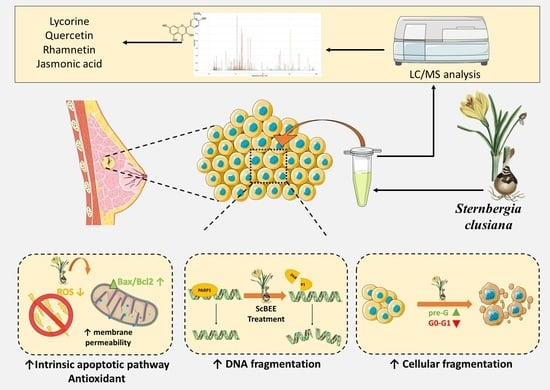
The Antioxidant and Proapoptotic Effects of Sternbergia clusiana Bulb Ethanolic Extract on Triple-Negative and Estrogen-Dependent Breast Cancer Cells In Vitro
Abstract
:1. Introduction
2. Results
2.1. ScBEE Exerts a Selective Antiproliferative Effect on MDA-MB-231 and MCF-7 Cells Compared to Its Effect on MSCs
2.2. ScBEE Induces Cellular Fragmentation of BC Cells
2.3. ScBEE Promotes DNA Fragmentation in Breast Cancer Cells
2.4. ScBEE Activates the Apoptotic Pathway via the Activation of the Mitochondrial Pathway
2.5. ScBEE Inhibits ROS Levels in Breast Cancer Cells
2.6. Chemical Characterization of Sternbergia Bulb Ethanolic Extract Using LC-MS/MS
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Sternbergia Bulb Ethanolic Extract (ScBEE) Preparation
4.3. Breast Cancer Cell Culture
4.4. Culture of Mesenchymal Stem Cells (MSCs) Isolated from Rat Bone Marrow
4.5. Cytotoxicity Assay
4.6. Cell Cycle Analysis
4.7. Cell Death ELISA
4.8. Protein Extraction and Quantification
4.9. Western Blot
4.10. Reactive Oxygen Species (ROS) Detection
4.11. Liquid Chromatograph-Tandem Mass Spectrometry (LC-MS/MS) Analysis
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the Global Cancer Incidence and Mortality in 2018: GLOBOCAN Sources and Methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malhotra, G.K.; Zhao, X.; Band, H.; Band, V. Histological, Molecular and Functional Subtypes of Breast Cancers. Cancer Biol. Ther. 2010, 10, 955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theodossiou, T.A.; Ali, M.; Grigalavicius, M.; Grallert, B.; Dillard, P.; Schink, K.O.; Olsen, C.E.; Wälchli, S.; Inderberg, E.M.; Kubin, A.; et al. Simultaneous Defeat of MCF7 and MDA-MB-231 Resistances by a Hypericin PDT–Tamoxifen Hybrid Therapy. NPJ Breast Cancer 2019, 5, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turashvili, G.; Brogi, E. Tumor Heterogeneity in Breast Cancer. Front. Med. 2017, 4, 227. [Google Scholar] [CrossRef] [Green Version]
- Chin, C.-Y.; Chen, Y.-H.; Wu, S.-C.; Liu, C.-T.; Lee, Y.-F.; Tsai, M.-Y. Complementary and Alternative Medicine Use in Breast Cancer Patients at a Medical Center in Taiwan: A Cross-Sectional Study. Integr. Cancer Ther. 2020, 19, 1534735420983910. [Google Scholar] [CrossRef]
- Jain, R.; Kosta, S.; Tiwari, A. Ayurveda and Cancer. Pharmacogn. Res. 2010, 2, 393–394. [Google Scholar] [CrossRef]
- Omara, T.; Kiprop, A.K.; Ramkat, R.C.; Cherutoi, J.; Kagoya, S.; Nyangena, D.M.; Tebo, T.A.; Nteziyaremye, P.; Karanja, L.N.; Jepchirchir, A.; et al. Medicinal Plants Used in Traditional Management of Cancer in Uganda: A Review of Ethnobotanical Surveys, Phytochemistry, and Anticancer Studies. Evid.-Based Complement. Altern. Med. Ecam 2020, 2020, 3529081. [Google Scholar] [CrossRef] [Green Version]
