Use of Under-Vine Living Mulches to Control Noxious Weeds in Irrigated Mediterranean Vineyards
Abstract
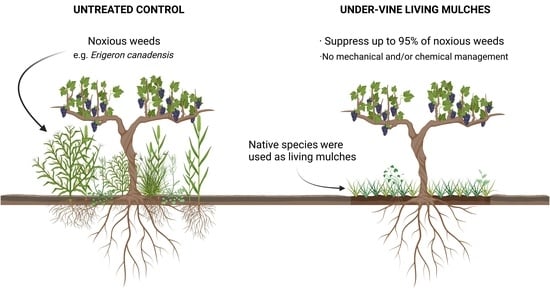
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Plant Species Selected for Living Mulches
2.3. Experimental Design
2.4. Field Data Collection
2.5. Statistical Analysis
3. Results
3.1. Establishment of Under-Vine Living Mulches
3.2. Effect of Under-Vine Living Mulches on Weed Density
3.3. Effect of Under-Vine Living Mulches on Weed Biomass
3.4. Relationships between Living Mulch Cover, Weed Density and Weed Biomass
3.5. Effect of Under-Vine Living Mulches on Grapevine Vegetative Growth
4. Discussion
4.1. Extend of Establishment of Under-Vine Living Mulches
4.2. Weed Suppression by Under-Vine Living Mulches
4.3. Control of Noxious Weeds by Under-Vine Living Mulches
4.4. Effect of Under-Vine Living Mulches on Grapevine Vegetative Growth
4.5. Implications for Vineyard Weed Management
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Biomass Estimation of Living Mulches
| Samples | FO | PO | PC | PL |
|---|---|---|---|---|
| 1 | 11.12 | 3.97 | 5.96 | 9.64 |
| 2 | 12.91 | 1.93 | 6.33 | 13.77 |
| 3 | 14.48 | 1.48 | 4.48 | 6.33 |
| 4 | 10.38 | 2.96 | 4.13 | 13.84 |
| 5 | 10.09 | 3.31 | 6.93 | 13.36 |
| 6 | 13.08 | 3.01 | 5.44 | 13.75 |
| 7 | 10.21 | 3.42 | 2.33 | 12.66 |
| 8 | 9.14 | 3.06 | 3.84 | 7.52 |
| 9 | 16.88 | 2.63 | 7.84 | 9.34 |
| 10 | 9.67 | 2.20 | 6.22 | 19.89 |
| Mean | 11.80 | 2.80 | 5.35 | 12.01 |
| SE | 2.48 | 0.75 | 1.65 | 3.93 |
| Biomass (g m−2) | 1083.20 | 256.84 | 491.28 | 1102.85 |
Appendix B
Sowing Cost Estimation of Two Under-Vine Living Mulches
References
- Limier, B.; Ivorra, S.; Bouby, L.; Figueiral, I.; Chabal, L.; Cabanis, M.; Ater, M.; Lacombe, T.; Ros, J.; Brémond, L.; et al. Documenting the History of the Grapevine and Viticulture: A Quantitative Eco-Anatomical Perspective Applied to Modern and Archaeological Charcoal. J. Archaeol. Sci. 2018, 100, 45–61. [Google Scholar] [CrossRef]
- OIV. State of the World Vitivinicultural Sector in 2020. 2020. Available online: https://www.oiv.int/public/medias/7909/oiv-state-of-the-world-vitivinicultural-sector-in-2020.pdf (accessed on 15 June 2022).
