Amaryllidaceae Alkaloids from Clivia miniata (Lindl.) Bosse (Amaryllidaceae): Isolation, Structural Elucidation, and Biological Activity
Abstract
:1. Introduction
2. Results and Discussion
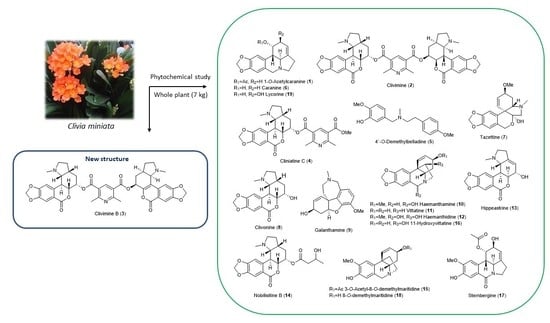
2.1. Phytochemical Study of Clivia miniata
2.2. Biological Activity of Isolated Amaryllidaceae Alkaloids
3. Conclusions
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Extraction and Isolation of Alkaloids
4.4. Inhibition of hAChE and hBuChE
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nair, J.J.; van Staden, J. Antiprotozoal alkaloid principles of the plant family Amaryllidaceae. Bioorg. Med. Chem. Lett. 2019, 29, 126642. [Google Scholar] [CrossRef] [PubMed]
- Cahlíková, L.; Kawano, I.; Řezáčová, M.; Blunden, G.; Hulcová, D.; Havelek, R. The Amaryllidaceae alkaloids haemanthamine, haemanthidine and their semisynthetic derivatives as potential drugs. Phytochem. Rev. 2021, 20, 303–323. [Google Scholar] [CrossRef]
- Nair, J.J.; Van Staden, J. The Amaryllidaceae, a chemically and biologically privileged plant family. S. Afr. J. Bot. 2021, 136, 18. [Google Scholar] [CrossRef]
- Al Shammari, L.; Hulcová, D.; Maříková, J.; Kučera, T.; Šafratová, M.; Nováková, L.; Schmidt, M.; Pulkrábková, L.; Janoušek, J.; Soukup, O.; et al. Amaryllidaceae alkaloids from Hippeastrum X Hybridum CV. Ferrari, and preparation of vittatine derivatives as potential ligands for Alzheimer’s disease. S. Afr. J. Bot. 2021, 136, 137–146. [Google Scholar] [CrossRef]
- Konrath, E.L.; Passos, C.D.S.; Klein-Júnior, L.C.; Henriques, A.T. Alkaloids as a source of potential anticholinesterase inhibitors for the treatment of Alzheimer’s disease. J. Pharm. Pharmacol. 2013, 65, 1701–1725. [Google Scholar] [CrossRef] [PubMed]
- Crouch, N.R.; Mulholland, D.A.; Pohl, T.L.; Ndlovu, E.; van Wyk, B.E. The ethnobotany and chemistry of the genus Clivia (Amaryllidaceae). S. Afr. J. Bot. 2003, 69, 144–147. [Google Scholar] [CrossRef] [Green Version]
- Musara, C.; Aladejana, E.B.; Aladejana, A.E. Clivia miniata (Lindl.) Bosse, (Amaryllidaceae): Botany, medicinal uses, phytochemistry and pharmacological properties. J. Appl. Pharm. Sci. 2021, 11, 012–018. [Google Scholar] [CrossRef]
- Omoruyi, S.I.; Delport, J.; Kangwa, T.S.; Ibrakaw, A.S.; Cupido, C.N.; Ekpo, O.E.; Hussein, A.A. In vitro neuroprotective potential of Clivia miniata and Nerine humilis (Amaryllidaceae) in MPP+-induced neuronal toxicity in SH-SY5Y neuroblastoma cells. S. Afr. J. Bot. 2021, 136, 110–117. [Google Scholar] [CrossRef]
- Rasethe, M.T.; Semenya, S.S.; Maroyi, A. Medicinal plants traded in informal herbal medicine markets of the Limpopo Province, South Africa. Evid.-Based Complement. Altern. Med. 2019, 2019, 2609532. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.A.; Ross, S.A.; El-Moghazy, A.M.; El-Moghazy, S.A. Clivonidine, a new alkaloid from Clivia miniata. J. Nat. Prod. 1983, 46, 350–352. [Google Scholar] [CrossRef]
