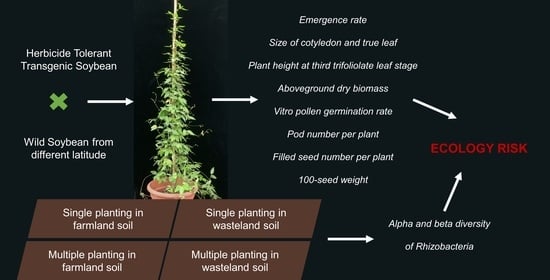
Fitness and Rhizobacteria of F2, F3 Hybrids of Herbicide-Tolerant Transgenic Soybean and Wild Soybean
Abstract
:1. Introduction
2. Results
2.1. Emergence Rate
2.2. Verification of Hybrids with cp4-Epsps Gene
2.3. Size of Cotyledon and True Leaf
2.4. Plant Height at Third Trifoliolate Leaf Stage
2.5. Aboveground Dry Biomass
2.6. Vitro Pollen Germination Rate
2.7. Pod Number and Filled-Seed Number Per Plant
2.8. 100-Seed Weight
2.9. Relative Composite Fitness
2.10. HLJHRB-1 F3 Rhizobacteria under Pure Planting Conditions in Farmland Soil
2.11. JSCZ F3 Rhizobacteria
3. Discussion
3.1. Germination, Cotyledon, and True Leave Size
3.2. Fitness and Rhizobacteria of HLJHRB-1 F2 and F3 Hybrid
3.3. Fitness and Rhizobacteria of JSCZ Hybrid
4. Materials and Methods
4.1. Seed Sowing and Emergence
4.2. Seedling Transplanting and Variables Measured
4.2.1. Without Weed Competition
4.2.2. With Weed Competition
4.3. Procedures to Verify Hybrids with cp4-Epsps Gene
4.4. Rhizosphere Soil Sampling and 16s rDNA High-Throughput Sequencing
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, C.; Hu, R.F.; Huang, J.K.; Huang, X.S.; Shi, G.M.; Li, Y.F.; Yin, Y.H.; Chen, Z.H. Health effect of agricultural pesticide use in China: Implications for the development of GM crops. Sci. Rep. 2016, 6, 34918. [Google Scholar] [CrossRef] [PubMed]
- ISAAA. Global Status of Commercialized Biotech/GM Crops in 2018: Biotech Crops Continue to Help Meet the Challenges of Increased Population and Climate Change; The International Service for the Acquisition of Agri-Biotech Applications (ISAAA): Ithaca, NY, USA, 2018; Volume 54. [Google Scholar]
- ISAAA. GM Approval Database. Available online: https://www.isaaa.org/gmapprovaldatabase/citations/default.asp (accessed on 17 August 2022).
- Liu, B.; Xue, K.; Liu, L.-P.; Zhou, Y.; Han, J. Progress on the Gene Flow from Genetically Modified Soybeans to Wild Soybeans. J. Ecol. Rural Environ. 2020, 36, 833–841. [Google Scholar] [CrossRef]
- Hu, Y.-Q.; Sheng, Z.-W.; Liu, J.-Y.; Liu, Q.; Qiang, S.; Song, X.-L.; Liu, B. Sexual compatibility of transgenic soybean and different wild soybean populations. J. Integr. Agric. 2022, 21, 36–48. [Google Scholar] [CrossRef]
- Kim, D.Y.; Heo, J.H.; Pack, I.S.; Park, J.-H.; Um, M.S.; Kim, H.J.; Park, K.W.; Nam, K.-H.; Oh, S.D.; Kim, J.K.; et al. Natural hybridization between transgenic and wild soybean genotypes. Plant Biotechnol. Rep. 2021, 15, 299–308. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, D.Y.; Moon, Y.S.; Pack, I.S.; Park, K.W.; Chung, Y.S.; Kim, Y.J.; Nam, K.-H.; Kim, C.-G. Gene flow from herbicide resistant transgenic soybean to conventional soybean and wild soybean. Appl. Biol. Chem. 2019, 62, 54. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.J.; Li, X.H. Interspecific gene flow and the origin of semi-wild soybean revealed by capturing the natural occurrence of introgression between wild and cultivated soybean populations. Plant Breed. 2011, 130, 117–127. [Google Scholar] [CrossRef]
