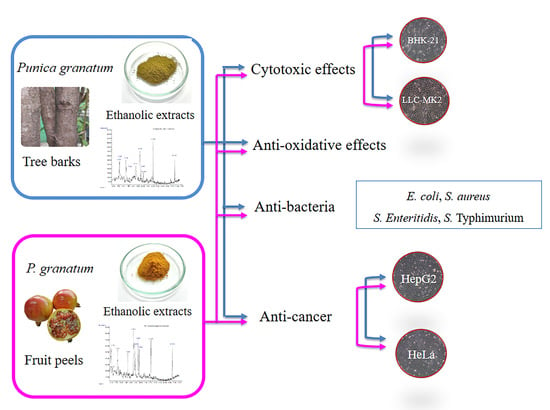
Natural Antioxidant, Antibacterial, and Antiproliferative Activities of Ethanolic Extracts from Punica granatum L. Tree Barks Mediated by Extracellular Signal-Regulated Kinase
Abstract
:1. Introduction
2. Results and Discussion
2.1. Total Phenolic Content, Flavonoid Content, and free radical scavenging activity
2.2. Chemical Composition
2.3. Antibacterial Effect
2.4. The Cytotoxic Effects of PBE and PPE on LLC-MK2 and BHK-21 Cells
2.5. Antiproliferative Effects of PBE and PPE on HeLa and HepG2 Cells
2.6. Immunoblotting
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Extracts Preparations
3.3. Determination of Total Phenolic Content
3.4. Determination of Flavonoid Content
3.5. Determination of DPPH• Free Radical Scavenging Activity
3.6. Gas Chromatography-Mass Spectrometry
3.7. Determination of Minimum Inhibitory Concentration of PPE and PBE
3.8. Cell Cultures and Treatments
3.9. Evaluation of Cytotoxicity and Antiproliferative Activity of PPE and PBE
3.10. Immunoblotting
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.A.; James, D.; Marzan, A.; Armaos, M. Cervical cancer: An overview of pathophysiology and management. Semin. Oncol. Nurs. 2019, 35, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Cubie, H.A.; Campbell, C. Cervical cancer screening—The challenges of complete pathways of care in low-income countries: Focus on Malawi. Womens Health 2020, 16. [Google Scholar] [CrossRef] [PubMed]
- Sawaya, G.F.; Smith-McCune, K.; Kuppermann, M. Cervical cancer screening: More choices in 2019. JAMA 2019, 321, 2018–2019. [Google Scholar] [CrossRef] [PubMed]
- Longerich, T. Hepatocellular carcinoma. Pathologe 2020, 41, 478–487. [Google Scholar] [CrossRef]
- Lichota, A.; Gwozdzinski, K. Anticancer activity of natural compounds from plant and marine environment. Int. J. Mol. Sci. 2018, 19, 3533. [Google Scholar] [CrossRef]
- Ishteyaque, S.; Mishra, A.; Mohapatra, S.; Singh, A.; Bhatta, R.S.; Tadigoppula, N.; Mugale, M.N. In vitro: Cytotoxicity, apoptosis and ameliorative potential of Lawsonia inermis extract in human lung, colon and liver cancer cell line. Cancer Investig. 2020, 38, 476–485. [Google Scholar] [CrossRef]
- Mandlik, D.S.; Mandlik, S.K. Herbal and natural dietary products: Upcoming therapeutic approach for prevention and treatment of hepatocellular carcinoma. Nutr. Cancer 2021, 73, 2130–2154. [Google Scholar] [CrossRef]
- Adewole, K.E. Nigerian antimalarial plants and their anticancer potential: A review. J. Integr. Med. 2020, 18, 92–113. [Google Scholar] [CrossRef]
- Moga, M.A.; Dimienescu, O.G.; Bălan, A.; Dima, L.; Toma, S.I.; Bîgiu, N.F.; Blidaru, A. Pharmacological and therapeutic properties of Punica granatum phytochemicals: Possible roles in breast cancer. Molecules 2021, 26, 1054. [Google Scholar] [CrossRef]
- Melgarejo-Sánchez, P.; Núñez-Gómez, D.; Martínez-Nicolás, J.J.; Hernández, F.; Legua, P.; Melgarejo, P. Pomegranate variety and pomegranate plant part, relevance from bioactive point of view: A review. Bioresour. Bioprocess. 2021, 8, 2. [Google Scholar] [CrossRef]