- El Khoury, M.; Haykal, T.; Hodroj, M.H.; Najem, S.A.; Sarkis, R.; Taleb, R.I.; Rizk, S. Malva Pseudolavatera Leaf Extract Promotes ROS Induction Leading to Apoptosis in Acute Myeloid Leukemia Cells In Vitro. Cancers 2020, 12, 435. [Google Scholar] [CrossRef] [Green Version]
- Nasser, M.; Damaj, Z.; Hijazi, A.; Merah, O.; Al-Khatib, B.; Hijazi, N.; Trabolsi, C.; Damaj, R.; Nasser, M. Pomegranate Juice Extract Decreases Cisplatin Toxicity on Peripheral Blood Mononuclear Cells. Medicines 2020, 7, 66. [Google Scholar] [CrossRef]
- Youssef, S.; Mahmood, A.; Vela, E. Sobre el género <em>Sternbergia</em> (Amaryllidaceae) en Iraq. An. Del Jardín Botánico De Madr. 2017, 74, e053. [Google Scholar] [CrossRef]
- Desgagné-Penix, I. Biosynthesis of Alkaloids in Amaryllidaceae Plants: A Review. Phytochem. Rev. 2021, 20, 409–431. [Google Scholar] [CrossRef]
- Martinez-Peinado, N.; Cortes-Serra, N.; Torras-Claveria, L.; Pinazo, M.-J.; Gascon, J.; Bastida, J.; Alonso-Padilla, J. Amaryllidaceae Alkaloids with Anti-Trypanosoma Cruzi Activity. Parasites Vectors 2020, 13, 299. [Google Scholar] [CrossRef] [PubMed]
- Hulcová, D.; Maříková, J.; Korábečný, J.; Hošťálková, A.; Jun, D.; Kuneš, J.; Chlebek, J.; Opletal, L.; De Simone, A.; Nováková, L.; et al. Amaryllidaceae Alkaloids from Narcissus Pseudonarcissus L. Cv. Dutch Master as Potential Drugs in Treatment of Alzheimer’s Disease. Phytochemistry 2019, 165, 112055. [Google Scholar] [CrossRef] [PubMed]
- Mammadov, R.; Kara, Y.; Vaizogullar, H.E. Study on the Phenolic Content, Antioxidant and Antimicrobial Effects of Sternbergia Clusiana. Asian J. Chem. 2011, 23, 5. [Google Scholar]
- Kaya, G.İ.; Sarıkaya, B.; Çiçek, D.; Somer, N.Ü. In Vitro Cytotoxic Activity of Sternbergia Sicula, S. Lutea and Pancratium Maritimum Extracts. Hacet. Univ. J. Fac. Pharm. 2010, 30, 41–48. [Google Scholar]
- Can Ağca, A.; Yazgan Ekici, A.N.; Yılmaz Sarıaltın, S.; Çoban, T.; Saltan İşcan, G.; Sever Yılmaz, B. Antioxidant, Anti-Inflammatory and Antidiabetic Activity of Two Sternbergia Taxons from Turkey. S. Afr. J. Bot. 2021, 136, 105–109. [Google Scholar] [CrossRef]
- Çitoğlu, G.S.; Sener, B.; Tankerl, M.; Çitoğlul, G.; Gümüşel2, B.; Şener3, B. Alkaloids of Sternbergia Clusiana and Their Analgesic Effects O Swets &Zeitlinger Aı,x,Tı,oids of srnnxnnrgia clusiana and their aniicusic effects. Artic. Pharm. Biol. 1996, 34, 194–197. [Google Scholar] [CrossRef]
- Haznedaroglu, M.; Gokce, G. Comparison of Anti-Acetylcholinesterase Activity of Bulb and Leaf Extracts of Sternbergia Candida Mathew & T. Baytop. Acta Biol. Hung. 2014, 65, 396–404. [Google Scholar] [CrossRef]
- Acikara, Ö.B.; Yilmaz, B.S.; Yazgan, D.; Işcan, G.S. Quantification of Galantamine in Sternbergia Species by High Performance Liquid Chromatography. Turk. J. Pharm. Sci. 2019, 16, 32–36. [Google Scholar] [CrossRef]