- Ellstrand, N.C.; Prentice, H.C.; Hancock, J.F. Gene flow and introgression from domesticated plants into their wild relatives. Annu. Rev. Ecol. Syst. 1999, 30, 539–563. [Google Scholar] [CrossRef]
- Yook, M.J.; Park, H.R.; Zhang, C.J.; Lim, S.H.; Jeong, S.C.; Chung, Y.S.; Kim, D.S. Environmental risk assessment of glufosinate-resistant soybean by pollen-mediated gene flow under field conditions in the region of the genetic origin. Sci. Total Environ. 2021, 762, 143073. [Google Scholar] [CrossRef]
- Kuroda, Y.; Kaga, A.; Tomooka, N.; Yano, H.; Takada, Y.; Kato, S.; Vaughan, D. QTL affecting fitness of hybrids between wild and cultivated soybeans in experimental fields. Ecol. Evol. 2013, 3, 2150–2168. [Google Scholar] [CrossRef]
- Zhao, N.; Ding, X.; Lian, T.; Wang, M.; Tong, Y.; Liang, D.; An, Q.; Sun, S.; Jackson, S.A.; Liu, B.; et al. The Effects of Gene Duplication Modes on the Evolution of Regulatory Divergence in Wild and Cultivated Soybean. Front. Genet. 2020, 11, 601003. [Google Scholar] [CrossRef]
- Wang, K.J.; Li, X.H. Phylogenetic relationships, interspecific hybridization and origin of some rare characters of wild soybean in the subgenus Glycine soja in China. Genet. Resour. Crop Evol. 2012, 59, 1673–1685. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, H.; Hu, Z.; Song, Q.; An, Y.C. Development of a versatile resource for post-genomic research through consolidating and characterizing 1500 diverse wild and cultivated soybean genomes. BMC Genom. 2022, 23, 250. [Google Scholar] [CrossRef]
- Mihelich, N.T.; Mulkey, S.E.; Stec, A.O.; Stupar, R.M. Characterization of genetic heterogeneity within accessions in the USDA soybean germplasm collection. Plant Genome 2020, 13, e20000. [Google Scholar] [CrossRef] [Green Version]
- Sedivy, E.J.; Wu, F.; Hanzawa, Y. Soybean domestication: The origin, genetic architecture and molecular bases. New Phytol. 2017, 214, 539–553. [Google Scholar] [CrossRef] [Green Version]
- Kofsky, J.; Zhang, H.; Song, B.H. Novel resistance strategies to soybean cyst nematode (SCN) in wild soybean. Sci. Rep. 2021, 11, 7967. [Google Scholar] [CrossRef]
- Aleem, M.; Raza, M.M.; Haider, M.S.; Atif, R.M.; Ali, Z.; Bhat, J.A.; Zhao, T. Comprehensive RNA-seq analysis revealed molecular pathways and genes associated with drought tolerance in wild soybean (Glycine soja Sieb. and Zucc.). Physiol. Plant 2020, 172, 707–732. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, X.; Yuan, X.; Shi, J.; Zhang, C.; Yan, N.; Jing, C. Comparison of Phenolic and Flavonoid Compound Profiles and Antioxidant and alpha-Glucosidase Inhibition Properties of Cultivated Soybean (Glycine max) and Wild Soybean (Glycine soja). Plants 2021, 10, 813. [Google Scholar] [CrossRef]
- Lu, B.R. Multidirectional gene flow among wild, weedy, and cultivated soybeans. In Crop Ferality and Volunteerism; CRC Press: Boca Raton, FL, USA, 2005; pp. 137–147. [Google Scholar] [CrossRef]