- Devanesan, S.; AlSalhi, M.S.; Balaji, R.V.; Ranjitsingh, A.J.A.; Ahamed, A.; Alfuraydi, A.A.; AlQahtani, F.Y.; Aleanizy, F.S.; Othman, A.H. Antimicrobial and cytotoxicity effects of synthesized silver nanoparticles from Punica granatum peel extract. Nanoscale Res. Lett. 2018, 13, 315. [Google Scholar] [CrossRef] [PubMed]
- Suručić, R.; Tubić, B.; Stojiljković, M.P.; Djuric, D.M.; Travar, M.; Grabež, M.; Šavikin, K.; Škrbić, R. Computational study of pomegranate peel extract polyphenols as potential inhibitors of SARS-CoV-2 virus internalization. Mol. Cell. Biochem. 2021, 476, 1179–1193. [Google Scholar] [CrossRef]
- Pirzadeh, M.; Caporaso, N.; Rauf, A.; Shariati, M.A.; Yessimbekov, Z.; Khan, M.U.; Imran, M.; Mubarak, M.S. Pomegranate as a source of bioactive constituents: A review on their characterization, properties and applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 982–999. [Google Scholar] [CrossRef] [PubMed]
- Modaeinama, S.; Abasi, M.; Abbasi, M.M.; Jahanban-Esfahlan, R. Anti tumoral properties of Punica granatum (Pomegranate) peel extract on different human cancer cells. Asian Pac. J. Cancer Prev. 2015, 16, 5697–56701. [Google Scholar] [CrossRef] [PubMed]
- Malviya, S.; Arvind; Jha, A.; Hettiarachchy, N. Antioxidant and antibacterial potential of pomegranate peel extracts. J. Food Sci. Technol. 2014, 51, 4132–4137. [Google Scholar] [CrossRef]
- Rosas-Burgos, E.C.; Burgos-Hernández, A.; Noguera-Artiaga, L.; Kačániová, M.; Hernández-García, F.; Cárdenas-López, J.L.; Carbonell-Barrachina, A.A. Antimicrobial activity of pomegranate peel extracts as affected by cultivar. J. Sci. Food Agric. 2017, 97, 802–810. [Google Scholar] [CrossRef]
- Magangana, T.P.; Makunga, N.P.; Fawole, O.A.; Stander, M.A.; Opara, U.L. Antioxidant, antimicrobial, and metabolomic characterization of blanched pomegranate peel extracts: Effect of cultivar. Molecules 2022, 27, 2979. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Li, M.; Wen, J.; Ren, F.; Yang, Z.; Jiang, X.; Chen, Y. The bioactivity and applications of pomegranate peel extract: A review. J. Food Biochem. 2022, 46, e14105. [Google Scholar] [CrossRef]
- Mo, Y.; Ma, J.; Gao, W.; Zhang, L.; Li, J.; Li, J.; Zang, J. Pomegranate peel as a source of bioactive compounds: A mini review on their physiological functions. Front. Nutr. 2022, 9, 887113. [Google Scholar] [CrossRef]
- Sreekumar, S.; Sithul, H.; Muraleedharan, P.; Azeez, J.M.; Sreeharshan, S. Pomegranate fruit as a rich source of biologically active compounds. BioMed Res. Int. 2014, 2014, 686921. [Google Scholar] [CrossRef]
- Tripathi, S.M.; Singh, D.K. Molluscicidal activity of Punica granatum bark and Canna indica root. Braz. J. Med. Biol. Res. 2000, 33, 1351–1355. [Google Scholar] [CrossRef] [PubMed]
- Lavaee, F.; Motaghi, D.; Jassbi, A.R.; Jafarian, H.; Ghasemi, F.; Badiee, P. Antifungal effect of the bark and root extracts of Punica granatum on oral Candida isolates. Curr. Med. Mycol. 2018, 4, 20–24. [Google Scholar] [PubMed]
- Abeyrathne, E.; Nam, K.; Huang, X.; Ahn, D.U. Plant- and animal-based antioxidants’ structure, efficacy, mechanisms, and applications: A review. Antioxidants 2022, 11, 1025. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural polyphenols: Chemical classification, definition of classes, subcategories, and structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef]