- Romero, P.; Navarro, J.M.; Ordaz, P.B. Towards a Sustainable Viticulture: The Combination of Deficit Irrigation Strategies and Agroecological Practices in Mediterranean Vineyards. A Review and Update. Agric. Water Manag. 2022, 259, 107216. [Google Scholar] [CrossRef]
- García-Ruiz, J.M.; López-Moreno, J.I.; Vicente-Serrano, S.M.; Lasanta–Martínez, T.; Beguería, S. Mediterranean Water Resources in a Global Change Scenario. Earth-Sci. Rev. 2011, 105, 121–139. [Google Scholar] [CrossRef] [Green Version]
- Tomás, M.; Medrano, H.; Pou, A.; Escalona, J.M.; Martorell, S.; Ribas-Carbó, M.; Flexas, J. Water-Use Efficiency in Grapevine Cultivars Grown under Controlled Conditions: Effects of Water Stress at the Leaf and Whole-Plant Level. Aust. J. Grape Wine Res. 2012, 18, 164–172. [Google Scholar] [CrossRef]
- Guerra, J.G.; Cabello, F.; Fernández-Quintanilla, C.; Dorado, J. A Trait-Based Approach in a Mediterranean Vineyard: Effects of Agricultural Management on the Functional Structure of Plant Communities. Agric. Ecosyst. Environ. 2021, 316, 107465. [Google Scholar] [CrossRef]
- Guerra, J.G.; Cabello, F.; Fernández-Quintanilla, C.; Peña, J.M.; Dorado, J. How Weed Management Influence Plant Community Composition, Taxonomic Diversity and Crop Yield: A Long-Term Study in a Mediterranean Vineyard. Agric. Ecosyst. Environ. 2022, 326, 107816. [Google Scholar] [CrossRef]
- Heap, I.; Duke, S.O. Overview of Glyphosate-Resistant Weeds Worldwide. Pest Manag. Sci. 2018, 74, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Prosdocimi, M.; Cerdà, A.; Tarolli, P. Soil Water Erosion on Mediterranean Vineyards: A Review. Catena 2016, 141, 1–21. [Google Scholar] [CrossRef]
- Martínez-Uña, A.; Martín, J.M.; Fernández-Quintanilla, C.; Dorado, J. Provisioning Floral Resources to Attract Aphidophagous Hoverflies (Diptera: Syrphidae) Useful for Pest Management in Central Spain. J. Econ. Entomol. 2013, 106, 2327–2335. [Google Scholar] [CrossRef]
- Winter, S.; Bauer, T.; Strauss, P.; Kratschmer, S.; Paredes, D.; Popescu, D.; Landa, B.; Guzmán, G.; Gómez, J.A.; Guernion, M.; et al. Effects of Vegetation Management Intensity on Biodiversity and Ecosystem Services in Vineyards: A Meta-Analysis. J. Appl. Ecol. 2018, 55, 2484–2495. [Google Scholar] [CrossRef] [Green Version]
- Novara, A.; Gristina, L.; Saladino, S.S.; Santoro, A.; Cerdà, A. Soil Erosion Assessment on Tillage and Alternative Soil Managements in a Sicilian Vineyard. Soil Tillage Res. 2011, 117, 140–147. [Google Scholar] [CrossRef] [Green Version]
- Hall, R.M.; Penke, N.; Kriechbaum, M.; Kratschmer, S.; Jung, V.; Chollet, S.; Guernion, M.; Nicolai, A.; Burel, F.; Fertil, A.; et al. Vegetation Management Intensity and Landscape Diversity Alter Plant Species Richness, Functional Traits and Community Composition across European Vineyards. Agric. Syst. 2020, 177, 102706. [Google Scholar] [CrossRef]
- Celette, F.; Gary, C. Dynamics of Water and Nitrogen Stress along the Grapevine Cycle as Affected by Cover Cropping. Eur. J. Agron. 2013, 45, 142–152. [Google Scholar] [CrossRef]
- Muscas, E.; Cocco, A.; Mercenaro, L.; Cabras, M.; Lentini, A.; Porqueddu, C.; Nieddu, G. Effects of Vineyard Floor Cover Crops on Grapevine Vigor, Yield, and Fruit Quality, and the Development of the Vine Mealybug under a Mediterranean Climate. Agric. Ecosyst. Environ. 2017, 237, 203–212. [Google Scholar] [CrossRef]
- Dry, P.r.; Loveys, B.r. Factors Influencing Grapevine Vigour and the Potential for Control with Partial Rootzone Drying. Aust. J. Grape Wine Res. 1998, 4, 140–148. [Google Scholar] [CrossRef]
- Guerra, B.; Steenwerth, K. Influence of Floor Management Technique on Grapevine Growth, Disease Pressure, and Juice and Wine Composition: A Review. Am. J. Enol. Vitic. 2012, 63, 149–164. [Google Scholar] [CrossRef]
- Creamer, N.G.; Bennett, M.A.; Stinner, B.R.; Cardina, J.; Regnier, E.E. Mechanisms of Weed Suppression in Cover Crop-Based Production Systems. HortScience 1996, 31, 410–413. [Google Scholar] [CrossRef]