- Nakayama, Y.; Yamaguchi, H. Natural hybridization in wild soybean (Glycine max ssp. soja) by pollen flow from cultivated soybean (Glycine max ssp. max) in a designed population. Weed Biol. Manag. 2002, 2, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Mizuguti, A.; Yoshimura, Y.; Matsuo, K. Flowering phenologies and natural hybridization of genetically modified and wild soybeans under field conditions. Weed Biol. Manag. 2009, 9, 93–96. [Google Scholar] [CrossRef]
- Wang, K.J.; Li, X.H. Genetic diversity and gene flow dynamics revealed in the rare mixed populations of wild soybean (Glycine soja) and semi-wild type (Glycine gracilis) in China. Genet. Resour. Crop Evol. 2013, 60, 2303–2318. [Google Scholar] [CrossRef]
- Liu, S.N.; Song, X.L.; Hu, Y.H.; Dai, W.M.; Qiang, S. Fitness of Hybrids between Two Types of Transgenic Rice and Six Japonica and Indica Weed Rice Accessions. Crop Sci. 2016, 56, 2751–2765. [Google Scholar] [CrossRef]
- Warwick, S.I.; Beckie, H.J.; Hall, L.M. Gene Flow, Invasiveness, and Ecological Impact of Genetically Modified Crops. Ann. N. Y. Acad. Sci. 2009, 1168, 72–99. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Sheng, Z.W.; Hu, Y.Q.; Liu, Q.; Qiang, S.; Song, X.L.; Liu, B. Fitness of F1 hybrids between 10 maternal wild soybean populations and transgenic soybean. Transgenic Res. 2021, 30, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, L.; Fu, J.; Shen, W.; Fang, Z.; Dai, Y.; Jia, R.; Liu, B.; Liang, J. Fitness and Ecological Risk of Hybrid Progenies of Wild and Herbicide-Tolerant Soybeans with EPSPS Gene. Front. Plant Sci. 2022, 13, 922215. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.J.; Zhang, P.F.; Wei, W.; Mi, X.C.; Kang, D.M.; Liu, B. Performance of hybrid progeny formed between genetically modified herbicide-tolerant soybean and its wild ancestor. AoB Plants 2015, 7, plv121. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.; Sudheer, S.; Usmani, Z.; Rani, R.; Gupta, P. Deciphering the Omics of Plant-Microbe Interaction: Perspectives and New Insights. Curr. Genom. 2020, 21, 343–362. [Google Scholar] [CrossRef]
- Phour, M.; Sehrawat, A.; Sindhu, S.S.; Glick, B.R. Interkingdom signaling in plant-rhizomicrobiome interactions for sustainable agriculture. Microbiol. Res. 2020, 241, 126589. [Google Scholar] [CrossRef]
- Moroenyane, I.; Tremblay, J.; Yergeau, E. Temporal and spatial interactions modulate the soybean microbiome. FEMS Microbiol. Ecol. 2020, 97, fiaa2062. [Google Scholar] [CrossRef]
- Lin, J.; Frank, M.; Reid, D. No Home without Hormones: How Plant Hormones Control Legume Nodule Organogenesis. Plant Commun. 2020, 1, 100104. [Google Scholar] [CrossRef]
- Rong, L.; Chen, H.; Yang, Z.; Yuan, S.; Zhou, X.A. Research status of soybean symbiosis nitrogen fixation. Oil Crop Sci. 2020, 5, 6–10. [Google Scholar] [CrossRef]