- Kaskatepe, B.; Yilmaz, B.S.; Acikara, O.B.; Iscan, G.S.; Vlainic, J.; Kosalec, I. Antifungal Activity of Some Sternbergia Taxa: Effects on Germ Tube and Biofilm Formation. Braz. J. Pharm. Sci. 2019, 55. [Google Scholar] [CrossRef] [Green Version]
- Nair, J.J.; van Staden, J. Cytotoxicity Studies of Lycorine Alkaloids of the Amaryllidaceae. Nat. Prod. Commun. 2014, 9, 1934578X1400900834. [Google Scholar] [CrossRef] [Green Version]
- Roy, M.; Liang, L.; Xiao, X.; Feng, P.; Ye, M.; Liu, J. Lycorine: A Prospective Natural Lead for Anticancer Drug Discovery. Biomed. Pharmacother. 2018, 107, 615–624. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, P.; Sun, Y.; Sharopov, F.S.; Yang, Q.; Chen, F.; Wang, P.; Liang, Z. Lycorine Possesses Notable Anticancer Potentials in On-Small Cell Lung Carcinoma Cells via Blocking Wnt/β-Catenin Signaling and Epithelial-Mesenchymal Transition (EMT). Biochem. Biophys. Res. Commun. 2018, 495, 911–921. [Google Scholar] [CrossRef]
- Younes, M.; Ammoury, C.; Haykal, T.; Nasr, L.; Sarkis, R.; Rizk, S. The Selective Anti-Proliferative and pro-Apoptotic Effect of A. Cherimola on MDA-MB-231 Breast Cancer Cell Line. BMC Complement. Med. Ther. 2020, 20, 343. [Google Scholar] [CrossRef] [PubMed]
- Fayad, C.; Audi, H.; Khnayzer, R.S.; Daher, C.F. The Anti-Cancer Effect of Series of Strained Photoactivatable Ru(II) Polypyridyl Complexes on Non-Small-Cell Lung Cancer and Triple Negative Breast Cancer Cells. JBIC J. Biol. Inorg. Chem. 2020, 26, 43–55. [Google Scholar] [CrossRef]
- Khoury, A.; Elias, E.; Mehanna, S.; Shebaby, W.; Deo, K.M.; Mansour, N.; Khalil, C.; Sayyed, K.; Sakoff, J.A.; Gilbert, J.; et al. Novel Platinum(II) and Platinum(IV) Antitumor Agents That Exhibit Potent Cytotoxicity and Selectivity. J. Med. Chem. 2022, 65, 16481–16493. [Google Scholar] [CrossRef] [PubMed]
- Tilaoui, M.; Ait Mouse, H.; Zyad, A. Update and New Insights on Future Cancer Drug Candidates From Plant-Based Alkaloids. Front. Pharmacol. 2021, 12, 3621. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A.; Hosseini, A. Cancer Therapy with Phytochemicals: Evidence from Clinical Studies. Avicenna J. Phytomedicine 2015, 5, 84–97. [Google Scholar] [CrossRef]
- Cahlíková, L.; Kawano, I.; Řezáčová, M.; Blunden, G.; Hulcová, D.; Havelek, R. The Amaryllidaceae Alkaloids Haemanthamine, Haemanthidine and Their Semisynthetic Derivatives as Potential Drugs. Phytochem. Rev. 2021, 20, 303–323. [Google Scholar] [CrossRef]
- Leporini, M.; Catinella, G.; Bruno, M.; Falco, T.; Tundis, R.; Loizzo, M.R. Investigating the Antiproliferative and Antioxidant Properties of Pancratium Maritimum L. (Amaryllidaceae) Stems, Flowers, Bulbs, and Fruits Extracts. Evid.-Based Complement. Altern. Med. 2018, 2018, e9301247. [Google Scholar] [CrossRef] [Green Version]