- Gutiérrez-Del-Río, I.; López-Ibáñez, S.; Magadán-Corpas, P.; Fernández-Calleja, L.; Pérez-Valero, Á.; Tuñón-Granda, M.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Terpenoids and polyphenols as natural antioxidant agents in food preservation. Antioxidants 2021, 10, 1264. [Google Scholar] [CrossRef] [PubMed]
- Derakhshan, Z.; Ferrante, M.; Tadi, M.; Ansari, F.; Heydari, A.; Hosseini, M.S.; Conti, G.O.; Adrabad, E.K. Antioxidant activity and total phenolic content of ethanolic extract of pomegranate peels, juice and seeds. Food Chem. Toxicol. 2018, 114, 108–111. [Google Scholar] [CrossRef]
- El-Badawi, A.; Sulieman, A.; Abd-Allah, I.; Rabie, M.; Mostfa, D.M. Oxidative stability of edible oils via addition of pomegranate and orange peel extracts. Foods Raw Mater. 2018, 6, 413–420. [Google Scholar]
- Fazio, A.; Iacopetta, D.; La Torre, C.; Ceramella, J.; Muià, N.; Catalano, A.; Caroccib, A.; Sinicropia, M.S. Finding solutions for agricultural wastes: Antioxidant and antitumor properties of pomegranate Akko peel extracts and β-glucan recovery. Food Funct. 2018, 9, 6618–6631. [Google Scholar] [CrossRef]
- Hasnaoui, N.; Wathelet, B.; Jiménez-Araujo, A. Valorization of pomegranate peel from 12 cultivars: Dietary fibre composition, antioxidant capacity and functional properties. Food Chem. 2014, 160, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Benchagra, L.; Berrougui, H.; Islam, M.O.; Ramchoun, M.; Boulbaroud, S.; Hajjaji, A.; Fulop, T.; Ferretti, G.; Khalil, A. Antioxidant effect of Moroccan pomegranate (Punica granatum L. Sefri Variety) extracts rich in punicalagin against the oxidative stress process. Foods 2021, 10, 2219. [Google Scholar] [CrossRef] [PubMed]
- Mastrogiovanni, F.; Bernini, R.; Basiricò, L.; Bernabucci, U.; Campo, M.; Romani, A.; Santi, L.; Lacetera, N. Antioxidant and anti-inflammatory effects of pomegranate peel extracts on bovine mammary epithelial cells BME-UV1. Nat. Prod. Res. 2020, 34, 1465–1469. [Google Scholar] [CrossRef] [PubMed]
- Keta, O.; Deljanin, M.; Petkovic, V.; Zdunić, G.; Janković, T.; Živković, J.; Ristić-Fira, A.; Petrović, I.; Šavikin, K. Pomegranate (Punica granatum L.) peel extract: Potential cytotoxic agent against different cancer cell lines. Rec. Nat. Prod. 2020, 14, 326–339. [Google Scholar] [CrossRef]
- Magangana, T.P.; Makunga, N.P.; Fawole, O.A.; Opara, U.L. Processing factors affecting the phytochemical and nutritional properties of pomegranate (Punica granatum L.) peel waste: A review. Molecules 2020, 25, 4690. [Google Scholar] [CrossRef]
- Campos, L.; Seixas, L.; Henriques, M.H.F.; Peres, A.M.; Veloso, A.C.A. Pomegranate peels and seeds as a source of phenolic compounds: Effect of cultivar, by-product, and extraction solvent. Int. J. Food Sci. 2022, 2022, 9189575. [Google Scholar] [CrossRef]
- Arkan, A.A.T.; Taiba, F.A.M. A chemical study by using GC-Mass spectrometry of the peel and seeds of Punica Granatum L. plant. SRP 2021, 12, 1414–1421. [Google Scholar]
