, J.; Sun, X.J.; Han, G.Q.; Ni, L.; Jiang, M.Y.; Yuan, J.H.; et al. BRASSINOSTEROID-SIGNALING KINASE 1 phosphorylating CALCIUM/CALMODULIN-DEPENDENT PROTEIN KINASE functions in drought tolerance in maize. New Phytol. 2021, 231, 695–712. [Google Scholar] [CrossRef]
- Huda, K.M.K.; Banu, M.S.A.; Yadav, S.; Sahoo, R.K.; Tuteja, R.; Tuteja, N. Salinity and drought tolerant OsACA6 enhances cold tolerance in transgenic tobacco by interacting with stress-inducible proteins. Plant Physiol. Biochem. 2014, 82, 229–238. [Google Scholar] [CrossRef]
- Mohanta, T.K.; Bashir, T.; Hashem, A.; Abd_Allah, E.F.; Khan, A.L.; Al-Harrasi, A.S. Early events in plant abiotic stress signaling: Interplay between calcium, reactive oxygen species and phytohormones. J. Plant Growth Regul. 2018, 37, 1033–1049. [Google Scholar] [CrossRef]
- Sanyal, S.K.; Kanwar, P.; Yadav, A.K.; Sharma, C.; Kumar, A.; Pandey, G.K. Arabidopsis CBL interacting protein kinase 3 interacts with ABR1, an APETALA2 domain transcription factor, to regulate ABA responses. Plant Sci. 2017, 254, 48–59. [Google Scholar] [CrossRef]
- Cuellar, T.; Pascaud, F.O.; Verdeil, J.L.; Torregrosa, L.; Adam-Blondon, A.F.; Thibaud, J.-B.; Sentenac, H.; Gaillard, I. A grapevine shaker inward K+ channel activated by the calcineurin B-like calcium sensor 1-protein kinase CIPK23 network is expressed in grape berries under drought stress conditions. Plant J. 2010, 61, 58–69. [Google Scholar] [CrossRef]
- Cheng, P.L.; Gao, J.J.; Feng, Y.T.; Zhang, Z.X.; Liu, Y.N.; Fang, W.M.; Chen, S.M.; Chen, F.D.; Jiang, J.F. The chrysanthemum leaf and root transcript profiling in response to salinity stress. Gene 2018, 674, 161–169. [Google Scholar] [CrossRef]
- Song, W.Y.; Zhang, Z.B.; Shao, H.B.; Guo, X.L.; Cao, H.X.; Zhao, H.B.; Fu, Z.Y.; Hu, X.J. Relationship between calcium decoding elements and plant abiotic-stress resistance. Int. J. Biol. Sci. 2008, 4, 116–125. [Google Scholar] [CrossRef]
- Weng, H.; Yoo, C.Y.; Gosney, M.J.; Hasegawa, P.M.; Mickelbart, M.V. Poplar GTL1 is a Ca2+/calmodulin-binding transcription factor that functions in plant water use efficiency and drought tolerance. PLoS ONE 2012, 7, e32925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durand, M.; Cohen, D.; Aubry, N.; Buré, C.; Tomášková, I.; Hummel, I.; Brendel, O.; Le Thiec, D. Element content and expression of genes of interest in guard cells are connected to spatiotemporal variations in stomatal conductance. Plant Cell Environ. 2020, 43, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Marin-Guirao, L.; Sandoval-Gil, J.M.; Garcia-Munoz, R.; Ruiz, J.M. The stenohaline seagrass posidonia oceanica can persist in natural environments under fluctuating hypersaline conditions. Estuaries Coasts 2017, 40, 1688–1704. [Google Scholar] [CrossRef]
- Hryvusevich, P.; Navaselsky, I.; Talkachova, Y.; Straltsova, D.; Keisham, M.; Viatoshkin, A.; Samokhina, V.; Smolich, I.; Sokolik, A.; Huang, X.; et al. Sodium influx and potassium efflux currents in sunflower root cells under high salinity. Front. Plant Sci. 2021, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Wegner, L.H.; De Boer, A.H. Properties of two outward-rectifying channels in root xylem parenchyma cells suggest a role in K+ homeostasis and long-distance signaling. Plant Physiol. 1997, 115, 1707–1719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahajan, S.; Pandey, G.K.; Tuteja, N. Calcium- and salt-stress signaling in plants: Shedding light on SOS pathway. Arch. Biochem. Biophys. 2008, 471, 146–158. [Google Scholar] [CrossRef]