- Isbilen, O.; Rizaner, N.; Volkan, E. Anti-Proliferative and Cytotoxic Activities of Allium Autumnale P. H. Davis (Amaryllidaceae) on Human Breast Cancer Cell Lines MCF-7 and MDA-MB-231. BMC Complement. Altern. Med. 2018, 18, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Havelek, R.; Seifrtova, M.; Kralovec, K.; Bruckova, L.; Cahlikova, L.; Dalecka, M.; Vavrova, J.; Rezacova, M.; Opletal, L.; Bilkova, Z. The Effect of Amaryllidaceae Alkaloids Haemanthamine and Haemanthidine on Cell Cycle Progression and Apoptosis in P53-Negative Human Leukemic Jurkat Cells. Phytomedicine 2014, 21, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Nair, J.J.; van Staden, J. Phenanthridone Alkaloids of the Amaryllidaceae as Activators of the Apoptosis-Related Proteolytic Enzymes, Caspases. Nat. Prod. Commun. 2018, 13, 1934578X1801301035. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, U.; Groscurth, P. Morphological Features of Cell Death. Physiology 2004, 19, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Mashimo, M.; Onishi, M.; Uno, A.; Tanimichi, A.; Nobeyama, A.; Mori, M.; Yamada, S.; Negi, S.; Bu, X.; Kato, J.; et al. The 89-KDa PARP1 Cleavage Fragment Serves as a Cytoplasmic PAR Carrier to Induce AIF-Mediated Apoptosis. J. Biol. Chem. 2021, 296, 100046. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Yu, X. Functions of PARylation in DNA Damage Repair Pathways. Genom. Proteom. Bioinform. 2016, 14, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Dumitraș, D.-A.; Andrei, S. Recent Advances in the Antiproliferative and Proapoptotic Activity of Various Plant Extracts and Constituents against Murine Malignant Melanoma. Molecules 2022, 27, 2585. [Google Scholar] [CrossRef]
- Nanni, V.; Di Marco, G.; Sacchetti, G.; Canini, A.; Gismondi, A. Oregano Phytocomplex Induces Programmed Cell Death in Melanoma Lines via Mitochondria and DNA Damage. Foods 2020, 9, 1486. [Google Scholar] [CrossRef]
- Fulda, S.; Debatin, K.-M. Extrinsic versus Intrinsic Apoptosis Pathways in Anticancer Chemotherapy. Oncogene 2006, 25, 4798–4811. [Google Scholar] [CrossRef] [Green Version]
- Khodapasand, E.; Jafarzadeh, N.; Farrokhi, F.; Kamalidehghan, B.; Houshmand, M. Is Bax/Bcl-2 Ratio Considered as a Prognostic Marker with Age and Tumor Location in Colorectal Cancer? Iran Biomed J. 2015, 19, 69–75. [Google Scholar] [CrossRef]
- Aydin, Ç.; ErmiŞ, A.; Mammadov, R. Phenolic Contents and Antioxidant Properties of Sternbergia lutea (L.) Ker-Gawl. Ex Sprengel Ethanol Extract. Int. J. Second. Metab. 2015, 2, 9. [Google Scholar]
- Jeong, C.-H.; Joo, S.H. Downregulation of Reactive Oxygen Species in Apoptosis. J. Cancer Prev. 2016, 21, 13–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolì, F.; Negro, C.; Vergine, M.; Aprile, A.; Nutricati, E.; Sabella, E.; Miceli, A.; Luvisi, A.; De Bellis, L. Evaluation of Phytochemical and Antioxidant Properties of 15 Italian Olea Europaea L. Cultivar Leaves. Molecules 2019, 24, 1998. [Google Scholar] [CrossRef]