- Russo, M.; Fanali, C.; Tripodo, G.; Dugo, P.; Muleo, R.; Dugo, L.; Gara, L.D.; Mondello, L. Analysis of phenolic compounds in different parts of pomegranate (Punica granatum) fruit by HPLC-PDA-ESI/MS and evaluation of their antioxidant activity: Application to different Italian varieties. Anal. Bioanal. Chem. 2018, 410, 3507–3520. [Google Scholar] [CrossRef]
- Laaraj, N.; Bouhrim, M.; Kharchoufa, L.; Tiji, S.; Bendaha, H.; Addi, M.; Drouet, S.; Hano, C.; Lorenzo, J.M.; Bnouham, M.; et al. Phytochemical analysis, α-Glucosidase and α-Amylase inhibitory activities and acute toxicity studies of extracts from pomegranate (Punica granatum) bark, a valuable agro-industrial by-product. Foods 2022, 11, 1353. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, S.M.; El-Shafea, A.Y.M.; Saleh, W.D.; Fathy, H.M. Chemical profiling, in vitro antimicrobial and antioxidant activities of pomegranate, orange and banana peel-extracts against pathogenic microorganisms. J. Genet. Eng. Biotechnol. 2021, 19, 80. [Google Scholar] [CrossRef]
- Menegazzo, F.; Ghedini, E.; Signoretto, M. 5-Hydroxymethylfurfural (HMF) production from real biomasses. Molecules 2018, 23, 2201. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chen, J.; Su, J.; Li, L.; Hu, S.; Li, B.; Zhang, X.; Xu, Z.; Chen, T. In vitro antioxidant and antiproliferative activities of 5-hydroxymethylfurfural. J. Agric. Food Chem. 2013, 6, 10604–10611. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, K.; Ramanathan, T. Antiquorum sensing and biofilm potential of 5-Hydroxymethylfurfural against Gram positive pathogens. Microb. Pathog. 2018, 125, 48–50. [Google Scholar] [CrossRef]
- Vijayakumar, K.; Ramanathan, T. Musa acuminata and its bioactive metabolite 5-Hydroxymethylfurfural mitigates quorum sensing (las and rhl) mediated biofilm and virulence production of nosocomial pathogen Pseudomonas aeruginosa in vitro. J. Ethnopharmacol. 2020, 10, 112242. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, K.; Thirunanasambandham, R. 5-Hydroxymethylfurfural inhibits Acinetobacter baumannii biofilms: An in vitro study. Arch. Microbiol. 2021, 203, 673–682. [Google Scholar] [CrossRef]
- Gu, H.; Jiang, Z.; Wang, M.; Jiang, H.; Zhao, F.; Ding, X.; Cai, B.; Zhan, Z. 5-Hydroxymethylfurfural from wine-processed Fructus corni inhibits hippocampal neuron apoptosis. Neural Regen. Res. 2013, 8, 2605–2614. [Google Scholar]
- Kong, F.; Lee, B.H.; Wei, K. 5-Hydroxymethylfurfural mitigates lipopolysaccharide-stimulated inflammation via suppression of MAPK, NF-κB and mTOR Activation in RAW 264.7 cells. Molecules 2019, 24, 275. [Google Scholar] [CrossRef]
- Ersus, S.; Akyüz, A.; Tekin, I. Hydroxymethyl furfural formation in grape and pomegranate juices over heating treatments. In Proceedings of the 1st International/11th National Food Engineering Congress, Antalya, Turkey, 7–9 December 2019. [Google Scholar]
- Yassin, M.T.; Mostafa, A.A.; Askar, A.A.A. In vitro evaluation of biological activities and phytochemical analysis of different solvent extracts of Punica granatum L. (Pomegranate) peels. Plants 2021, 10, 2742. [Google Scholar] [CrossRef]