- Ma, L.; Ye, J.; Yang, Y.; Lin, H.; Yue, L.; Luo, J.; Long, Y.; Fu, H.; Liu, X.; Zhang, Y.; et al. The SOS2-SCaBP8 complex generates and fine-tunes an AtANN4-dependent calcium signature under salt stress. Dev. Cell 2019, 48, 697–709. [Google Scholar] [CrossRef] [Green Version]
- Ma, D.-M.; Xu, W.-R.; Li, H.-W.; Jin, F.-X.; Guo, L.-N.; Wang, J.; Dai, H.-J.; Xu, X. Co-expression of the Arabidopsis SOS genes enhances salt tolerance in transgenic tall fescue (Festuca arundinacea Schreb.). Protoplasma 2014, 251, 219–231. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhang, Y.; Deng, C.; Deng, S.; Li, N.; Zhao, C.; Zhao, R.; Liang, S.; Chen, S. The Arabidopsis Ca2+-dependent protein kinase CPK12 is involved in plant response to salt stress. Int. J. Mol. Sci. 2018, 19, 4062. [Google Scholar] [CrossRef] [Green Version]
- Kurusu, T.; Sakurai, Y.; Miyao, A.; Hirochika, H.; Kuchitsu, K. Identification of a putative voltage-gated Ca2+-permeable channel (OSTPC1) involved in Ca2+ influx and regulation of growth and development in rice. Plant Cell Physiol. 2004, 45, 693–702. [Google Scholar] [CrossRef] [Green Version]
- Cousson, A.; Vavasseur, A. Two potential Ca2+-dependent transduction pathways in stomatal closing in response to abscisic acid. Plant Physiol. Biochem. 1998, 36, 257–262. [Google Scholar] [CrossRef]
- Qudeimat, E.; Frank, W. Ca2+ signatures The role of Ca2+-ATPases. Plant Signal. Behav. 2009, 4, 350–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Z.H.; Zhou, X.P.; Tao, M.; Yuan, F.; Liu, L.L.; Wu, F.H.; Wu, X.M.; Xiang, Y.; Niu, Y.; Liu, F.; et al. Plant cell-surface GIPC sphingolipids sense salt to trigger Ca2+ influx. Nature 2019, 572, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Dai, X.Y.; Xu, Y.Y.; Luo, W.; Zheng, X.M.; Zeng, D.L.; Pan, Y.J.; Lin, X.L.; Liu, H.H.; Zhang, D.J.; et al. COLD1 confers chilling tolerance in rice. Cell 2015, 160, 1209–1221. [Google Scholar] [CrossRef] [Green Version]
- Yang, N.; Peng, C.L.; Cheng, D.; Huang, Q.; Xu, G.H.; Gao, F.; Chen, L.B. The over-expression of calmodulin from Antarctic notothenioid fish increases cold tolerance in tobacco. Gene 2013, 521, 32–37. [Google Scholar] [CrossRef]
- Luo, Q.; Wei, Q.; Wang, R.; Zhang, Y.; Zhang, F.; He, Y.; Yang, G.; He, G. Ectopic expression of BdCIPK31 confers enhanced low-temperature tolerance in transgenic tobacco plants. Acta Biochim. Biophys. Sin. 2018, 50, 199–208. [Google Scholar] [CrossRef] [Green Version]
- Wood, N.T.; Allan, A.C.; Haley, A.; Viry-Moussaïd, M.; Trewavas, A.J. The characterization of differential calcium signalling in tobacco guard cells. Plant J. 2000, 24, 335–344. [Google Scholar] [CrossRef]
- Li, Z.G.; Gong, M.; Xie, H.; Yang, L.; Li, J. Hydrogen sulfide donor sodium hydrosulfide-induced heat tolerance in tobacco (Nicotiana tabacum L.) suspension cultured cells and involvement of Ca2+ and calmodulin. Plant Sci. 2012, 185, 185–189. [Google Scholar] [CrossRef]
- Li, W.; Sun, Z.-H.; Zhang, W.-C.; Ma, X.-T.; Liu, D.-H. Role of Ca2+ and calmodulin on freezing tolerance of citrus protoplasts. Acta Biochim. Biophys. Sin. 1997, 23, 262–266. [Google Scholar]