- Singh, A.; Chauhan, S.S.; Tripathi, V. Quinic Acid Attenuates Oral Cancer Cell Proliferation by Downregulating Cyclin D1 Expression and Akt Signaling. Pharmacogn. Mag. 2018, 14, 14. [Google Scholar] [CrossRef]
- Kang, K.A.; Piao, M.J.; Ryu, Y.S.; Hyun, Y.J.; Park, J.E.; Shilnikova, K.; Zhen, A.X.; Kang, H.K.; Koh, Y.S.; Jeong, Y.J.; et al. Luteolin Induces Apoptotic Cell Death via Antioxidant Activity in Human Colon Cancer Cells. Int. J. Oncol. 2017, 51, 1169–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, S.; Yang, Y.; Liu, D.; Luo, Y.; Ye, X.; Liu, Y.; Chen, X.; Wang, S.; Wu, H.; Wang, Y.; et al. Flavonoids and Tannins from Smilax China L. Rhizome Induce Apoptosis Via Mitochondrial Pathway and MDM2-P53 Signaling in Human Lung Adenocarcinoma Cells. Am. J. Chin. Med. 2017, 45, 369–384. [Google Scholar] [CrossRef]
- Siddiqui, S.S.; Rahman, S.; Rupasinghe, H.P.V.; Vazhappilly, C.G. Dietary Flavonoids in P53—Mediated Immune Dysfunctions Linking to Cancer Prevention. Biomedicines 2020, 8, 286. [Google Scholar] [CrossRef]
- Lan, L.; Wang, Y.; Pan, Z.; Wang, B.; Yue, Z.; Jiang, Z.; Li, L.; Wang, C.; Tang, H. Rhamnetin Induces Apoptosis in Human Breast Cancer Cells via the MiR-34a/Notch-1 Signaling Pathway. Oncol. Lett. 2019, 17, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, S.; Somasagara, R.R.; Hegde, M.; Nishana, M.; Tadi, S.K.; Srivastava, M.; Choudhary, B.; Raghavan, S.C. Quercetin, a Natural Flavonoid Interacts with DNA, Arrests Cell Cycle and Causes Tumor Regression by Activating Mitochondrial Pathway of Apoptosis. Sci. Rep. 2016, 6, 24049. [Google Scholar] [CrossRef] [Green Version]
- Galluzzo, P.; Martini, C.; Bulzomi, P.; Leone, S.; Bolli, A.; Pallottini, V.; Marino, M. Quercetin-Induced Apoptotic Cascade in Cancer Cells: Antioxidant versus Estrogen Receptor Alpha-Dependent Mechanisms. Mol. Nutr. Food Res. 2009, 53, 699–708. [Google Scholar] [CrossRef]
- Yeruva, L.; Elegbede, J.A.; Carper, S.W. Methyl Jasmonate Decreases Membrane Fluidity and Induces Apoptosis via Tumor Necrosis Factor Receptor 1 in Breast Cancer Cells. Anticancer. Drugs 2008, 19, 766–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.; Yu, H.; Ma, Q.; Shen, S.; Das, U.N. Linoleic Acid Suppresses Colorectal Cancer Cell Growth by Inducing Oxidant Stress and Mitochondrial Dysfunction. Lipids Health Dis. 2010, 9, 106. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Zhang, L.; Yuan, X.; Ou, Y.; Zhu, X.; Cheng, Z.; Zhang, P.; Wu, X.; Meng, Y.; Zhang, L. The Relationship between the Bcl-2/Bax Proteins and the Mitochondria-Mediated Apoptosis Pathway in the Differentiation of Adipose-Derived Stromal Cells into Neurons. PLoS ONE 2016, 11, e0163327. [Google Scholar] [CrossRef]