- Chen, W.F.; Wang, E.T.; Ji, Z.J.; Zhang, J.J. Recent development and new insight of diversification and symbiosis specificity of legume rhizobia: Mechanism and application. J. Appl. Microbiol. 2020, 131, 553–563. [Google Scholar] [CrossRef]
- Lu, G.H.; Zhu, Y.L.; Kong, L.R.; Cheng, J.; Tang, C.Y.; Hua, X.M.; Meng, F.F.; Pang, Y.J.; Yang, R.W.; Qi, J.L.; et al. Impact of a Glyphosate-Tolerant Soybean Line on the Rhizobacteria, Revealed by Illumina MiSeq. J. Microbiol. Biotechnol. 2017, 27, 561–572. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Romero, E.; Aguirre-Noyola, J.L.; Taco-Taype, N.; Martinez-Romero, J.; Zuniga-Davila, D. Plant microbiota modified by plant domestication. Syst. Appl. Microbiol. 2020, 43, 126106. [Google Scholar] [CrossRef]
- Calado, J.M.G.; Basch, G.; Carvalho, M. Weed Emergence in Autumn under Temperate Conditions. Planta Daninha 2011, 29, 343–349. [Google Scholar] [CrossRef] [Green Version]
- Chu, L.; Gao, Y.P.; Chen, L.L.; McCullough, P.E.; Jespersen, D.; Sapkota, S.; Bagavathiannan, M.; Yu, J.L. Impact of Environmental Factors on Seed Germination and Seedling Emergence of White Clover (Trifolium repens L.). Agronomy 2022, 12, 190. [Google Scholar] [CrossRef]
- Singh, R.J.; Hymowitz, T. The Genomic Relationship between Glycine-Max (L) Merr and Glycine-Soja Sieb and Zucc as Revealed by Pachytene Chromosome Analysis. Theor. Appl. Genet. 1988, 76, 705–711. [Google Scholar] [CrossRef]
- Mikkelsen, T.R.; Jensen, J.; Jorgensen, R.B. Inheritance of oilseed rape (Brassica napus) RAPD markers in a backcross progeny with Brassica campestris. Theor. Appl. Genet. 1996, 92, 492–497. [Google Scholar] [CrossRef]
- De Jong, T.J.; Hesse, E. Selection against hybrids in mixed populations of Brassica rapa and Brassica napus: Model and synthesis. New Phytol. 2012, 194, 1134–1142. [Google Scholar] [CrossRef]
- Susko, D.J.; Cavers, P.B. Seed size effects and competitive ability in Thlaspi arvense L. (Brassicaccae). Botany 2008, 86, 259–267. [Google Scholar] [CrossRef]
- Hu, X.W.; Zhang, R.; Wu, Y.P.; Baskin, C.C. Seedling tolerance to cotyledon removal varies with seed size: A case of five legume species. Ecol. Evol. 2017, 7, 5948–5955. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, D.W. Associations among Cotyledon Developmental Stability, Canalization, and Phenotypic Plasticity in Response to Shading and Burial Depth in Five Herbaceous Species at Early Seedling Stage. Int. J. Plant Sci. 2022, 183, 630–637. [Google Scholar] [CrossRef]
- Singh, R.K.; Bhatia, V.S.; Bhat, K.V.; Mohapatra, T.; Singh, N.K.; Bansal, K.C.; Koundal, K.R. SSR and AFLP based genetic diversity of soybean germplasm differing in photoperiod sensitivity. Genet. Mol. Biol. 2010, 33, 319–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Xu, C.; Sun, J.; Dong, L.; Li, M.; Liu, Y.; Wang, J.; Zhang, X.; Li, D.; Sun, J.; et al. GmRAV confers ecological adaptation through photoperiod control of flowering time and maturity in soybean. Plant Physiol. 2021, 187, 361–377. [Google Scholar] [CrossRef] [PubMed]