- Chai, W.M.; Liu, X.; Hu, Y.H.; Feng, H.L.; Jia, Y.L.; Guo, Y.J.; Zhou, H.T.; Hen, Q.X. Antityrosinase and antimicrobial activities of furfuryl alcohol, furfural and furoic acid. Int. J. Biol. Macromol. 2013, 57, 151–155. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Buzón-Durán, L.; Cuchí-Oterino, J.A.; Martín-Gil, J.; Lorenzo-Vidal, B.; Martín-Ramos, P. Dwarf pomegranate (Punica granatum L. var. nana): Source of 5-HMF and bioactive compounds with applications in the protection of woody crops. Plants 2022, 11, 550. [Google Scholar] [CrossRef]
- Nasr, M. Development of an optimized hyaluronic acid-based lipidic nanoemulsion co-encapsulating two polyphenols for nose to brain delivery. Drug Deliv. 2016, 23, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Bintsis, T. Foodborne pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef] [PubMed]
- Peršurić, Ž.; Martinović, L.S.; Malenica, M.; Gobin, I.; Pedisić, S.; Dragović-Uzelac, V.; Pavelić, S.K. Assessment of the biological activity and phenolic composition of ethanol extracts of pomegranate (Punica granatum L.) peels. Molecules 2020, 25, 5916. [Google Scholar] [CrossRef] [PubMed]
- Al-Zoreky, N.S. Antimicrobial activity of pomegranate (Punica granatum L.) fruit peels. Int. J. Food Microbiol. 2009, 134, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.G.; Kang, O.H.; Lee, Y.S.; Chae, H.S.; Oh, Y.C.; Brice, O.O.; Kim, M.S.; Sohn, D.H.; Kim, H.S.; Park, H.; et al. In vitro and in vivo antibacterial activity of Punica granatum peel ethanol extract against Salmonella. Evid.-Based Complement. Altern. Med. 2011, 2011, 690518. [Google Scholar]
- Pagliarulo, C.; De Vito, V.; Picariello, G.; Colicchio, R.; Pastore, G.; Salvatore, P.; Volpe, M.G. Inhibitory effect of pomegranate (Punica granatum L.) polyphenol extracts on the bacterial growth and survival of clinical isolates of pathogenic Staphylococcus aureus and Escherichia coli. Food Chem. 2016, 190, 824–831. [Google Scholar] [CrossRef]
- Bandele, O.J.; Clawson, S.J.; Osheroff, N. Dietary polyphenols as topoisomerase II poisons: B ring and C ring substituents determine the mechanism of enzyme-mediated DNA cleavage enhancement. Chem. Res. Toxicol. 2008, 21, 1253–1260. [Google Scholar] [CrossRef]
- Yang, N.J.; Hinner, M.J. Getting across the cell membrane: An overview for small molecules, peptides, and proteins. Methods Mol. Biol. 2015, 1266, 29–53. [Google Scholar]
- Yoda, Y.; Hu, Z.Q.; Zhao, W.H.; Shimamura, T. Different susceptibilities of Staphylococcus and Gram-negative rods to epigallocatechin gallate. J. Infect. Chemother. 2004, 10, 55–58. [Google Scholar] [CrossRef]
- Nakayama, M.; Shimatani, K.; Ozawa, T.; Shigemune, N.; Tsugukuni, T.; Tomiyama, D.; Kurahachi, M.; Nonaka, A.; Miyamoto, T. A study of the antibacterial mechanism of catechins: Isolation and identification of Escherichia coli cell surface proteins that interact with epigallocatechin gallate. Food Control 2013, 33, 433–439. [Google Scholar] [CrossRef]
- Mori, A.; Nishino, C.; Enoki, N.; Tawata, S. Antibacterial activity and mode of action of plant flavonoids against Proteus vulgaris and Staphylococcus aureus. Phytochemistry 1987, 26, 2231–2234. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Rao, S.; Sun, J.; Ma, C.; Li, J. p-Coumaric acid kills bacteria through dual damage mechanisms. Food Control 2012, 25, 550–554. [Google Scholar] [CrossRef]