- Balijepalli, M.K.; Tandra, S.; Pichika, M.R. Antiproliferative Activity and Induction of Apoptosis in Estrogen Receptor-Positive and Negative Human Breast Carcinoma Cell Lines by Gmelina Asiatica Roots. Pharmacogn. Res. 2010, 2, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Keene, S.; Azuelos, C.; Majumdar, S.K. Sensitivity Evaluation of Two Human Breast Cancer Cell Lines to Tamoxifen through Apoptosis Induction. Open J. Apoptosis 2014, 3, 70–77. [Google Scholar] [CrossRef] [Green Version]
- Ko, C.H.; Shen, S.-C.; Hsu, C.-S.; Chen, Y.-C. Mitochondrial-Dependent, Reactive Oxygen Species-Independent Apoptosis by Myricetin: Roles of Protein Kinase C, Cytochrome c, and Caspase Cascade. Biochem. Pharm. 2005, 69, 913–927. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J.; Xing, G. Lycorine Inhibits the Growth and Metastasis of Breast Cancer through the Blockage of STAT3 Signaling Pathway. Acta Biochim. Biophys. Sin. 2017, 49, 771–779. [Google Scholar] [CrossRef] [Green Version]
- Ying, X.; Huang, A.; Xing, Y.; Lan, L.; Yi, Z.; He, P. Lycorine Inhibits Breast Cancer Growth and Metastasis via Inducing Apoptosis and Blocking Src/FAK-Involved Pathway. Sci. China Life Sci. 2017, 60, 417–428. [Google Scholar] [CrossRef]
- Ilijeva, R.; Buchbauer, G. Biological Properties of Some Volatile Phenylpropanoids. Nat. Prod. Commun. 2016, 11, 1934578X1601101041. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Lin, D.; Jiang, R.; Li, H.; Wan, J.; Li, H. Ferulic Acid Exerts Antitumor Activity and Inhibits Metastasis in Breast Cancer Cells by Regulating Epithelial to Mesenchymal Transition. Oncol. Rep. 2016, 36, 271–278. [Google Scholar] [CrossRef] [Green Version]
- Anwar, M.M.; Abd El-Karim, S.S.; Mahmoud, A.H.; Amr, A.E.-G.E.; Al-Omar, M.A. A Comparative Study of the Anticancer Activity and PARP-1 Inhibiting Effect of Benzofuran–Pyrazole Scaffold and Its Nano-Sized Particles in Human Breast Cancer Cells. Molecules 2019, 24, 2413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shebaby, W.N.; Mroueh, M.; Bodman-Smith, K.; Mansour, A.; Taleb, R.I.; Daher, C.F.; El-Sibai, M. Daucus Carota Pentane-Based Fractions Arrest the Cell Cycle and Increase Apoptosis in MDA-MB-231 Breast Cancer Cells. BMC Complement. Altern. Med. 2014, 14, 387. [Google Scholar] [CrossRef] [Green Version]
- Haykal, T.; Nasr, P.; Hodroj, M.H.; Taleb, R.I.; Sarkis, R.; Moujabber, M.N.E.; Rizk, S. Annona Cherimola Seed Extract Activates Extrinsic and Intrinsic Apoptotic Pathways in Leukemic Cells. Toxins 2019, 11, 506. [Google Scholar] [CrossRef]
- Khalil, C. In Vitro UVB Induced Cellular Damage Assessment Using Primary Human Skin Derived Fibroblasts—MedCrave Online. MOJ Toxicol. 2015, 1, 138–143. [Google Scholar] [CrossRef]