- Cheong, Y.H.; Kim, K.-N.; Pandey, G.K.; Gupta, R.; Grant, J.J.; Luan, S. CBL1, a calcium sensor that differentially regulates salt, drought, and cold responses in Arabidopsis. Plant Cell 2003, 15, 1833–1845. [Google Scholar] [CrossRef] [Green Version]
- Cheong, Y.H.; Pandey, G.K.; Grant, J.J.; Batistic, O.; Li, L.; Kim, B.G.; Lee, S.C.; Kudla, J.; Luan, S. Two calcineurin B-like calcium sensors, interacting with protein kinase CIPK23, regulate leaf transpiration and root potassium uptake in Arabidopsis. Plant J. 2007, 52, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Q.; Chen, S.; Jua, Z.; Yao, Y. Joint transcriptomic and metabolomic analysis reveals the mechanism of low-temperature tolerance in Hosta ventricosa. PLoS ONE 2021, 16, e0259455. [Google Scholar] [CrossRef] [PubMed]
- Wan, B.L.; Lin, Y.J.; Mou, T.M. Expression of rice Ca2+-dependent protein kinases (CDPKs) genes under different environmental stresses. FEBS Lett. 2007, 581, 1179–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, G.; Shen, S.; Yang, S.; Komatsu, S. OsCDPK13, a calcium-dependent protein kinase gene from rice, is induced in response to cold and gibberellin. Plant Physiol. Biochem. 2003, 41, 369–374. [Google Scholar] [CrossRef]
- Abo-El-Saad, M.; Wu, R. A rice membrane calcium-dependent protein kinase is induced by gibberellin. Plant Physiol. 1995, 108, 787–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, D.; Brosnan, J.M.; Muir, S.R.; Allen, G.; Crofts, A.; Johannes, E. Ion channels and calcium signalling in plants: Multiple pathways and cross-talk. Biochem. Soc. Symp. 1994, 60, 183–197. [Google Scholar]
- Islam, M.M.; Munemasa, S.; Hossain, M.A.; Nakamura, Y.; Mori, I.C.; Murata, Y. Roles of AtTPC1, vacuolar two pore channel 1, in Arabidopsis stomatal closure. Plant Cell Physiol. 2010, 51, 302–311. [Google Scholar] [CrossRef]
- Agurla, S.; Gahir, S.; Munemasa, S.; Murata, Y.; Raghavendra, A.S. Mechanism of stomatal closure in plants exposed to drought and cold stress. In Survival Strategies in Extreme Cold and Desiccation: Adaptation Mechanisms and Their Applications; IwayaInoue, M., Sakurai, M., Uemura, M., Eds.; Springer Singapore Pte Ltd.: Singapore, 2018; Volume 1081, pp. 215–232. [Google Scholar]
- Knight, J.R.P.; Bastide, A.; Roobol, A.; Roobol, J.; Jackson, T.J.; Utami, W.; Barrett, D.A.; Smales, C.M.; Willis, A.E. Eukaryotic elongation factor 2 kinase regulates the cold stress response by slowing translation elongation. Biochem. J. 2015, 465, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, N.; Katano, K. Coordination between ROS regulatory systems and other pathways under heat stress and pathogen attack. Front. Plant Sci. 2018, 9, 8. [Google Scholar] [CrossRef]
- Li, T.-L.; Li, M.; Sun, Z.-P. Regulation effect of calcium and salicylic acid on defense enzyme activities in tomato leaves under sub-high temperature stress. Yingyong Shengtai Xuebao 2009, 20, 586–590. [Google Scholar]