- Zhao, W.H.; Hu, Z.Q.; Okubo, S.; Hara, Y.; Shimamura, T. Mechanism of synergy between epigallocatechin gallate and beta-lactams against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2001, 45, 1737–1742. [Google Scholar] [CrossRef]
- Ollila, F.; Halling, K.; Vuorela, P.; Vuorela, H.; Slotte, J.P. Characterization of flavonoid–biomembrane interactions. Arch. Biochem. Biophys. 2002, 399, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Dadi, P.K.; Ahmad, M.; Ahmad, Z. Inhibition of ATPase activity of Escherichia coli ATP synthase by polyphenols. Int. J. Biol. Macromol. 2009, 45, 72–79. [Google Scholar] [CrossRef]
- Packiavathy, I.A.; Priya, S.; Pandian, S.K.; Ravi, A.V. Inhibition of biofilm development of uropathogens by curcumin—An anti-quorum sensing agent from Curcuma longa. Food Chem. 2014, 148, 453–460. [Google Scholar] [CrossRef]
- Gopu, V.; Meena, C.; Shetty, P.H. Quercetin influences quorum sensing in food borne bacteria: In-vitro and in-silico evidence. PLoS ONE 2015, 10, e0134684. [Google Scholar] [CrossRef]
- Joel, O.O.; Maharjan, R. Effects of 5-hydroxymethylfurfural isolated from Cola hispida on oral adenosquamous carcinoma and MDR Staphylococcus aureus. JMPHTR 2021, 8, 1–7. [Google Scholar]
- Wafa, B.A.; Makni, M.; Ammar, S.; Khannous, L.; Hassana, A.B.; Bouaziz, M.; Es-Safi, N.E.; Gdoura, R. Antimicrobial effect of the Tunisian Nana variety Punica granatum L. extracts against Salmonella enterica (serovars Kentucky and Enteritidis) isolated from chicken meat and phenolic composition of its peel extract. Int. J. Food Microbiol. 2017, 241, 123–131. [Google Scholar] [CrossRef]
- Gullón, P.; Astray, G.; Gullón, B.; Tomasevic, I.; Lorenzo, J.M. Pomegranate peel as suitable source of high-added value bioactives: Tailored functionalized meat products. Molecules 2020, 25, 2859. [Google Scholar] [CrossRef]
- Sineh Sepehr, K.; Baradaran, B.; Mazandarani, M.; Khori, V.; Shahneh, F.Z. Studies on the cytotoxic activities of Punica granatum L. var. spinosa (Apple Punice) extract on prostate cell line by induction of apoptosis. ISRN Pharm. 2012, 2012, 547942. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, G. Targeting the Ras/Raf/MEK/ERK pathway in hepatocellular carcinoma. Oncol. Lett. 2017, 13, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Maik-Rachline, G.; Hacohen-Lev-Ran, A.; Seger, R. Nuclear ERK: Mechanism of translocation, substrates, and role in cancer. Int. J. Mol. Sci. 2019, 20, 1194. [Google Scholar] [CrossRef]
- Degirmenci, U.; Wang, M.; Hu, J. Targeting aberrant RAS/RAF/MEK/ERK signaling for cancer therapy. Cells 2020, 9, 198. [Google Scholar] [CrossRef]
- Vališ, K.; Novák, P. Targeting ERK-Hippo interplay in cancer therapy. Int. J. Mol. Sci. 2020, 21, 3236. [Google Scholar] [CrossRef] [PubMed]
- Boonmasawai, S.; Leesombun, A.; Chaichoun, K.; Taowan, J.; Sariya, L.; Thongjuy, O. Effects of the three flavonoids; kaempferol, quercetin, and myricetin on Baby hamster kidney (BHK-21) cells and Human hepatocellular carcinoma cell (HepG2) cells proliferations and total Erk1/2 protein expression. J. Appl. Anim. Sci. 2017, 10, 23–34. [Google Scholar]
