Production and Secretion of Isowighteone in Hairy Root Cultures of Pigeon Pea (Cajanus cajan) Co-Treated with Multiple Elicitors
Abstract
:1. Introduction
2. Results and Discussion
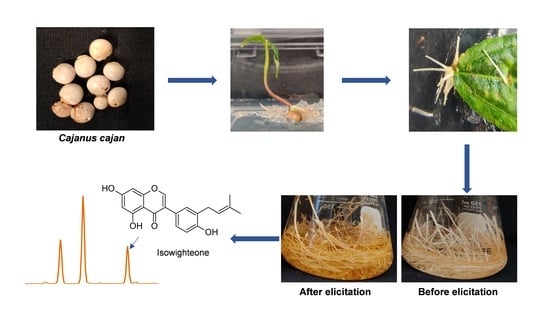
2.1. Development and Characterization of Pigeon Pea Hairy Root Culture
2.2. Elicitation of Hairy Root Cultures of Pigeon Pea and Identification of Isowighteone
2.3. Scanning Electron Microscopy of Non-Elicited and Elicited Hairy Roots
2.4. Time Course Accumulation of Isowighteone in the Culture Medium
2.5. Yield of isowighteone in the Hairy Root Culture System
3. Materials and Methods
3.1. Seed Sterilization and Germination of Pigeon Pea
3.2. Establishment of Hairy Root Lines of Pigeon Pea
3.3. Growth Kinetics of Pigeon Pea Hairy Root Line
3.4. Elicitation of Pigeon Pea Hairy Root Cultures
3.5. Extraction and Analysis of Phenolic Compounds
3.6. Identification and Purification of Isowighteone
3.7. Scanning Electron Microscopy
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grover, J.K.; Yadav, S.; Vats, V. Medicinal plants of India with anti-diabetic potential. J. Ethnopharmacol. 2002, 81, 81–100. [Google Scholar] [CrossRef]
- Kong, Y.; Fu, Y.-J.; Zu, Y.-G.; Liu, W.; Wang, W.; Hua, X.; Yang, M. Ethanol modified supercritical fluid extraction and antioxidant activity of cajaninstilbene acid and pinostrobin from pigeon pea [Cajanus cajan (L.) Millsp.] leaves. Food Chem. 2009, 117, 152–159. [Google Scholar] [CrossRef]
- Liu, W.; Fu, Y.; Zu, Y.; Kong, Y.; Zhang, L.; Zu, B.; Efferth, T. Negative-pressure cavitation extraction for the determination of flavonoids in pigeon pea leaves by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 3841–3850. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Kong, Y.; Zu, Y.; Fu, Y.; Luo, M.; Zhang, L.; Li, J. Determination and quantification of active phenolic compounds in pigeon pea leaves and its medicinal product using liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 4723–4731. [Google Scholar]
- Dahiya, J.S.; Strange, R.N.; Bilyard, K.G.; Cooksey, C.J.; Garratt, P.J. Two isoprenylated isoflavone phytoalexins from Cajanus cajan. Phytochemistry 1984, 23, 871–873. [Google Scholar]
- Dahiya, J.S. Reversed-phase high-performance liquid chromatography of Cajanus cajan phytoalexins. J. Chromatogr. A 1987, 409, 355–359. [Google Scholar]
- Hatano, T.; Shintani, Y.; Aga, Y.; Shiota, S.; Tsuchiya, T.; Yoshida, T. Phenolic Constituents of Licorice. VIII. Structures of Glicophenone and Glicoisoflavanone, and Effects of Licorice Phenolics on Methicillin-Resistant Staphylococcus aureus. Chem. Pharm. Bull. 2000, 121, 1286–1296. [Google Scholar]
- Araya-Cloutier, C.; Vincken, J.-P.; van de Schans, M.G.M.; Hageman, J.; Schaftenaar, G.; den Besten, H.M.W.; Gruppen, H. QSAR-based molecular signatures of prenylated (iso)flavonoids underlying antimicrobial potency against and membrane-disruption in Gram positive and Gram negative bacteria. Sci. Rep. 2018, 8, 9267. [Google Scholar]
- Yao, J.; Wang, Z.; Wang, R.; Wang, Y.; Xu, J.; He, X. Anti-proliferative and anti-inflammatory prenylated isoflavones and coumaronochromones from the fruits of Ficus altissima. Bioorg. Chem. 2021, 113, 104996. [Google Scholar] [CrossRef]