- Hu, Z.J.; Li, J.X.; Ding, S.T.; Cheng, F.; Li, X.; Jiang, Y.P.; Yu, J.Q.; Foyer, C.H.; Shi, K. The protein kinase CPK28 phosphorylates ascorbate peroxidase and enhances thermotolerance in tomato. Plant Physiol. 2021, 186, 1302–1317. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.J.; Ding, Y.R.; Jin, J.Y.; Song, A.P.; Chen, S.M.; Chen, F.D.; Fang, W.M.; Jiang, J.F. Physiological and transcripts analyses reveal the mechanism by which melatonin alleviates heat stress in chrysanthemum seedlings. Front. Plant Sci. 2021, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.O.; Xv, K.P.; Meng, Q.W.; Li, G.; Yang, X.H. Potato plants ectopically expressing Arabidopsis thaliana CBF3 exhibit enhanced tolerance to high-temperature stress. Plant Cell Environ. 2015, 38, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.Y.; Wang, W.L.; Xu, K.; Xu, Y.; Ji, D.H.; Chen, C.S.; Xie, C.T. Ca2+ influences heat shock signal transduction in Pyropia haitanensis. Aquaculture 2020, 516, 8. [Google Scholar] [CrossRef]
- Virdi, A.S.; Singh, S.; Singh, P. Abiotic stress responses in plants: Roles of calmodulin-regulated proteins. Front. Plant Sci. 2015, 6, 19. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, A.; Singh, P.; Khadim, S.R.; Singh, A.K.; Singh, U.; Singh, P.; Asthana, R.K. Role of Ca2+ as protectant under heat stress by regulation of photosynthesis and membrane saturation in Anabaena PCC 7120. Protoplasma 2019, 256, 681–691. [Google Scholar] [CrossRef]
- Tripp, J.; Mishra, S.K.; Scharf, K.D. Functional dissection of the cytosolic chaperone network in tomato mesophyll protoplasts. Plant Cell Environ. 2009, 32, 123–133. [Google Scholar] [CrossRef]
- Barbero, F.; Guglielmotto, M.; Islam, M.; Maffei, M.E. Extracellular fragmented self-DNA is involved in plant responses to biotic stress. Front. Plant Sci. 2021, 12, 17. [Google Scholar] [CrossRef]
- Doubnerova, V.; Ryslava, H. Roles of HSP70 in Plant Abiotic Stress; CRC Press-Taylor & Francis Group: Boca Raton, FL, USA, 2014; pp. 44–66. [Google Scholar]
- Peng, X.; Zhang, X.N.; Li, B.; Zhao, L.Q. Cyclic nucleotide-gated ion channel 6 mediates thermotolerance in Arabidopsis seedlings by regulating nitric oxide production via cytosolic calcium ions. BMC Plant Biol. 2019, 19, 368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.T.; Zhang, L.L.; Geng, B.; Feng, J.R.; Zhu, S.H. Interactive effects of abscisic acid and nitric oxide on chilling resistance and active oxygen metabolism in peach fruit during cold storage. J. Sci. Food Agric. 2019, 99, 3367–3380. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Du, H.W.; Wang, Y.K.; Wang, H.L.; Yang, S.Y.; Li, C.H.; Chen, N.; Yang, H.; Zhang, Y.H.; Zhu, Y.L.; et al. The calcium-dependent protein kinase ZmCDPK7 functions in heat-stress tolerance in maize. J. Integr. Plant Biol. 2021, 63, 510–527. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.B.L.; Di Benedette, J.P.T.; Morais, F.V.; Ovalle, R.; Nobrega, M.P. Evidence for the role of calcineurin in morphogenesis and calcium homeostasis during mycelium-to-yeast dimorphism of Paracoccidioides brasiliensis. Eukaryot. Cell 2008, 7, 1856–1864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moustaka, J.; Ouzounidou, G.; Baycu, G.; Moustakas, M. Aluminum resistance in wheat involves maintenance of leaf Ca2+ and Mg2+ content, decreased lipid peroxidation and Al accumulation, and low photosystem II excitation pressure. Biometals 2016, 29, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, R.K. The forms and distribution of aluminum adsorbed onto maize and soybean roots. J. Soils Sediments. 2015, 15, 491–502. [Google Scholar] [CrossRef]