- Ort, N.W.W.; Morrison, M.J.; Cober, E.R.; Samanfar, B.; Lawley, Y.E. Photoperiod Affects Node Appearance Rate and Flowering in Early Maturing Soybean. Plants 2022, 11, 871. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.E.; Nakamichi, N. Plant clock modifications for adapting flowering time to local environments. Plant Physiol. 2022, 190, 952–967. [Google Scholar] [CrossRef]
- Jiang, B.; Nan, H.; Gao, Y.; Tang, L.; Yue, Y.; Lu, S.; Ma, L.; Cao, D.; Sun, S.; Wang, J.; et al. Allelic combinations of soybean maturity Loci E1, E2, E3 and E4 result in diversity of maturity and adaptation to different latitudes. PLoS ONE 2014, 9, e106042. [Google Scholar] [CrossRef]
- Adeyemi, T.A.; Jolaosho, A.O.; Dele, P.A.; Adekoya, A.T.; Oloyede, F.A.; Ojo, V.O.A.; Okukenu, O.A.; Amisu, A.A. Intraspecific pod and seed trait variation of two herbaceous legume seeds in response to competing neighbours and nutrient resource abundance. Acta Oecol. 2021, 111, 103741. [Google Scholar] [CrossRef]
- Zhong, Y.; Xun, W.; Wang, X.; Tian, S.; Zhang, Y.; Li, D.; Zhou, Y.; Qin, Y.; Zhang, B.; Zhao, G.; et al. Root-secreted bitter triterpene modulates the rhizosphere microbiota to improve plant fitness. Nat. Plants 2022, 8, 887–896. [Google Scholar] [CrossRef]
- Islam, M.; Al-Hashimi, A.; Ayshasiddeka, M.; Ali, H.; El Enshasy, H.A.; Dailin, D.J.; Sayyed, R.Z.; Yeasmin, T. Prevalence of mycorrhizae in host plants and rhizosphere soil: A biodiversity aspect. PLoS ONE 2022, 17, e0266403. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, S.; Jiang, C.; Wu, C.; Gao, M.; Wang, Q. A review of root exudates and rhizosphere microbiome for crop production. Environ. Sci. Pollut. Res. Int. 2021, 28, 54497–54510. [Google Scholar] [CrossRef]
- Lucke, M.; Correa, M.G.; Levy, A. The Role of Secretion Systems, Effectors, and Secondary Metabolites of Beneficial Rhizobacteria in Interactions with Plants and Microbes. Front. Plant Sci. 2020, 11, 589416. [Google Scholar] [CrossRef]
- Swarnalakshmi, K.; Yadav, V.; Tyagi, D.; Dhar, D.W.; Kannepalli, A.; Kumar, S. Significance of Plant Growth Promoting Rhizobacteria in Grain Legumes: Growth Promotion and Crop Production. Plants 2020, 9, 1596. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Babalola, O.O. Exploring the potentialities of beneficial endophytes for improved plant growth. Saudi J. Biol. Sci. 2020, 27, 3622–3633. [Google Scholar] [CrossRef]
- Cattelan, A.J.; Hartel, P.G.; Fuhrmann, J.J. Screening for plant growth-promoting rhizobacteria to promote early soybean growth. Soil Sci. Soc. Am. J. 1999, 63, 1670–1680. [Google Scholar] [CrossRef]
- Sijilmassi, B.; Filali-Maltouf, A.; Boulahyaoui, H.; Kricha, A.; Boubekri, K.; Udupa, S.; Kumar, S.; Amri, A. Assessment of Genetic Diversity and Symbiotic Efficiency of Selected Rhizobia Strains Nodulating Lentil (Lens culinaris Medik.). Plants 2020, 10, 15. [Google Scholar] [CrossRef]