- Hirasawa, Y.; Tanaka, T.; Hirasawa, S.; Wong, C.P.; Uchiyama, N.; Kaneda, T.; Goda, Y.; Morita, H. Cliniatines A–C, new Amaryllidaceae alkaloids from Clivia miniata, inhibiting Acetylcholinesterase. J. Nat. Med. 2022, 76, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Döpke, W.; Bienert, M.; Burlingame, A.L.; Schnoes, H.K.; Jeffs, P.W.; Farrier, D.S. Structures and stereochemistry of clivonine and clivimine. Tetrahedron Lett. 1967, 8, 451–457. [Google Scholar] [CrossRef]
- Döpke, W.; Bienert, M. Struktur und stereochemie des miniatins. Tetrahedron Lett. 1970, 11, 745–747. [Google Scholar] [CrossRef]
- Ieven, M.; Vlietinick, A.J.; Berghe, D.V.; Totte, J.; Dommisse, R.; Esmans, E.; Alderweireldt, F. Plant antiviral agents. III. Isolation of alkaloids from Clivia miniata Regel (Amaryllidaceae). J. Nat. Prod. 1982, 45, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.S.; Shen, L.L.; Zhu, C.; Bu, X.L.; Liu, Y.H.; Liu, C.H.; Yao, X.Q.; Zhang, L.L.; Zhou, H.D.; Walker, D.G.; et al. Brain-derived neurotrophic factor protects against tau-related neurodegeneration of Alzheimer’s disease. Transl. Psychiatry 2016, 6, e907. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Zhang, Y.; Sterling, K.; Song, W. Brain-derived neurotrophic factor in Alzheimer’s disease and its pharmaceutical potential. Transl. Neurodegener. 2022, 11, 4. [Google Scholar] [CrossRef]
- De Vita, D.; Pandolfi, F.; Ornano, L.; Feroci, M.; Chiarotto, I.; Sileno, I.; Pepi, F.; Costi, R.; Di Santo, R.; Scipione, L. New N, N-dimethylcarbamate inhibitors of acetylcholinesterase: Design synthesis and biological evaluation. J. Enzyme Inhib. Med. Chem. 2016, 31, 106–113. [Google Scholar] [CrossRef] [Green Version]
- Al Mamun, A.; Maříková, J.; Hulcová, D.; Janoušek, J.; Šafratová, M.; Nováková, L.; Kučera, T.; Hrabinová, M.; Kuneš, J.; Korábečný, J.; et al. Amaryllidaceae alkaloids of belladine-type from Narcissus pseudonarcissus cv. Carlton as new selective inhibitors of butyrylcholinesterase. Biomolecules 2020, 10, 800. [Google Scholar] [CrossRef]
- Hulcová, D.; Maříková, J.; Korábečný, J.; Hošťálková, A.; Jun, D.; Kuneš, J.; Chlebek, J.; Opletal, L.; De Simone, A.; Novakova, L.; et al. Amaryllidaceae alkaloids from Narcissus pseudonarcissus L. cv. Dutch Master as potential drugs in treatment of Alzheimer’s disease. Phytochemistry 2019, 165, 112055. [Google Scholar] [CrossRef]
- Vaněčková, N.; Hošt‘álková, A.; Šafratová, M.; Kuneš, J.; Hulcová, D.; Hrabinová, M.; Doskočil, I.; Štěpánková, Š.; Opletal, L.; Nováková, L.; et al. Isolation of Amaryllidaceae alkaloids from Nerine bowdenii W. Watson and their biological activities. RSC Adv. 2016, 6, 80114–80120. [Google Scholar] [CrossRef]
- Cho, N.; Du, Y.; Valenciano, A.L.; Fernández-Murga, M.L.; Goetz, M.; Clement, J.; Cassera, M.B.; Kingston, D.G. Antiplasmodial alkaloids from bulbs of Amaryllis belladonna Steud. Bioorg. Med. Chem. Lett. 2018, 28, 40–42. [Google Scholar] [CrossRef] [PubMed]