- Brennan, E.B.; Smith, R.F. Winter Cover Crop Growth and Weed Suppression on the Central Coast of California. Weed Technol. 2005, 19, 1017–1024. [Google Scholar] [CrossRef]
- Kruidhof, H.M.; Bastiaans, L.; Kropff, M.J. Ecological Weed Management by Cover Cropping: Effects on Weed Growth in Autumn and Weed Establishment in Spring. Weed Res. 2008, 48, 492–502. [Google Scholar] [CrossRef]
- Baraibar, B.; Mortensen, D.A.; Hunter, M.C.; Barbercheck, M.E.; Kaye, J.P.; Finney, D.M.; Curran, W.S.; Bunchek, J.; White, C.M. Growing Degree Days and Cover Crop Type Explain Weed Biomass in Winter Cover Crops. Agron. Sustain. Dev. 2018, 38, 65. [Google Scholar] [CrossRef] [Green Version]
- Osipitan, O.A.; Dille, J.A.; Assefa, Y.; Radicetti, E.; Ayeni, A.; Knezevic, S.Z. Impact of Cover Crop Management on Level of Weed Suppression: A Meta-Analysis. Crop Sci. 2019, 59, 833–842. [Google Scholar] [CrossRef]
- Hartwig, N.L.; Ammon, H.U. Cover Crops and Living Mulches. Weed Sci. 2002, 50, 688–699. [Google Scholar] [CrossRef]
- Paušič, A.; Tojnko, S.; Lešnik, M. Permanent, Undisturbed, in-Row Living Mulch: A Realistic Option to Replace Glyphosate-Dominated Chemical Weed Control in Intensive Pear Orchards. Agric. Ecosyst. Environ. 2021, 318, 107502. [Google Scholar] [CrossRef]
- Ilnicki, R.D.; Enache, A.J. Subterranean Clover Living Mulch: An Alternative Method of Weed Control. Agric. Ecosyst. Environ. 1992, 40, 249–264. [Google Scholar] [CrossRef]
- Hiltbrunner, J.; Liedgens, M.; Bloch, L.; Stamp, P.; Streit, B. Legume Cover Crops as Living Mulches for Winter Wheat: Components of Biomass and the Control of Weeds. Eur. J. Agron. 2007, 26, 21–29. [Google Scholar] [CrossRef]
- Bhaskar, V.; Bellinder, R.R.; DiTommaso, A.; Walter, M.F. Living Mulch Performance in a Tropical Cotton System and Impact on Yield and Weed Control. Agriculture 2018, 8, 19. [Google Scholar] [CrossRef] [Green Version]
- Gerhards, R. Weed Suppression Ability and Yield Impact of Living Mulch in Cereal Crops. Agriculture 2018, 8, 39. [Google Scholar] [CrossRef] [Green Version]
- Nakamoto, T.; Tsukamoto, M. Abundance and Activity of Soil Organisms in Fields of Maize Grown with a White Clover Living Mulch. Agric. Ecosyst. Environ. 2006, 115, 34–42. [Google Scholar] [CrossRef]
- Qian, X.; Gu, J.; Pan, H.; Zhang, K.; Sun, W.; Wang, X.; Gao, H. Effects of Living Mulches on the Soil Nutrient Contents, Enzyme Activities, and Bacterial Community Diversities of Apple Orchard Soils. Eur. J. Soil Biol. 2015, 70, 23–30. [Google Scholar] [CrossRef]
- Karl, A.D.; Merwin, I.A.; Brown, M.G.; Hervieux, R.A.; Heuvel, J.E.V. Under-Vine Management Impacts Soil Properties and Leachate Composition in a New York State Vineyard. HortScience 2016, 51, 941–949. [Google Scholar] [CrossRef] [Green Version]
- Altieri, M.A.; Wilson, R.C.; Schmidt, L.L. The Effects of Living Mulches and Weed Cover on the Dynamics of Foliage- and Soil-Arthropod Communities in Three Crop Systems. Crop Prot. 1985, 4, 201–213. [Google Scholar] [CrossRef]
- Las Casas, G.; Ciaccia, C.; Iovino, V.; Ferlito, F.; Torrisi, B.; Lodolini, E.M.; Giuffrida, A.; Catania, R.; Nicolosi, E.; Bella, S. Effects of Different Inter-Row Soil Management and Intra-Row Living Mulch on Spontaneous Flora, Beneficial Insects, and Growth of Young Olive Trees in Southern Italy. Plants 2022, 11, 545. [Google Scholar] [CrossRef] [PubMed]
- Fleishman, S.M.; Eissenstat, D.M.; Centinari, M. Rootstock Vigor Shifts Aboveground Response to Groundcover Competition in Young Grapevines. Plant Soil 2019, 440, 151–165. [Google Scholar] [CrossRef]
- Marks, J.N.J.; Lines, T.E.P.; Penfold, C.; Cavagnaro, T.R. Cover Crops and Carbon Stocks: How under-Vine Management Influences SOC Inputs and Turnover in Two Vineyards. Sci. Total Environ. 2022, 831, 154800. [Google Scholar] [CrossRef]
- Giese, G.; Velasco-Cruz, C.; Roberts, L.; Heitman, J.; Wolf, T.K. Complete Vineyard Floor Cover Crops Favorably Limit Grapevine Vegetative Growth. Sci. Hortic. 2014, 170, 256–266. [Google Scholar] [CrossRef]