- Lan, T.; You, J.F.; Kong, L.N.; Yu, M.; Liu, M.H.; Yang, Z.M. The interaction of salicylic acid and Ca2+ alleviates aluminum toxicity in soybean (Glycine max L.). Plant Physiol. Biochem. 2016, 98, 146–154. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Smyth, T.J.; Hesterberg, D.; Israel, D.W. Soybean root growth in relation to ionic composition in magnesium-amended acid subsoils: Implications on root citrate ameliorating aluminum constraints. Soil Sci. Plant Nutr. 2007, 53, 753–763. [Google Scholar] [CrossRef]
- Cousson, A. Two calcium mobilizing pathways implicated within abscisic acid-induced stomatal closing in Arabidopsis thaliana. Biol. Plant 2007, 51, 285–291. [Google Scholar] [CrossRef]
- Kinraide, T.B. Three mechanisms for the calcium alleviation of mineral toxicities. Plant Physiol. 1998, 118, 513–520. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.L.; Huang, H.J. ROS and CDPK-like kinase-mediated activation of MAP kinase in rice roots exposed to lead. Chemosphere 2008, 71, 1377–1385. [Google Scholar] [CrossRef]
- Maksymiec, W.; Baszynski, T. Are calcium ions and calcium channels involved in the mechanisms of Cu2+ toxicity in bean plants? The influence of leaf age. Photosynthetica 1999, 36, 267–278. [Google Scholar] [CrossRef]
- Fang, H.H.; Jing, T.; Liu, Z.Q.; Zhang, L.P.; Jin, Z.P.; Pei, Y.X. Hydrogen sulfide interacts with calcium signaling to enhance the chromium tolerance in Setaria italica. Cell Calcium 2014, 56, 472–481. [Google Scholar] [CrossRef]
- Tian, W.; Wang, C.; Gao, Q.F.; Li, L.G.; Luan, S. Calcium spikes, waves and oscillations in plant development and biotic interactions. Nat. Plants 2020, 6, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Pawelek, A.; Duszyn, M.; Swiezawska, B.; Szmidt-Jaworska, A.; Jaworski, K. Transcriptional response of a novel HpCDPK1 kinase gene from Hippeastrum x hybr. to wounding and fungal infection. J. Plant Physiol. 2017, 216, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Uemura, T.; Wang, J.Q.; Aratani, Y.; Gilroy, S.; Toyota, M. Wide-field, real-time imaging of local and systemic wound signals in Arabidopsis. J. Vis. Exp. 2021, 172, e62114. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, T.K.; Occhipinti, A.; Zebelo, S.A.; Foti, M.; Fliegmann, J.; Bossi, S.; Maffei, M.E.; Bertea, C.M. Ginkgo biloba responds to herbivory by activating early signaling and direct defenses. PLoS ONE 2012, 7, e32822. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Luoni, L.; Marrano, C.A.; Hashimoto, K.; Koster, P.; Giacometti, S.; De Michelis, M.I.; Kudla, J.; Bonza, M.C. Ca2+-dependent phosphoregulation of the plasma membrane Ca2+-ATPase ACA8 modulates stimulus-induced calcium signatures. J. Exp. Bot. 2017, 68, 3215–3230. [Google Scholar] [CrossRef] [Green Version]
- Farmer, E.E.; Gao, Y.Q.; Lenzoni, G.; Wolfender, J.L.; Wu, Q. Wound- and mechanostimulated electrical signals control hormone responses. New Phytol. 2020, 227, 1037–1050. [Google Scholar] [CrossRef]
- Moyen, C.; Hammond-Kosack, K.E.; Jones, J.; Knight, M.R.; Johannes, E. Systemin triggers an increase of cytoplasmic calcium in tomato mesophyll cells: Ca2+ mobilization from intra- and extracellular compartments. Plant Cell Environ. 1998, 21, 1101–1111. [Google Scholar] [CrossRef]