- Limper, C.; Wang, Y.; Ruhl, S.; Wang, Z.; Lou, Y.; Totzke, F.; Kubbutat, M.H.G.; Chovolou, Y.; Proksch, P.; Wätjen, W. Compounds isolated from Psoralea corylifolia seeds inhibit protein kinase activity and induce apoptotic cell death in mammalian cells. J. Pharm. Pharmacol. 2013, 65, 1393–1408. [Google Scholar] [CrossRef]
- Dias, M.I.; Sousa, M.J.; Alves, R.C.; Ferreira, I.C.F.R. Exploring plant tissue culture to improve the production of phenolic compounds: A review. Ind. Crops Prod. 2016, 82, 9–22. [Google Scholar] [CrossRef]
- Hussain, M.J.; Abbas, Y.; Nazli, N.; Fatima, S.; Drouet, S.; Hano, C.; Abbasi, B.H. Root cultures, a boon for the production of valuable compounds: A comparative review. Plants 2022, 11, 439. [Google Scholar] [CrossRef]
- Condori, J.; Sivakumar, G.; Hubstenberger, J.; Dolan, M.C.; Sobolev, V.S.; Medina-Bolivar, F. Induced biosynthesis of resveratrol and the prenylated stilbenoids arachidin-1 and arachidin-3 in hairy root cultures of peanut: Effects of culture medium and growth stage. Plant Physiol. Biochem. 2010, 48, 310–318. [Google Scholar] [CrossRef]
- Jiao, J.; Gai, Q.-Y.; Wang, X.; Liu, J.; Lu, Y.; Wang, Z.-Y.; Xu, X.-J.; Fu, Y.-J. Effective production of phenolic compounds with health benefits in pigeon pea [Cajanus cajan (L.) Millsp.] hairy root cultures. J. Agric. Food Chem. 2020, 68, 8350–8361. [Google Scholar] [CrossRef]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.M.; Palazon, J. Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef]
- Fang, L.; Yang, T.; Medina-Bolivar, F. Production of prenylated stilbenoids in hairy root cultures of peanut (Arachis hypogaea) and its wild relatives A. ipaensis and A. duranensis via an optimized elicitation procedure. Molecules 2020, 25, 509. [Google Scholar] [CrossRef]
- Nopo-Olazabal, C.; Hubstenberger, J.; Nopo-Olazabal, L.; Medina-Bolivar, F. Antioxidant activity of selected stilbenoids and their bioproduction in hairy root cultures of muscadine grape (Vitis rotundifolia Michx.). J. Agric. Food Chem. 2013, 61, 11744–11758. [Google Scholar] [CrossRef]
- Gai, Q.-Y.; Lu, Y.; Jiao, J.; Fu, J.-X.; Xu, X.-J.; Yao, L.; Fu, Y.-J. Application of UV-B radiation for enhancing the accumulation of bioactive phenolic compounds in pigeon pea [Cajanus cajan (L.) Millsp.] hairy root cultures. J. Photochem. Photobiol. 2022, 228, 112406. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Fang, L.; Nopo-Olazabal, C.; Condori, J.; Nopo-Olazabal, L.; Balmaceda, C.; Medina-Bolivar, F. Enhanced production of resveratrol, piceatannol, arachidin-1, and arachidin-3 in hairy root cultures of peanut co-treated with methyl jasmonate and cyclodextrin. J. Agric. Food Chem. 2015, 63, 3942–3950. [Google Scholar] [CrossRef] [PubMed]
- Barba-Espín, G.; Martínez-Jiménez, C.; Izquierdo-Martínez, A.; Acosta-Motos, J.R.; Hernández, J.A.; Díaz-Vivancos, P. H2O2-elicitation of black carrot hairy roots induces a controlled oxidative burst leading to increased anthocyanin production. Plants 2021, 10, 2753. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chang, P.F.L.; Liu, D.; Narasimhan, M.L.; Raghothama, K.G.; Hasegawa, P.M.; Bressan, R.A. Plant defense genes are synergistically induced by ethylene and methyl jasmonate. Plant Cell 1994, 6, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.-H.; Ko, T.-P.; Wang, A.H.J. Structure, mechanism and function of prenyltransferases. Eur. J. Biochem. 2002, 269, 3339–3354. [Google Scholar] [CrossRef]