- Lamoral-Theys, D.; Andolfi, A.; Van Goietsenoven, G.; Cimmino, A.; Le Calvé, B.; Wauthoz, N.; Mégalizzi, V.; Gras, T.; Bruyère, C.; Dubois, J.; et al. Lycorine, the main phenanthridine Amaryllidaceae alkaloid, exhibits significant antitumor activity in cancer cells that display resistance to proapoptotic stimuli: An investigation of structure-activity relationship and mechanistic insight. J. Med. Chem. 2009, 52, 6244–6256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, J.D.; Crain, W.O., Jr.; Wildman, W.C. Nuclear magnetic resonance spectroscopy. Carbon-13 spectra of nicotine, quinine, and some Amaryllidaceae alkaloids. J. Am. Chem. Soc. 1971, 93, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.K.; Lin, F.H.; Tseng, L.H.; Jiang, C.L.; Lee, S.S. Comprehensive study of alkaloids from Crinum asiaticum var. sinicum assisted by HPLC-DAD-SPE-NMR. J. Nat. Prod. 2011, 74, 411–419. [Google Scholar] [CrossRef]
- Chen, J.Q.; Xie, J.H.; Bao, D.H.; Liu, S.; Zhou, Q.L. Total synthesis of (−)-galanthamine and (−)-lycoramine via catalytic asymmetric hydrogenation and intramolecular reductive Heck cyclization. Org. Lett. 2012, 14, 2714–2717. [Google Scholar] [CrossRef]
- Das, M.K.; Kumar, N.; Bisai, A. Catalytic asymmetric total syntheses of naturally occurring Amarylidaceae alkaloids,(−)-crinine,(−)-epi-crinine,(−)-oxocrinine,(+)-epi-elwesine,(+)-vittatine, and (+)-epi-vittatine. Org. Lett. 2018, 20, 4421–4424. [Google Scholar] [CrossRef]
- Bastida, J.; Lavilla, R.; Viladomat, F. Chapter 3 chemical and biological aspects of Narcissus. In Alkaloids. The Alkaloids: Chemistry and Biology, 1st ed.; Cordell, A.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 63, pp. 87–179. [Google Scholar] [CrossRef]
- Jeffs, P.W.; Abou-Donia, A.; Campau, D.; Staiger, D. Structures of 9-O-demethylhomolycorine and 5α-hydroxyhomolycorine. alkaloids of Crinum defixum, C. scabrum, and C. latifolium. Assignment of aromatic substitution patterns from 1H-coupled 13C spectra. J. Org. Chem. 1985, 50, 1732–1737. [Google Scholar] [CrossRef]
- Evidente, A.; Abou-Donia, A.H.; Darwish, F.A.; Amer, M.E.; Kassem, F.F.; Hammoda, H.A.; Motta, A. Nobilisitine A and B, two masanane-type alkaloids from Clivia nobilis. Phytochemistry 1999, 51, 1151–1155. [Google Scholar] [CrossRef]
- Lan, P.; Banwell, M.G.; Willis, A.C. Application of electrocyclic ring-opening and desymmetrizing nucleophilic trappings of meso-6,6-dibromobicyclo [3.1.0] hexanes to total syntheses of crinine and haemanthamine alkaloids. J. Org. Chem. 2019, 84, 3431–3466. [Google Scholar] [CrossRef]
- Evidente, A.; Iasiello, I.; Randazzo, G. Isolation of sternbergine, a new alkaloid from bulbs of Sternbergia lutea. J. Nat. Prod. 1984, 47, 1003–1008. [Google Scholar] [CrossRef]
- Pabuççuoglu, V.; Richomme, P.; Gözler, T.; Kivçak, B.; Freyer, A.J.; Shamma, M. Four new crinine-type alkaloids from Sternbergia species. J. Nat. Prod. 1989, 52, 785–791. [Google Scholar] [CrossRef]