- Chou, M.-Y.; Heuvel, J.E.V. Annual Under-Vine Cover Crops Mitigate Vine Vigor in a Mature and Vigorous Cabernet Franc Vineyard. Am. J. Enol. Vitic. 2018, 70, 98–108. [Google Scholar] [CrossRef]
- Coniberti, A.; Ferrari, V.; Disegna, E.; Garcia Petillo, M.; Lakso, A.N. Under-Trellis Cover Crop and Planting Density to Achieve Vine Balance in a Humid Climate. Sci. Hortic. 2018, 227, 65–74. [Google Scholar] [CrossRef]
- Neri, D.; Polverigiani, S.; Zucchini, M.; Giorgi, V.; Marchionni, F.; Mia, M.J. Strawberry Living Mulch in an Organic Vineyard. Agronomy 2021, 11, 1643. [Google Scholar] [CrossRef]
- Abad, J.; Diana, M.; Gonzaga, S.L.; Félix, C.J.; Ana, S. Under-Vine Cover Crops: Impact on Weed Development, Yield and Grape Composition. OENO One 2020, 54, 975–983. [Google Scholar] [CrossRef]
- Valencia-Gredilla, F.; Royo-Esnal, A.; Juárez-Escario, A.; Recasens, J. Different Ground Vegetation Cover Management Systems to Manage Cynodon Dactylon in an Irrigated Vineyard. Agronomy 2020, 10, 908. [Google Scholar] [CrossRef]
- Mercenaro, L.; Nieddu, G.; Pulina, P.; Porqueddu, C. Sustainable Management of an Intercropped Mediterranean Vineyard. Agric. Ecosyst. Environ. 2014, 192, 95–104. [Google Scholar] [CrossRef]
- Lopes, C.M.; Santos, T.P.; Monteiro, A.; Rodrigues, M.L.; Costa, J.M.; Chaves, M.M. Combining Cover Cropping with Deficit Irrigation in a Mediterranean Low Vigor Vineyard. Sci. Hortic. 2011, 129, 603–612. [Google Scholar] [CrossRef] [Green Version]
- Doisy, D.; Colbach, N.; Roger-Estrade, J.; Médiène, S. Weed Seed Rain Interception by Grass Cover Depends on Seed Traits. Weed Res. 2014, 54, 593–602. [Google Scholar] [CrossRef]
- Bishop, G.F.; Davy, A.J. Hieracium Pilosella L. (Pilosella Officinarum F. Schultz & Schultz-Bip.). J. Ecol. 1994, 82, 195–210. [Google Scholar] [CrossRef]
- Winkler, E.; Stöcklin, J. Sexual and Vegetative Reproduction of Hieracium Pilosella L. under Competition and Disturbance: A Grid-based Simulation Model. Ann. Bot. 2002, 89, 525–536. [Google Scholar] [CrossRef] [Green Version]
- French, K. Invasion by Hawkweeds. Biol. Invasions 2021, 23, 3641–3652. [Google Scholar] [CrossRef]
- Treskonova, M. Changes in the structure of tall tussock grasslands and infestation by species of Hieracium in the Mackenzie Country, New Zealand. N. Z. J. Ecol. 1991, 15, 65–78. [Google Scholar]
- Cipriotti, P.A.; Rauber, R.B.; Collantes, M.B.; Braun, K.; Escartín, C. Hieracium Pilosella Invasion in the Tierra Del Fuego Steppe, Southern Patagonia. Biol. Invasions 2010, 12, 2523–2535. [Google Scholar] [CrossRef]
- Miglécz, T.; Valkó, O.; Török, P.; Deák, B.; Kelemen, A.; Donkó, Á.; Drexler, D.; Tóthmérész, B. Establishment of Three Cover Crop Mixtures in Vineyards. Sci. Hortic. 2015, 197, 117–123. [Google Scholar] [CrossRef]
- Hodgson, J.G.; Wilson, P.J.; Hunt, R.; Grime, J.P.; Thompson, K. Allocating C-S-R Plant Functional Types: A Soft Approach to a Hard Problem. Oikos 1999, 85, 282–294. [Google Scholar] [CrossRef]
- Andújar, D.; Ribeiro, A.; Carmona, R.; Fernández-Quintanilla, C.; Dorado, J. An Assessment of the Accuracy and Consistency of Human Perception of Weed Cover. Weed Res. 2010, 50, 638–647. [Google Scholar] [CrossRef]
- Finney, D.M.; White, C.M.; Kaye, J.P. Biomass Production and Carbon/Nitrogen Ratio Influence Ecosystem Services from Cover Crop Mixtures. Agron. J. 2016, 108, 39–52. [Google Scholar] [CrossRef] [Green Version]
- MacLaren, C.; Swanepoel, P.; Bennett, J.; Wright, J.; Dehnen-Schmutz, K. Cover Crop Biomass Production Is More Important than Diversity for Weed Suppression. Crop Sci. 2019, 59, 733–748. [Google Scholar] [CrossRef] [Green Version]
- Castroviejo, S. (coord. gen.), 1986–2012. Flora ibérica 1-8, 10-15, 17-18, 21. Real Jardín Botánico, CSIC, Madrid. Available online: http://www.floraiberica.org/ (accessed on 22 June 2022).
- IPNI. International Plant Names Index. The Royal Botanic Gardens, Kew, Harvard University Herbaria & Libraries and Australian National Botanic Gardens. 2022. Available online: http://www.ipni.org (accessed on 22 June 2022).