- Ludwig, A.A.; Saitoh, H.; Felix, G.; Freymark, G.; Miersch, O.; Wasternack, C.; Boller, T.; Jones, J.D.G.; Romeis, T. Ethylene-mediated cross-talk between calcium-dependent protein kinase and MAPK signaling controls stress responses in plants. Proc. Natl. Acad. Sci. USA 2005, 102, 10736–10741. [Google Scholar] [CrossRef] [Green Version]
- Fichman, Y.; Mittler, R. Integration of electric, calcium, reactive oxygen species and hydraulic signals during rapid systemic signaling in plants. Plant J. 2021, 107, 7–20. [Google Scholar] [CrossRef]
- Hander, T.; Fernandez-Fernandez, A.D.; Kumpf, R.P.; Willems, P.; Schatowitz, H.; Rombaut, D.; Staes, A.; Nolf, J.; Pottie, R.; Yao, P.F.; et al. Damage on plants activates Ca2+-dependent metacaspases for release of immunomodulatory peptides. Science 2019, 363, eaar7486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, S.G.; Yin, C.C.; He, P. Cleave and Unleash: Metacaspases Prepare Peps for Work. Trends Plant Sci. 2019, 24, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Matschi, S.; Hake, K.; Herde, M.; Hause, B.; Romeis, T. The calcium-dependent protein kinase CPK28 regulates development by inducing growth phase-specific, spatially restricted alterations in jasmonic acid levels independent of defense responses in Arabidopsis. Plant Cell 2015, 27, 591–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vodeneev, V.; Akinchits, E.; Sukhov, V. Variation potential in higher plants: Mechanisms of generation and propagation. Plant Signal. Behav. 2015, 10, 7. [Google Scholar] [CrossRef]
- Choi, W.G.; Hilleary, R.; Swanson, S.J.; Kim, S.H.; Gilroy, S. Rapid, long-distance electrical and calcium signaling in plants. In Annual Review of Plant Biology; Merchant, S.S., Ed.; Annual Reviews: Palo Alto, CA, USA, 2016; Volume 67, pp. 287–307. [Google Scholar]
- Takahashi, F.; Mizoguchi, T.; Yoshida, R.; Ichimura, K.; Shinozaki, K. Calmodulin-dependent activation of MAP kinase for ROS homeostasis in Arabidopsis. Mol. Cell 2011, 41, 649–660. [Google Scholar] [CrossRef]
- Steinhorst, L.; Kudla, J. Calcium and reactive oxygen species rule the waves of signaling. Plant Physiol. 2013, 163, 471–485. [Google Scholar] [CrossRef] [Green Version]
- Bonaventure, G.; Gfeller, A.; Proebsting, W.M.; Hortensteiner, S.; Chetelat, A.; Martinoia, E.; Farmer, E.E. A gain-of-function allele of TPC1 activates oxylipin biogenesis after leaf wounding in Arabidopsis. Plant J. 2007, 49, 889–898. [Google Scholar] [CrossRef]
- Yan, C.; Fan, M.; Yang, M.; Zhao, J.P.; Zhang, W.H.; Su, Y.; Xiao, L.T.; Deng, H.T.; Xie, D.X. Injury activates Ca2+/Calmodulin-dependent phosphorylation of JAV1-JAZ8-WRKY51 complex for jasmonate biosynthesis. Mol. Cell 2018, 70, 136–149. [Google Scholar] [CrossRef] [Green Version]
- Ho, V.T.; Tran, A.N.; Cardarelli, F.; Perata, P.; Pucciariello, C. A calcineurin B-like protein participates in low oxygen signalling in rice. Funct. Plant Biol. 2017, 44, 917–928. [Google Scholar] [CrossRef]
- Ou, L.J.; Liu, Z.B.; Zhang, Y.P.; Zou, X.X. Effects of exogenous Ca2+ on photosynthetic characteristics and fruit quality of pepper under waterlogging stress. Chil. J. Agric. Res. 2017, 77, 126–133. [Google Scholar] [CrossRef] [Green Version]