- Wang, C.D.; Chen, Q.; Shin, S.; Cho, C.G. Total Synthesis of (±)-Clivonine via Diels–Alder Reactions of 3,5-Dibromo-2-pyrone. J. Org. Chem. 2020, 85, 10035–10049. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Banwell, M.G.; Willis, A.C.; Ward, J.S. A chemoenzymatic route to the (+)-form of the amaryllidaceae alkaloid narseronine. Aust. J. Chem. 2014, 68, 241–247. [Google Scholar] [CrossRef]
- Schwartz, B.D.; Jones, M.T.; Banwell, M.G.; Cade, I.A. Synthesis of the enantiomer of the structure assigned to the natural product nobilisitine A. Org. Lett. 2010, 12, 5210–5213. [Google Scholar] [CrossRef] [PubMed]
- Lodewyk, M.W.; Tantillo, D.J. Prediction of the structure of nobilisitine A using computed NMR chemical shifts. J. Nat. Prod. 2011, 74, 1339–1343. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, B.D.; White, L.V.; Banwell, M.G.; Willis, A.C. Structure of the lycorinine alkaloid nobilisitine A. J. Org. Chem. 2011, 76, 8560–8563. [Google Scholar] [CrossRef] [PubMed]
- Breiterová, K.; Koutová, D.; Maříková, J.; Havelek, R.; Kuneš, J.; Majorošová, M.; Opletal, L.; Hošťálková, A.; Jenčo, J.; Řezáčová, M.; et al. Amaryllidaceae alkaloids of different structural types from Narcissus L. cv. professor einstein and their cytotoxic activity. Plants 2020, 9, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cahlíková, L.; Hrabinová, M.; Kulhánková, A.; Benešová, N.; Chlebek, J.; Jun, D.; Novák, Z.; Macáková, K.; Kuneš, J.; Kuča, K.; et al. Alkaloids from Chlidanthus fragrans and their acetylcholinesterase, butyrylcholinesterase and prolyl oligopeptidase activities. Nat. Prod. Commun. 2013, 8, 1541–1545. [Google Scholar] [CrossRef] [Green Version]
- Šafratová, M.; Hošťálková, A.; Hulcová, D.; Breiterová, K.; Hrabcová, V.; Machado, M.; Fontinha, D.; Prudêncio, M.; Kuneš, J.; Chlebek, J.; et al. Alkaloids from Narcissus poeticus cv. Pink Parasol of various structural types and their biological activity. Arch. Pharm. Res. 2018, 41, 208–218. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]





| Position | 3 | ||
|---|---|---|---|
| δH | δC | Type | |
| 2 | 3.46–3.38, m; 2.57–2.46, m | 52.5 | CH2 |
| 3 | 2.08–1.97, m | 30.8 | CH2 |
| 3a | 2.44–2.35, m | 32.8 | CH |
| 4 | 2.44–2.35, m; 1.93–1.89, m | 27.8 | CH2 |
| 5 | 5.83, ddd (3.1, 3.1, 2.9) | 70.5 | CH |
| 5a | 4.38, dd (12.7, 2.9) | 79.0 | CH |
| 7 | 164.2 | C=O | |
| 7a | 119.4 | C | |
| 8 | 7.66, s | 109.1 | CH |
| 9 | 147.2 | C | |
| 10 | 152.8 | C | |
| 11 | 7.78, s | 107.6 | CH |
| 11a | 141.0 | C | |
| 11b | 3.39, dd (12.7, 10.0) | 34.9 | CH |
| 11c | 2.83, dd (10.0, 6.5) | 69.6 | CH |
| 12 | 2.33, s | 44.5 | CH3 |
| 13 | 6.14, d, overlap (3.9); 6.14, d, overlap (3.9) | 102.6 | CH2 |
| 2′ | 3.07–2.98, m; 2.57–2.46, m | 54.4 | CH2 |
| 3′ | 1.97–1.93, m; 1.53–1.44, m | 29.0 | CH2 |
| 3′a | 2.44–2.35, m | 36.1 | CH |
| 4′ | 2.35–2.28, m; 2.14–2.08, m | 32.2 | CH2 |