- Ferrari, S.; Cribari-Neto, F. Beta Regression for Modelling Rates and Proportions. J. Appl. Stat. 2004, 31, 799–815. [Google Scholar] [CrossRef]
- Cribari-Neto, F.; Zeileis, A. Beta Regression in R. J. Stat. Softw. 2010, 34, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Mächler, M.; Bolker, B.M. GlmmTMB Balances Speed and Flexibility among Packages for Zero-Inflated Generalized Linear Mixed Modeling. R. J. 2017, 9, 378–400. [Google Scholar] [CrossRef] [Green Version]
- Lüdecke, D.; Ben-Shachar, M.S.; Patil, I.; Waggoner, P.; Makowski, D. Performance: An R Package for Assessment, Comparison and Testing of Statistical Models. J. Open Source Softw. 2021, 6, 3139. [Google Scholar] [CrossRef]
- Scheurwater, I.; Cornelissen, C.; Dictus, F.; Welschen, R.; Lambers, H. Why Do Fast- and Slow-Growing Grass Species Differ so Little in Their Rate of Root Respiration, Considering the Large Differences in Rate of Growth and Ion Uptake? Plant Cell Environ. 1998, 21, 995–1005. [Google Scholar] [CrossRef]
- Cabrera-Pérez, C.; Recasens, J.; Baraibar, B.; Royo-Esnal, A. Emergence Modelling of 18 Species Susceptible to Be Used as Cover Crops in Mediterranean Semiarid Vineyards. Eur. J. Agron. 2022, 132, 126413. [Google Scholar] [CrossRef]
- Villellas, J.; Ehrlén, J.; Olesen, J.M.; Braza, R.; García, M.B. Plant Performance in Central and Northern Peripheral Populations of the Widespread Plantago Coronopus. Ecography 2013, 36, 136–145. [Google Scholar] [CrossRef] [Green Version]
- Villellas, J.; García, M.B. Intrinsic and Extrinsic Drivers of Recruitment across the Distribution Range of a Seed-Dimorphic Herb. Plant Ecol. 2017, 218, 529–539. [Google Scholar] [CrossRef]
- Steinmaus, S.; Elmore, C.L.; Smith, R.J.; Donaldson, D.; Weber, E.A.; Roncoroni, J.A.; Miller, P.R.M. Mulched Cover Crops as an Alternative to Conventional Weed Management Systems in Vineyards. Weed Res. 2008, 48, 273–281. [Google Scholar] [CrossRef] [Green Version]
- Yasin, M.; Rosenqvist, E.; Andreasen, C. The Effect of Reduced Light Intensity on Grass Weeds. Weed Sci. 2017, 65, 603–613. [Google Scholar] [CrossRef]
- Colbach, N.; Gardarin, A.; Moreau, D. The Response of Weed and Crop Species to Shading: Which Parameters Explain Weed Impacts on Crop Production? Field Crops Res. 2019, 238, 45–55. [Google Scholar] [CrossRef]
- Dietz, M.; Machill, S.; Hoffmann, H.C.; Schmidtke, K. Inhibitory Effects of Plantago Lanceolata L. on Soil N Mineralization. Plant Soil 2013, 368, 445–458. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Y.; Chen, Y.; Wang, Q.; Jiang, L.; Luo, Y. Allelopathic Potential of Invasive Plantago Virginica on Four Lawn Species. PLoS ONE 2015, 10, e0125433. [Google Scholar] [CrossRef]
- Powles, S.B. Evolved Glyphosate-Resistant Weeds around the World: Lessons to Be Learnt. Pest Manag. Sci. 2008, 64, 360–365. [Google Scholar] [CrossRef]
- Hanson, B.D.; Shrestha, A.; Shaner, D.L. Distribution of Glyphosate-Resistant Horseweed (Conyza Canadensis) and Relationship to Cropping Systems in The Central Valley of California. Weed Sci. 2009, 57, 48–53. [Google Scholar] [CrossRef]
- Alcorta, M.; Fidelibus, M.W.; Steenwerth, K.L.; Shrestha, A. Competitive Effects of Glyphosate-Resistant and Glyphosate-Susceptible Horseweed (Conyza Canadensis) on Young Grapevines (Vitis Vinifera). Weed Sci. 2011, 59, 489–494. [Google Scholar] [CrossRef]
- Pittman, K.B.; Barney, J.N.; Flessner, M.L. Horseweed (Conyza Canadensis) Suppression from Cover Crop Mixtures and Fall-Applied Residual Herbicides. Weed Technol. 2019, 33, 303–311. [Google Scholar] [CrossRef]