- He, L.Z.; Yu, L.; Li, B.; Du, N.S.; Guo, S.R. The effect of exogenous calcium on cucumber fruit quality, photosynthesis, chlorophyll fluorescence, and fast chlorophyll fluorescence during the fruiting period under hypoxic stress. BMC Plant Biol. 2018, 18, 180. [Google Scholar] [CrossRef] [PubMed]
- Steffens, B.; Kovalev, A.; Gorb, S.N.; Sauter, M. Emerging roots alter epidermal cell fate through mechanical and reactive oxygen species signaling. Plant Cell 2012, 24, 3296–3306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.F.; Chen, Z.H.; Liu, X.H.; Colmer, T.D.; Shabala, L.; Salih, A.; Zhou, M.X.; Shabala, S. Revealing the roles of GORK channels and NADPH oxidase in acclimation to hypoxia in Arabidopsis. J. Exp. Bot. 2017, 68, 3191–3204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yemelyanov, V.V.; Shishova, M.F.; Chirkova, T.V.; Lindberg, S.M. Anoxia-induced elevation of cytosolic Ca2+ concentration depends on different Ca2+ sources in rice and wheat protoplasts. Planta 2011, 234, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Sedbrook, J.C.; Kronebusch, P.J.; Trewavas, G.G.B.A.J.; Masson, P.H. Transgenic AEQUORIN reveals organ-specific cytosolic Ca2+ responses to anoxia in Arabidopsis thaliana seedlings. Plant Physiol. 1996, 111, 243–257. [Google Scholar] [CrossRef] [Green Version]
- Peng, R.Y.; Bian, Z.Y.; Zhou, L.N.; Cheng, W.; Hai, N.; Yang, C.Q.; Yang, T.; Wang, X.Y.; Wang, C.Y. Hydrogen sulfide enhances nitric oxide-induced tolerance of hypoxia in maize (Zea mays L.). Plant Cell Rep. 2016, 35, 2325–2340. [Google Scholar] [CrossRef]
- Li, Y.Q.; Sun, D.; Xu, K.; Jin, L.B.; Peng, R.Y. Hydrogen sulfide enhances plant tolerance to waterlogging stress. Plants 2021, 10, 1928. [Google Scholar] [CrossRef]
- Wu, Q.; Su, N.N.; Huang, X.; Cui, J.; Shabala, L.; Zhou, M.X.; Yu, M.; Shabala, S. Hypoxia-induced increase in GABA content is essential for restoration of membrane potential and preventing ROS-induced disturbance to ion homeostasis. Plant Commun. 2021, 2, 12. [Google Scholar] [CrossRef]
- Frohnmeyer, H.; Loyall, L.; Blatt, M.R.; Grabov, A. Millisecond UV-B irradiation evokes prolonged elevation of cytosolic-free Ca2+ and stimulates gene expression in transgenic parsley cell cultures. Plant J. 1999, 20, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.X.; Tang, X.X.; Wang, M.; Zhang, W.; Zhou, B.; Wang, Y. ROS and calcium signaling mediated pathways involved in stress responses of the marine microalgae Dunaliella salina to enhanced UV-B radiation. J. Photochem. Photobiol. B-Biol. 2017, 173, 360–367. [Google Scholar] [CrossRef]
- Yu, G.H.; Li, W.; Yuan, Z.Y.; Cui, H.Y.; Lv, C.G.; Gao, Z.P.; Han, B.; Gong, Y.Z.; Chen, G.X. The effects of enhanced UV-B radiation on photosynthetic and biochemical activities in super-high-yield hybrid rice Liangyoupeijiu at the reproductive stage. Photosynthetica 2013, 51, 33–44. [Google Scholar] [CrossRef]
- Barabas, K.N.; Szegletes, Z.; Pestenacz, A.; Fulop, K.; Erdei, L. Effects of excess UV-B irradiation on the antioxidant defence mechanisms in wheat (Triticum aestivum L.) seedlings. J. Plant Physiol. 1998, 153, 146–153. [Google Scholar] [CrossRef]
- Christie, J.M.; Jenkins, G.I. Distinct UV-B and UV-A/blue light signal transduction pathways induce chalcone synthase gene expression in Arabidopsis cells. Plant Cell 1996, 8, 1555–1567. [Google Scholar] [PubMed] [Green Version]