| 5′ | 6.20, dd (8.4, 6.2) | 69.3 | CH |
| 5′a | 149.7 | C | |
| 7′ | 160.9 | C=O | |
| 7′a | 116.5 | C | |
| 8′ | 7.84, s | 107.4 | CH |
| 9′ | 148.9 | C | |
| 10′ | 154.1 | C | |
| 11′ | 7.45, s | 103.7 | CH |
| 11′a | 135.2 | C | |
| 11′b | 112.6 | C | |
| 11′c | 3.57, d (5.3) | 60.8 | CH |
| 12′ | 2.30, s | 41.1 | CH3 |
| 13′ | 6.23, d, overlap (6.5); 6.23, d, overlap (6.5) | 103.3 | CH2 |
| 2″ | 163.41 or 163.38 | C | |
| 3″ | 122.78 or 122.53 | C | |
| 4″ | 8.84, s | 140.5 | CH |
| 5″ | 122.78 or 122.53 | C | |
| 6″ | 163.41 or 163.38 | C | |
| 7″ | 3.01, s or 3.00, s | 25.33 or 25.29 | CH3 |
| 8″ | 164.7 | C | |
| 9″ | 165.1 | C | |
| 10″ | 3.01, s or 3.00, s | 25.33 or 25.29 | CH3 |
| Compound | % Inhibition hAChE ± SEMa | IC50 hAChE ± SEM (µM) b | % Inhibition hBuChE ± SEM a | IC50 hBuChE ± SEM (µM) b |
|---|---|---|---|---|
| clivimine (2) | 9.62 ± 1.75 | >100 | 15.22 ± 8.23 | >100 |
| clivimine B (3) | 10.09 ± 5.37 | >100 | 7.69 ± 2.56 | >100 |
| cliniatine C (4) | 5.72 ± 0.53 | >100 | 9.43 ± 1.23 | >100 |
| clivonine (6) | 34.43 ± 1.68 | >100 | 28.22 ± 6.63 | >100 |
| 3-O-acetyl-8-O-demethylmaritidine (12) | 12.35 ± 1.29 | >100 | 10.31 ± 2.06 | >100 |
| nobilisitine B (14) | 40.03 ± 1.13 | >100 | 12.57 ± 0.53 | >100 |
| sternbergine (17) | 1.25 ± 0.23 | >100 | 23.50 ± 2.28 | >100 |
| galanthamine c | 94.88 ± 0.43 | 2.01 ± 0.14 | 68.23 ± 1.24 | 29.31 ± 3.49 |
| eserine c | 99.98 ± 0.02 | 0.20 ± 0.0.01 | 99.78 ± 0.04 | 0.30 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šafratová, M.; Křoustková, J.; Maafi, N.; Suchánková, D.; Vrabec, R.; Chlebek, J.; Kuneš, J.; Opletal, L.; Bucar, F.; Cahlíková, L. Amaryllidaceae Alkaloids from Clivia miniata (Lindl.) Bosse (Amaryllidaceae): Isolation, Structural Elucidation, and Biological Activity. Plants 2022, 11, 3034. https://doi.org/10.3390/plants11223034
Šafratová M, Křoustková J, Maafi N, Suchánková D, Vrabec R, Chlebek J, Kuneš J, Opletal L, Bucar F, Cahlíková L. Amaryllidaceae Alkaloids from Clivia miniata (Lindl.) Bosse (Amaryllidaceae): Isolation, Structural Elucidation, and Biological Activity. Plants. 2022; 11(22):3034. https://doi.org/10.3390/plants11223034
Chicago/Turabian StyleŠafratová, Marcela, Jana Křoustková, Negar Maafi, Daniela Suchánková, Rudolf Vrabec, Jakub Chlebek, Jiří Kuneš, Lubomír Opletal, Franz Bucar, and Lucie Cahlíková. 2022. "Amaryllidaceae Alkaloids from Clivia miniata (Lindl.) Bosse (Amaryllidaceae): Isolation, Structural Elucidation, and Biological Activity" Plants 11, no. 22: 3034. https://doi.org/10.3390/plants11223034
APA StyleŠafratová, M., Křoustková, J., Maafi, N., Suchánková, D., Vrabec, R., Chlebek, J., Kuneš, J., Opletal, L., Bucar, F., & Cahlíková, L. (2022). Amaryllidaceae Alkaloids from Clivia miniata (Lindl.) Bosse (Amaryllidaceae): Isolation, Structural Elucidation, and Biological Activity. Plants, 11(22), 3034. https://doi.org/10.3390/plants11223034