- Milberg, P.; Andersson, L.; Thompson, K. Large-Seeded Spices Are Less Dependent on Light for Germination than Small-Seeded Ones. Seed Sci. Res. 2000, 10, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Nandula, V.K.; Eubank, T.W.; Poston, D.H.; Koger, C.H.; Reddy, K.N. Factors Affecting Germination of Horseweed (Conyza Canadensis). Weed Sci. 2006, 54, 898–902. [Google Scholar] [CrossRef]
- Boldt, P.E.; Rosenthal, S.S.; Srinivasan, R. Distribution of Field Bindweed and Hedge Bindweed in the USA. J. Prod. Agric. 1998, 11, 377–381. [Google Scholar] [CrossRef]
- Juárez-Escario, A.; Valls, J.; Solé-Senan, X.o.; Conesa, J.a. A Plant-Traits Approach to Assessing the Success of Alien Weed Species in Irrigated Mediterranean Orchards. Ann. Appl. Biol. 2013, 162, 200–213. [Google Scholar] [CrossRef]
- Juárez-Escario, A.; Solé-Senan, X.o.; Recasens, J.; Taberner, A.; Conesa, J.a. Long-Term Compositional and Functional Changes in Alien and Native Weed Communities in Annual and Perennial Irrigated Crops. Ann. Appl. Biol. 2018, 173, 42–54. [Google Scholar] [CrossRef]
- Ayuda, M.-I.; Esteban, E.; Martín-Retortillo, M.; Pinilla, V. The Blue Water Footprint of the Spanish Wine Industry: 1935–2015. Water 2020, 12, 1872. [Google Scholar] [CrossRef]
- Vanden Heuvel, J.; Centinari, M. Under-Vine Vegetation Mitigates the Impacts of Excessive Precipitation in Vineyards. Front. Plant Sci. 2021, 12, 1542. [Google Scholar] [CrossRef]
- Jordan, L.M.; Björkman, T.; Heuvel, J.E.V. Annual Under-Vine Cover Crops Did Not Impact Vine Growth or Fruit Composition of Mature Cool-Climate ‘Riesling’ Grapevines. Horttechnology 2016, 26, 36–45. [Google Scholar] [CrossRef] [Green Version]
- Karl, A.; Merwin, I.A.; Brown, M.G.; Hervieux, R.A.; Heuvel, J.E.V. Impact of Undervine Management on Vine Growth, Yield, Fruit Composition, and Wine Sensory Analyses in Cabernet Franc. Am. J. Enol. Vitic. 2016, 67, 269–280. [Google Scholar] [CrossRef]
- Vukicevich, E.; Lowery, T.; Hart, M. Effects of Living Mulch on Young Vine Growth and Soil in a Semi-Arid Vineyard. Vitis-J. Grapevine Res. 2019, 58, 113–122. [Google Scholar] [CrossRef]
- Abad, J.; de Mendoza, I.H.; Marín, D.; Orcaray, L.; Santesteban, L.G. Cover Crops in Viticulture. A Systematic Review (2):Implications on Vineyard Agronomic Performance. OENO One 2021, 55, 1–27. [Google Scholar] [CrossRef]
- Linares Torres, R.; De La Fuente Lloreda, M.; Junquera Gonzalez, P.; Lissarrague García-Gutierrez, J.r.; Baeza Trujillo, P. Effect of Soil Management Strategies on the Characteristics of the Grapevine Root System in Irrigated Vineyards under Semi-Arid Conditions. Aust. J. Grape Wine Res. 2018, 24, 439–449. [Google Scholar] [CrossRef]
- Delpuech, X.; Metay, A. Adapting Cover Crop Soil Coverage to Soil Depth to Limit Competition for Water in a Mediterranean Vineyard. Eur. J. Agron. 2018, 97, 60–69. [Google Scholar] [CrossRef]







| Farm A | Farm B | |||||
|---|---|---|---|---|---|---|
| Tmean (°C) | R (mm) | ΔR (%) | Tmean (°C) | R (mm) | ΔR (%) | |
| October 2018–May 2019 | 11.2 | 172.0 | −45 | 9.7 | 240.4 | −33 |
| June–September 2019 | 24.8 | 150.0 | 177 | 22.4 | 68.6 | 11 |
| October 2019–May 2020 | 12.2 | 318.4 | 1 | 10.3 | 424.4 | 18 |
| June–September 2020 | 24.3 | 21.0 | −61 | 22.0 | 73.2 | 19 |
| October 2020–May 2021 | 11.0 | 220.6 | −30 | 9.4 | 286.2 | −20 |
| June–September 2021 | 24.2 | 123.6 | 129 | 21.9 | 149.4 | 142 |
| EPPO Code | Biomass | RLF | PHV | LA | LM | LDMC | SLA | CSR | |