- An, L.; Feng, H.; Tang, X.; Wang, X. Changes of microsomal membrane properties in spring wheat leaves (Triticum aestivum L.) exposed to enhanced ultraviolet-B radiation. J. Photochem. Photobiol. B Biol. 2000, 57, 60–65. [Google Scholar] [CrossRef]
- Chen, Z.J.; Ma, Y.; Yang, R.Q.; Gu, Z.X.; Wang, P. Effects of exogenous Ca2+ on phenolic accumulation and physiological changes in germinated wheat (Triticum aestivum L.) under UV-B radiation. Food Chem. 2019, 288, 368–376. [Google Scholar] [CrossRef]
- Gao, L.M.; Wang, X.F.; Shen, Z.H.; Li, Y.F. The application of exogenous gibberellic acid enhances wheat seedlings UV-B tolerance by ameliorating DNA damage and manipulating UV-absorbing compound biosynthesis in wheat seedling leaves. Pak. J. Bot. 2018, 50, 2167–2172. [Google Scholar]
- Wang, J.X.; Ding, H.D.; Zhang, A.; Ma, F.F.; Cao, J.M.; Jiang, M.Y. A novel mitogen-activated protein kinase gene in maize (Zea mays), ZmMPK3, is involved in response to diverse environmental cues. J. Integr. Plant Biol. 2010, 52, 442–452. [Google Scholar] [CrossRef]
- Du, L.Q.; Poovaiah, B.W. A novel family of Ca2+/calmodulin-binding proteins involved in transcriptional regulation: Interaction with fsh/Ring3 class transcription activators. Plant Mol. Biol. 2004, 54, 549–569. [Google Scholar] [CrossRef]
- Marcec, M.J.; Gilroy, S.; Poovaiah, B.W.; Tanaka, K. Mutual interplay of Ca2+ and ROS signaling in plant immune response. Plant Sci. 2019, 283, 343–354. [Google Scholar] [CrossRef]
- Gong, Z.Z.; Xiong, L.M.; Shi, H.Z.; Yang, S.H.; Herrera-Estrella, L.R.; Xu, G.H.; Chao, D.Y.; Li, J.R.; Wang, P.Y.; Qin, F.; et al. Plant abiotic stress response and nutrient use efficiency. Sci. China-Life Sci. 2020, 63, 635–674. [Google Scholar]
- Wang, Y.; Wu, W.H. Potassium transport and signaling in higher plants. In Annual Review of Plant Biology; Merchant, S.S., Ed.; Annual Reviews: Palo Alto, CA, USA, 2013; Volume 64, pp. 451–476. [Google Scholar]
- Li, L.G.; Kim, B.G.; Cheong, Y.H.; Pandey, G.K.; Luan, S. A Ca2+ signaling pathway regulates a K+ channel for low-K response in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 12625–12630. [Google Scholar] [PubMed] [Green Version]
- Qian, D.; Xiang, Y. Actin cytoskeleton as actor in upstream and downstream of calcium signaling in plant cells. Int. J. Mol. Sci. 2019, 20, 16. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Liu, Y.; Jin, L.; Peng, R. Crosstalk between Ca2+ and Other Regulators Assists Plants in Responding to Abiotic Stress. Plants 2022, 11, 1351. https://doi.org/10.3390/plants11101351
Li Y, Liu Y, Jin L, Peng R. Crosstalk between Ca2+ and Other Regulators Assists Plants in Responding to Abiotic Stress. Plants. 2022; 11(10):1351. https://doi.org/10.3390/plants11101351
Chicago/Turabian StyleLi, Yaoqi, Yinai Liu, Libo Jin, and Renyi Peng. 2022. "Crosstalk between Ca2+ and Other Regulators Assists Plants in Responding to Abiotic Stress" Plants 11, no. 10: 1351. https://doi.org/10.3390/plants11101351
APA StyleLi, Y., Liu, Y., Jin, L., & Peng, R. (2022). Crosstalk between Ca2+ and Other Regulators Assists Plants in Responding to Abiotic Stress. Plants, 11(10), 1351. https://doi.org/10.3390/plants11101351