- Semchenko, M.; Barry, K.E.; de Vries, F.T.; Mommer, L.; Moora, M.; Macia-Vicente, J.G. Deciphering the role of specialist and generalist plant-microbial interactions as drivers of plant-soil feedback. New Phytol. 2022, 234, 1929–1944. [Google Scholar] [CrossRef]
- Boyle, J.A.; Simonsen, A.K.; Frederickson, M.E.; Stinchcombe, J.R. Priority effects alter interaction outcomes in a legume-rhizobium mutualism. Proc. Biol. Sci. 2021, 288, 20202753. [Google Scholar] [CrossRef]
- Windisch, S.; Sommermann, L.; Babin, D.; Chowdhury, S.P.; Grosch, R.; Moradtalab, N.; Walker, F.; Hoglinger, B.; El-Hasan, A.; Armbruster, W.; et al. Impact of Long-Term Organic and Mineral Fertilization on Rhizosphere Metabolites, Root-Microbial Interactions and Plant Health of Lettuce. Front. Microbiol. 2020, 11, 597745. [Google Scholar] [CrossRef]
- Wagner, A. Competition for nutrients increases invasion resistance during assembly of microbial communities. Mol. Ecol. 2022, 31, 4188–4203. [Google Scholar] [CrossRef]
- Do Nascimento, T.R.; Sena, P.T.S.; Oliveira, G.S.; da Silva, T.R.; Dias, M.A.M.; de Freitas, A.D.S.; Martins, L.M.V.; Fernandes-Junior, P.I. Co-inoculation of two symbiotically efficient Bradyrhizobium strains improves cowpea development better than a single bacterium application. 3 Biotech 2021, 11, 4. [Google Scholar] [CrossRef]
- Cerezini, P.; Kuwano, B.H.; Grunvald, A.K.; Hungria, M.; Nogueira, M.A. Soybean tolerance to drought depends on the associated Bradyrhizobium strain. Braz. J. Microbiol. 2020, 51, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, R.M.; Ford, B.L.; Li, L.; Aroney, S.T.N.; Knights, H.E.; Ledermann, R.; East, A.K.; Ramachandran, V.K.; Poole, P.S. Lifestyle adaptations of Rhizobium from rhizosphere to symbiosis. Proc. Natl. Acad. Sci. USA 2020, 117, 23823–23834. [Google Scholar] [CrossRef] [PubMed]
- Belimov, A.A.; Shaposhnikov, A.I.; Syrova, D.S.; Kichko, A.A.; Guro, P.V.; Yuzikhin, O.S.; Azarova, T.S.; Sazanova, A.L.; Sekste, E.A.; Litvinskiy, V.A.; et al. The Role of Symbiotic Microorganisms, Nutrient Uptake and Rhizosphere Bacterial Community in Response of Pea (Pisum sativum L.) Genotypes to Elevated Al Concentrations in Soil. Plants 2020, 9, 1801. [Google Scholar] [CrossRef] [PubMed]













| Hybrids | PNW | PNWO | TSR | χ2 | p |
|---|---|---|---|---|---|
| HLJHRB-1 F2 | 216 | 84 | 3:1 | 1.28 | >0.05 |
| JSCZ F2 | 105 | 45 | 3:1 | 1.74 | >0.05 |
| HLJHRB-1 F3 | 241 | 59 | 5:1 | 1.73 | >0.05 |
| JSCZ F3 | 119 | 31 | 5:1 | 1.45 | >0.05 |
| Soybeans | Coverage | Chao1 | Shannoneven | Shannon |
|---|---|---|---|---|
| CK | 0.98 | 5144.64 ± 275.4 a | 0.64 ± 0.02 a | 5.4 ± 0.22 a |
| HLJHRB-1 F3 | 0.97 | 3786.07 ± 401.95 b | 0.43 ± 0.05 c | 3.42 ± 0.4 c |
| HLJHRB-1 | 0.97 | 5175.57 ± 205.72 a | 0.56 ± 0.02 ab | 4.61 ± 0.17 ab |
| Planting Conditions | Soybeans | Coverage (%) | Richness Index | Evenness Index | Diversity Index |
|---|---|---|---|---|---|