|---|---|---|---|---|---|---|---|---|---|
| (g m−2) | - | (cm) | (mm2) | (g) | (mg g−1) | (mm2 mg−1) | - | ||
| Festuca ovina | FESOV | 1083.2 | H | 17.1 | 46.7 | 1.9 | 422.1 | 14.0 | S/CSR |
| Pilosella officinarum | HIEPI | 256.8 | H | 7.3 | 258.8 | 18.9 | 207.0 | 16.0 | S/CSR |
| Plantago coronopus | PLACO | 491.3 | H/Th | 10.2 | 195.9 | 13.4 | 124.8 | 18.3 | SR/CSR |
| Plantago lanceoalata | PLALA | 1102.8 | H | 18.3 | 1653.3 | 95.4 | 178.6 | 18.9 | SC/CSR |
| EPPO Code | Scientific Name | Farm A | Farm B | ||
|---|---|---|---|---|---|
| May | September | May | September | ||
| EPHCH | Euphorbia prostrata Aiton | 0.01 | 0.02 | 0.25 | 0.71 |
| ERICA * | Erigeron canadensis L. | 0.43 | 0.35 | − | − |
| BROMA | Bromus madritensis L. | 0.20 | 0.07 | 0.11 | 0.04 |
| CONAR * | Convolvulus arvensis L. | 0.01 | 0.01 | 0.11 | 0.14 |
| CYPRO * | Cyperus rotundus L. | 0.05 | 0.17 | − | − |
| FESAR | Lolium arundinaceum (Schreb.) Darbysh. | − | 0.21 | − | − |
| SONAS * | Sonchus asper (L.) Hill | 0.06 | 0.01 | 0.11 | 0.03 |
| GALPR | Galium parisiense L. | 0.01 | − | 0.13 | − |
| CYNDA * | Cynodon dactylon (L.) Pers. | 0.05 | 0.06 | − | − |
| DIPER * | Diplotaxis erucoides (L.) DC. | − | − | 0.07 | 0.03 |
| MEDMI | Medicago minima (L.) Bartal. | − | + | 0.09 | + |
| CHEAL * | Chenopodium album L. | 0.03 | 0.03 | − | − |
| BRODI * | Bromus diandrus Roth | 0.04 | − | 0.01 | − |
| LACSE* | Lactuca serriola L. | 0.01 | + | 0.02 | 0.01 |
| SOLNI * | Solanum nigrum L. | 0.02 | 0.02 | − | + |
| SETVI | Setaria viridis (L.) P.Beauv. | 0.02 | 0.01 | − | − |
| ANGAR | Lysimachia arvensis (L.) U.Manns & Anderb. | 0.01 | 0.01 | − | − |
| POLAV | Polygonum aviculare L. | + | + | − | 0.02 |
| LOLRI | Lolium rigidum Gaudin | 0.02 | − | 0.01 | − |
| SONOL * | Sonchus oleraceus L. | + | − | + | − |
| HORMU | Hordeum murinum L. | + | − | 0.02 | − |
| MEDOR | Medicago orbicularis (L.) Bartal. | - | − | 0.02 | − |
| ASAHA | Astragalus hamosus L. | + | − | 0.01 | − |
| CIRAR * | Cirsium arvense (L.) Scop. | − | − | + | 0.01 |
| AMAAL * | Amaranthus albus L. | + | + | + | + |
| POROL | Portulaca oleracea L. | − | 0.01 | − | − |
| SASKA * | Salsola kali L. | + | + | − | + |
| AVEST | Avena sterilis L. | − | − | 0.01 | − |
| ECHCG | Echinochloa crus-galli (L.) P.Beauv. | − | + | − | − |
| BROTE | Bromus tectorum L. | − | − | + | − |
| FILPY | Filago pyramidata L. | − | − | + | − |
| PAPRH | Papaver rhoeas L. | + | + | − | + |
| LPHCR | Rostraria cristata (L.) Tzvelev | + | − | + | − |
| MALSI * | Malva sylvestris L. | − | − | + | + |
| TRKMO | Medicago monspeliaca (L.) Trautv. | − | − | + | − |
| VEBOF | Verbena officinalis L. | − | − | + | − |
| VLPMY | Festuca myuros L. | − | − | + | − |
| HEOEU | Heliotropium europaeum L. | + | + | − | − |
| GERMO | Geranium molle L. | − | − | + | + |
| FUMOF | Fumaria officinalis L. | + | − | − | − |
| VERHE | Veronica hederifolia L. | + | − | − | − |
| CENME | Centaurea melitensis L. | − | − | + | − |
| CVPVT | Crepis vesicaria subsp. taraxacifolia (Thuill.) Thell. | − | − | + | − |
| LAMAM | Lamium amplexicaule L. | − | − | + | − |
| SCVLA | Scorzonera laciniata L. | − | − | + | − |
| TROPS | Tragopogon porrifolius L. | − | − | + | − |
| ASAST | Astragalus stella L. | − | − | + | − |
| BRORU | Bromus rubens L. | − | − | + | − |
| ERXCA | Eryngium campestre L. | − | − | + | − |
| HESCO | Sulla coronaria (L.) B.H.Choi & H.Ohashi | − | − | + | − |
| TANCR | Taeniatherum caput-medusae (L.) Nevski | − | − | + | − |