- Sheridan, C.; Brumatti, G.; Elgendy, M.; Brunet, M.; Martin, S.J. An ERK-dependent pathway to Noxa expression regulates apoptosis by platinum-based chemotherapeutic drugs. Oncogene 2010, 29, 6428–6441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boonmasawai, S.; Sariya, L.; Leesombun, A.; Chaichoun, K.; Taowan, J.; Thongjuy, O. Anti-proliferative and total ERK1/2 inhibitory effects of plant flavonols on Human cervical cancer (HeLa) cells. TJVM 2018, 48, 541–549. [Google Scholar]
- Li, H.; Shi, B.; Li, Y.; Yin, F. Polydatin inhibits cell proliferation and induces apoptosis in laryngeal cancer and HeLa cells via suppression of the PDGF/AKT signaling pathway. J. Biochem. Mol. Toxicol. 2017, 31, e21900. [Google Scholar] [CrossRef]
- Yang, T.; Shi, X.; Kang, Y.; Zhu, M.; Fan, M.; Zhang, D.; Zhang, Y. Novel compounds TAD-1822-7-F2 and F5 inhibited HeLa cells growth through the JAK/Stat signaling pathway. Biomed. Pharmacother. 2018, 103, 118–126. [Google Scholar] [CrossRef]
- Xia, X.; Xiang, X.; Huang, F.; Zheng, M.; Zhang, Z.; Han, L. Dietary canolol induces apoptosis in human cervical carcinoma HeLa cells through ROS-MAPK mediated mitochondrial signaling pathway: In vitro and in vivo. Chem. Biol. Interact. 2019, 300, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.; Liem, A.; Miller, J.A.; Tannenbaum, S.R. 5-Sulfooxymethylfurfural as a possible ultimate mutagenic and carcinogenic metabolite of the Maillard reaction product, 5-hydroxymethylfurfural. Carcinogenesis 1994, 15, 2375–2377. [Google Scholar] [CrossRef] [PubMed]
- Florian, S.; Bauer-Marinovic, M.; Taugner, F.; Dobbernack, G.; Monien, B.H.; Meinl, W.; Glatt, H. Study of 5-hydroxymethylfurfural and its metabolite 5-sulfooxymethylfurfural on induction of colonic aberrant crypt foci in wild-type mice and transgenic mice expressing human sulfotransferases 1A1 and 1A2. Mol. Nutr. Food Res. 2012, 56, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Uchida, R.; Kato, M.; Hattori, Y.; Kikuchi, H.; Watanabe, E.; Kobayashi, K.; Nishida, K. Identification of 5-Hydroxymethylfurfural (5-HMF) as an active component Citrus Jabara that suppresses FcεRI-mediated mast cell activation. Int. J. Mol. Sci. 2020, 21, 2472. [Google Scholar] [CrossRef] [PubMed]
- Matić, P.; Sabljić, M.; Jakobek, L. Validation of spectrophotometric methods for the determination of total polyphenol and total flavonoid content. J. AOAC Int. 2019, 100, 1795–1803. [Google Scholar] [CrossRef]
- CLSI Approved Standard M100-S15; Clinical and Laboratory Standards. Performance Standards for Antimicrobial Susceptibility Testing. Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2018.
- Elshikh, M.; Ahmed, S.; Funston, S.; Dunlop, P.; McGaw, M.; Marchant, R.; Banat, I.M. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol. Lett. 2016, 38, 1015–1019. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Orellana, E.A.; Kasinski, A.L. Sulforhodamine B (SRB) assay in cell culture to investigate cell proliferation. Bio-Protocol 2016, 6, e1984. [Google Scholar] [CrossRef] [Green Version]





| Samples | Total Phenolic Content | Flavonoid Content | DPPH• Assay |
|---|---|---|---|
| (mg GAE/g sample) | (mg QE/g sample) | IC50 (µg/mL) | |
| PBE | 574.64 | 52.98 | 4.10 |
| PPE | 242.60 | 23.08 | 9.60 |