| Chao1 | Shannoneven | Shannon | |||
| PW | CK-W | 97.48% | 8652.69 ± 534.61 a | 0.79 ± 0.00 a | 6.88 ± 0.08 a |
| JSCZ | 97.84% | 8451.91 ± 225.01 Ba | 0.76 ± 0.00 Cb | 6.62 ± 0.02 Cb | |
| JSCZ F3 | 97.66% | 8562.90 ± 155.92 Aa | 0.79 ± 0.00 Aa | 6.87 ± 0.03 Ba | |
| PF | CK-F | 97.30% | 9057.88 ± 175.51 a | 0.80 ± 0.00 a | 6.98 ± 0.03 a |
| JSCZ | 98.25% | 7764.78 ± 146.50 Cab | 0.76 ± 0.00 Cc | 6.56 ± 0.04 Cc | |
| JSCZ F3 | 97.91% | 7043.98 ± 832.06 Bb | 0.79 ± 0.01 Ab | 6.68 ± 0.02 Cb | |
| MW | CK-W | 97.48% | 8652.69 ± 534.61 b | 0.79 ± 0.00 b | 6.88 ± 0.08 b |
| JSCZ | 96.91% | 9462.12 ± 256.79 Aab | 0.81 ± 0.01 Aa | 7.13 ± 0.06 Aa | |
| JSCZ F3 | 97.71% | 9891.08 ± 236.07 Aa | 0.80 ± 0.01 Aab | 7.07 ± 0.02 Aa | |
| MF | CK-F | 97.30% | 9057.88 ± 175.51 a | 0.80 ± 0.00 a | 6.98 ± 0.03 a |
| JSCZ | 97.54% | 8784.09 ± 214.60 Ba | 0.79 ± 0.00 Ba | 6.92 ± 0.03 Ba | |
| JSCZ F3 | 97.83% | 8754.58 ± 229.05 Aa | 0.79 ± 0.00 Aa | 6.89 ± 0.01 Ba |
| Population | Collecting Site | Latitude and Longitude |
|---|---|---|
| HLJHRB-1 | Harbin City, Heilongjiang Province | N46°06′34″, E127°21′43″ |
| JSCZ | Changzhou City, Jiangsu Province | N31°37′13″, E119°29′53″ |
| Hybrids | Planting Years | Hybrids | Planting Years |
|---|---|---|---|
| HLJHRB-1 F2 | 2018 | HLJHRB-1 F3 | 2019 |
| JSCZ F2 | 2019 | JSCZ F3 | 2020 |
| Year | Soil Conditions | Organic Matter g/kg | Total Nitrogen g/kg | Total Phosphorus g/kg | Total Potassium g/kg | Available Phosphorus mg/kg | Alkali-Hydrolyzable Nitrogen mg/kg |
|---|---|---|---|---|---|---|---|
| 2018 | Wasteland | 2.79 | 0.37 | 0.56 | 22.04 | 22.39 | 44.15 |
| 2018 | Farmland | 38.51 | 2.2 | 1.76 | 18.94 | 47.81 | 163.74 |
| 2019 | Wasteland | 4.82 | 0.27 | 0.17 | 9.79 | 0.10 | 10.71 |
| 2019 | Farmland | 9.74 | 0.37 | 0.26 | 10.07 | 1.68 | 23.59 |
| 2020 | Wasteland | 7.78 | 0.72 | 0.25 | 20.94 | 9.99 | 51.91 |
| 2020 | Farmland | 11.19 | 1.06 | 0.36 | 21.09 | 28.21 | 145.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, R.; Ji, X.; Sheng, Z.; Liu, J.; Qiang, S.; Song, X. Fitness and Rhizobacteria of F2, F3 Hybrids of Herbicide-Tolerant Transgenic Soybean and Wild Soybean. Plants 2022, 11, 3184. https://doi.org/10.3390/plants11223184
Liang R, Ji X, Sheng Z, Liu J, Qiang S, Song X. Fitness and Rhizobacteria of F2, F3 Hybrids of Herbicide-Tolerant Transgenic Soybean and Wild Soybean. Plants. 2022; 11(22):3184. https://doi.org/10.3390/plants11223184
Chicago/Turabian StyleLiang, Rong, Xueqin Ji, Zewen Sheng, Jinyue Liu, Sheng Qiang, and Xiaoling Song. 2022. "Fitness and Rhizobacteria of F2, F3 Hybrids of Herbicide-Tolerant Transgenic Soybean and Wild Soybean" Plants 11, no. 22: 3184. https://doi.org/10.3390/plants11223184
APA StyleLiang, R., Ji, X., Sheng, Z., Liu, J., Qiang, S., & Song, X. (2022). Fitness and Rhizobacteria of F2, F3 Hybrids of Herbicide-Tolerant Transgenic Soybean and Wild Soybean. Plants, 11(22), 3184. https://doi.org/10.3390/plants11223184