| TAROV | Taraxacum obovatum (Willd.) DC. | − | − | + | − |
| CAPBP | Capsella bursa-pastoris (L.) Medik. | + | − | − | − |
| PLAMA | Plantago major L. | + | − | − | − |
| PLAOV | Plantago ovata Forssk. | + | − | − | − |
| RBITI * | Rubia tinctorum L. | + | − | − | − |
| TRBTE | Tribulus terrestris L. | − | + | − | − |
| HEQGL | Herniaria glabra L. | − | − | + | − |
| MEDRI | Medicago rigidula (L.) All. | − | − | + | − |
| Farm A | Farm B | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2m | p-Value | UC | FO | PO | PC | PL | R2m | p-Value | UC | FO | PO | PC | PL | |
| BROMA | 0.40 | *** | 33.67 a | 1.53 b | 27.17 ab | 17.98 ab | 1.53 b | 0.32 | *** | 17.98 a | 3.44 b | 9.18 ab | 3.83 b | 0.77 b |
| CHEAL * | 0.09 | ns | 1.53 a | 0.38 a | 10.33 a | 4.97 a | 2.30 a | − | − | − | − | − | − | − |
| CONAR * | − | − | − | − | − | − | − | 0.20 | *** | 16.45 a | 13.39 ab | 12.63 ab | 17.60 a | 4.59 b |
| CYNDA * | 0.16 | *** | 4.21 b | 0.77 b | 21.04 a | 3.83 b | 6.89 ab | − | − | − | − | − | − | − |
| CYPRO * | 0.34 | ** | 14.54 ab | 2.68 b | 22.96 ab | 30.61 a | 0.38 b | − | − | − | − | − | − | − |
| DIPER * | − | − | − | − | − | − | − | 0.02 | ns | 3.44 a | 9.18 a | 3.83 a | 4.97 a | 4.59 a |
| EPHCH | − | − | − | − | − | − | − | 0.07 | *** | 81.11 a | 77.67 a | 61.98 ab | 32.52 ab | 10.33 b |
| ERICA * | 0.42 | *** | 128.18 a | 10.71 c | 66.57 b | 36.35 bc | 3.44 c | − | − | − | − | − | − | − |
| FESAR | 0.06 | ns | 30.23 a | 9.57 a | 26.78 a | 4.21 a | 1.91 a | − | − | − | − | − | − | − |
| GALPR | − | − | − | − | − | − | − | 0.34 | ** | 16.45 a | 1.53 b | 7.65 ab | 1.91 b | 0.00 b |
| MEDMI | − | − | − | − | − | − | − | 0.35 | * | 7.27 a | 6.50 a | 2.68 ab | 1.53 ab | 0.00 b |
| SONAS * | 0.26 | ** | 8.80 a | 0.77 b | 8.80 a | 3.06 ab | 0.38 b | 0.43 | *** | 10.71 a | 7.65 ab | 3.83 bc | 3.83 bc | 1.15 c |
| Overall | ||
| Weed density | Weed biomass | |
| Living mulch cover | −0.60 *** | −0.69 *** |
| Farm A | ||
| Weed density | Weed biomass | |
| Living mulch cover | −0.72 *** | −0.79 *** |
| Farm B | ||
| Weed density | Weed biomass | |
| Living mulch cover | −0.45 *** | −0.57 *** |
| Farm A | Farm B | |||||
|---|---|---|---|---|---|---|
| Living Mulch Cover (%) | Weed Supression (%) a | Ratio (g Plants−1) b | Living Mulch Cover (%) | Weed Supression (%) a | Ratio (g Plants−1) b | |
| FO | 77 | 93 | 0.31 | 71 | 61 | 0.61 |
| PO | 41 | 29 | 1.01 | 83 | 68 | 0.71 |
| PC | 33 | 43 | 1.17 | 54 | 71 | 0.97 |
| PL | 77 | 95 | 0.40 | 84 | 93 | 0.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerra, J.G.; Cabello, F.; Fernández-Quintanilla, C.; Peña, J.M.; Dorado, J. Use of Under-Vine Living Mulches to Control Noxious Weeds in Irrigated Mediterranean Vineyards. Plants 2022, 11, 1921. https://doi.org/10.3390/plants11151921
Guerra JG, Cabello F, Fernández-Quintanilla C, Peña JM, Dorado J. Use of Under-Vine Living Mulches to Control Noxious Weeds in Irrigated Mediterranean Vineyards. Plants. 2022; 11(15):1921. https://doi.org/10.3390/plants11151921
Chicago/Turabian StyleGuerra, Jose G., Félix Cabello, César Fernández-Quintanilla, José Manuel Peña, and José Dorado. 2022. "Use of Under-Vine Living Mulches to Control Noxious Weeds in Irrigated Mediterranean Vineyards" Plants 11, no. 15: 1921. https://doi.org/10.3390/plants11151921
APA StyleGuerra, J. G., Cabello, F., Fernández-Quintanilla, C., Peña, J. M., & Dorado, J. (2022). Use of Under-Vine Living Mulches to Control Noxious Weeds in Irrigated Mediterranean Vineyards. Plants, 11(15), 1921. https://doi.org/10.3390/plants11151921