| No. | Retention Time (min) | Compound | Peak Area (%) | Similarity Index (%) |
|---|---|---|---|---|
| 1 | 5.15 | Decane | 9.53 | 76 |
| 2 | 5.24 | Cyclotetrasiloxane, octamethyl- | 9.43 | 86 |
| 3 | 6.54 | Undecane | 8.26 | 93 |
| 4 | 7.31 | Cyclopentasiloxane, decamethyl- | 4.00 | 78 |
| 5 | 8.53 | 5-Hydroxymethylfurfural | 23.76 | 93 |
| 6 | 9.41 | Phenol, 2-methyl-5-(1-methylethyl)- | 15.31 | 90 |
| 7 | 9.64 | Cyclohexasiloxane, dodecamethyl- | 5.59 | 72 |
| 8 | 10.07 | Pseudopelletierine | 3.53 | 72 |
| 9 | 12.05 | 2,4-Di-tert-butylphenol | 11.91 | 95 |
| 10 | 16.48 | Hexadecanoic acid, methyl ester | 6.51 | 93 |
| Total | 97.83 |
| No. | Retention Time (min) | Compound | Peak Area (%) | Similarity Index (%) |
|---|---|---|---|---|
| 1 | 3.70 | Furfural | 8.13 | 72 |
| 2 | 4.18 | 4-Cyclopentene-1,3-dione | 3.83 | 72 |
| 3 | 5.27 | Cyclotetrasiloxane, octamethyl- | 6.86 | 86 |
| 4 | 7.35 | Cyclopentasiloxane, decamethyl- | 5.33 | 91 |
| 5 | 8.50 | 5-Hydroxymethylfurfural | 33.19 | 93 |
| 6 | 8.84 | Benzene, 1,3-bis(1,1-dimethylethyl)- | 8.40 | 74 |
| 7 | 9.46 | Phenol, 2-methyl-5-(1-methylethyl)- | 12.62 | 90 |
| 8 | 9.69 | Cyclohexasiloxane, dodecamethyl- | 6.98 | 87 |
| 9 | 12.10 | 2,4-Di-tert-butylphenol | 6.09 | 95 |
| 10 | 16.52 | Hexadecanoic acid, methyl ester | 4.80 | 98 |
| Total | 96.23 |
| Bacterial Strains | PBE | PPE |
|---|---|---|
| MIC (mg/mL) | MIC (mg/mL) | |
| E. coli ATCC 25922 | 6.25 | 25 |
| S. aureus ATCC 29213 | 1.56 | 6.25 |
| S. Enteritidis ATCC 13076 | 1.56 | 3.125 |
| S. Typhimurium ATCC 14028. | 3.125 | 3.125 |
| Plant Extracts | IC50 Value (µg/mL) | |||
|---|---|---|---|---|
| HeLa | HepG2 | |||
| MTT Assay | SRB Assay | MTT Assay | SRB Assay | |
| PBE | 57.5 | 52.8 | 18.6 | 15.9 |
| PPE | 63.2 | 71.4 | 19.5 | 24.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leesombun, A.; Sariya, L.; Taowan, J.; Nakthong, C.; Thongjuy, O.; Boonmasawai, S. Natural Antioxidant, Antibacterial, and Antiproliferative Activities of Ethanolic Extracts from Punica granatum L. Tree Barks Mediated by Extracellular Signal-Regulated Kinase. Plants 2022, 11, 2258. https://doi.org/10.3390/plants11172258
Leesombun A, Sariya L, Taowan J, Nakthong C, Thongjuy O, Boonmasawai S. Natural Antioxidant, Antibacterial, and Antiproliferative Activities of Ethanolic Extracts from Punica granatum L. Tree Barks Mediated by Extracellular Signal-Regulated Kinase. Plants. 2022; 11(17):2258. https://doi.org/10.3390/plants11172258
Chicago/Turabian StyleLeesombun, Arpron, Ladawan Sariya, Jarupha Taowan, Chowalit Nakthong, Orathai Thongjuy, and Sookruetai Boonmasawai. 2022. "Natural Antioxidant, Antibacterial, and Antiproliferative Activities of Ethanolic Extracts from Punica granatum L. Tree Barks Mediated by Extracellular Signal-Regulated Kinase" Plants 11, no. 17: 2258. https://doi.org/10.3390/plants11172258
APA StyleLeesombun, A., Sariya, L., Taowan, J., Nakthong, C., Thongjuy, O., & Boonmasawai, S. (2022). Natural Antioxidant, Antibacterial, and Antiproliferative Activities of Ethanolic Extracts from Punica granatum L. Tree Barks Mediated by Extracellular Signal-Regulated Kinase. Plants, 11(17), 2258. https://doi.org/10.3390/plants11172258