- Gajurel, G.; Hasan, R.; Medina-Bolivar, F. Antioxidant assessment of prenylated stilbenoid-rich extracts from elicited hairy root cultures of three cultivars of peanut (Arachis hypogaea). Molecules 2021, 26, 6778. [Google Scholar] [CrossRef]
- Ingham, J.L.; Tahara, S.; Shibaki, S.; Mizutani, J. Isoflavonoids from the Root Bark of Piscidia erythrina and a Note on the Structure of Piscidone. Z. Naturforsch. C Biosci. 1989, 44, 905–913. [Google Scholar] [CrossRef]
- Marsh, Z.; Yang, T.; Nopo-Olazabal, L.; Wu, S.; Ingle, T.; Joshee, N.; Medina-Bolivar, F. Effect of light, methyl jasmonate and cyclodextrin on production of phenolic compounds in hairy root cultures of Scutellaria lateriflora. Phytochemistry 2014, 107, 50–60. [Google Scholar] [CrossRef]
- Ruiz-May, E.; De-la-Peña, C.; Galaz-Ávalos, R.M.; Lei, Z.; Watson, B.S.; Sumner, L.W.; Loyola-Vargas, V.M. Methyl jasmonate induces ATP biosynthesis deficiency and accumulation of proteins related to secondary metabolism in Catharanthus roseus (l.) g. hairy roots. Plant Cell Physiol. 2011, 52, 1401–1421. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Fang, L.; Sanders, S.; Jayanthi, S.; Rajan, G.; Podicheti, R.; Thallapuranam, S.K.; Mockaitis, K.; Medina-Bolivar, F. Stilbenoid prenyltransferases define key steps in the diversification of peanut phytoalexins. J. Biol. Chem. 2018, 293, 28–46. [Google Scholar] [CrossRef]
- Medina-Bolivar, F.; Condori, J.; Rimando, A.M.; Hubstenberger, J.; Shelton, K.; O’Keefe, S.F.; Bennett, S.; Dolan, M.C. Production and secretion of resveratrol in hairy root cultures of peanut. Phytochemistry 2007, 68, 1992–2003. [Google Scholar] [CrossRef] [PubMed]






| tR (min) | UV Max (nm) | [M − H]− | MS2 Ions | [M + H]+ | MS2 Ions |
|---|---|---|---|---|---|
| 31.84 | 261 | 337 | 268, 281, 294 | 339 | 283, 271, 255 |
| Source | Yield of isowighteone (μg/g DW) | |
|---|---|---|
| Control | Elicitor Treatment | |
| Hairy root tissue | 29.08 ± 3.7 | 346.35 ± 63.65 |
| Culture medium | bDL a | 7712.27 ± 441.23 |
| Total yield of isowighteone | 29.08 ± 3.7 | 8058.618 ± 445.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajurel, G.; Nopo-Olazabal, L.; Hendrix, E.; Medina-Bolivar, F. Production and Secretion of Isowighteone in Hairy Root Cultures of Pigeon Pea (Cajanus cajan) Co-Treated with Multiple Elicitors. Plants 2022, 11, 834. https://doi.org/10.3390/plants11060834
Gajurel G, Nopo-Olazabal L, Hendrix E, Medina-Bolivar F. Production and Secretion of Isowighteone in Hairy Root Cultures of Pigeon Pea (Cajanus cajan) Co-Treated with Multiple Elicitors. Plants. 2022; 11(6):834. https://doi.org/10.3390/plants11060834
Chicago/Turabian StyleGajurel, Gaurav, Luis Nopo-Olazabal, Emily Hendrix, and Fabricio Medina-Bolivar. 2022. "Production and Secretion of Isowighteone in Hairy Root Cultures of Pigeon Pea (Cajanus cajan) Co-Treated with Multiple Elicitors" Plants 11, no. 6: 834. https://doi.org/10.3390/plants11060834
APA StyleGajurel, G., Nopo-Olazabal, L., Hendrix, E., & Medina-Bolivar, F. (2022). Production and Secretion of Isowighteone in Hairy Root Cultures of Pigeon Pea (Cajanus cajan) Co-Treated with Multiple Elicitors. Plants, 11(6), 834. https://doi.org/10.3390/plants11060834