- Idriss, M.; Hodroj, M.H.; Fakhoury, R.; Rizk, S. Beta-Tocotrienol Exhibits More Cytotoxic Effects than Gamma-Tocotrienol on Breast Cancer Cells by Promoting Apoptosis via a P53-Independent PI3-Kinase Dependent Pathway. Biomolecules 2020, 10, 577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haykal, T.; Younes, M.; El Khoury, M.; Ammoury, C.; Tannous, S.; Hodroj, M.H.; Sarkis, R.; Gasilova, N.; Menin, L.; Rizk, S. The Pro-Apoptotic Properties of a Phytonutrient Rich Infusion of A. Cherimola Leaf Extract on AML Cells. Biomed. Pharmacother. 2021, 140, 111592. [Google Scholar] [CrossRef]
- Ghanem, P.; Zouein, A.; Mohamad, M.; Hodroj, M.H.; Haykal, T.; Abou Najem, S.; Naim, H.Y.; Rizk, S. The Vitamin E Derivative Gamma Tocotrienol Promotes Anti-Tumor Effects in Acute Myeloid Leukemia Cell Lines. Nutrients 2019, 11, 2808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalife, R.; Hodroj, M.H.; Fakhoury, R.; Rizk, S. Thymoquinone from Nigella Sativa Seeds Promotes the Antitumor Activity of Noncytotoxic Doses of Topotecan in Human Colorectal Cancer Cells in Vitro. Planta Med. 2016, 82, 312–321. [Google Scholar] [CrossRef]
- Hodroj, M.H.; Jardaly, A.; Raad, S.A.; Zouein, A.; Rizk, S. Andrographolide Potentiates the Antitumor Effect of Topotecan in Acute Myeloid Leukemia Cells through an Intrinsic Apoptotic Pathway. Cancer Manag. Res. 2018, 10, 1079–1088. [Google Scholar] [CrossRef] [Green Version]
- Tannous, S.; Haykal, T.; Dhaini, J.; Hodroj, M.H.; Rizk, S. The Anti-Cancer Effect of Flaxseed Lignan Derivatives on Different Acute Myeloid Leukemia Cancer Cells. Biomed. Pharmacother. 2020, 132, 110884. [Google Scholar] [CrossRef]






| IC50 Value | ||
|---|---|---|
| Breast Cancer Cell Line | 24 h | 48 h |
| MDA-MB-231 | 0.926% v/v (≈21.55 µg/mL) | 0.289% v/v (≈6.73 µg/mL) |
| MCF-7 | 0.992% v/v (≈23.08 µg/mL) | 0.160% v/v (≈3.72 µg/mL) |
| Name | Formula | RT (min) | Area (max) | Negative Run | Positive Run |
|---|---|---|---|---|---|
| Bis(4-ethylbenzylidene) sorbitol | C24H30O6 | 37.878 | 3,174,434,618 | 3,174,434,618 | |
| 4-oxo-4,5,6,7-tetrahydrobenzo[b]furan-3-carboxylic acid | C9H8O4 | 11.438 | 2,230,875,723 | 2,230,875,723 | |
| Lycorine | C16H17NO4 | 2.837 | 2,150,797,725 | 2,150,797,725 | |
| 3-oxoindane-1-carboxylic acid | C10H8O3 | 31.823 | 1,490,616,529 | 1,490,616,529 | |
| L-Phenylalanine | C9H11NO2 | 2.793 | 1,081,816,069 | 1,081,816,069 | |
| (9Z,11E,15Z)-13-hydroxyoctadeca-9,11,15-trienoic acid | C18H30O3 | 38.526 | 926,272,408.7 | 926,272,408.7 | |
| (10E,12E)-9-hydroperoxyoctadeca-10,12-dienoic acid | C18H32O4 | 38.535 | 890,320,782.9 | 890,320,782.9 | |
| 2-Amino-1,3,4-octadecanetriol | C18H39NO3 | 31.808 | 610,188,836.2 | 610,188,836.2 | |
| 13,14-dihydro Prostaglandin F1α | C20H38O5 | 35.606 | 511,502,894.1 | 511,502,894.1 | |
| 9S,13R-12-Oxophytodienoic acid | C18H28O3 | 39.803 | 461,820,295.2 | 68,310,411.97 | 461,820,295.2 |
| Cetrimonium | C19H41N | 39.798 | 420,064,536.9 | 420,064,536.9 | |
| 3-hydroxy-4-(3-hydroxyphenyl)-1,2-dihydroquinolin-2-one | C15H11NO3 | 9.26 | 308,498,766.5 | 308,498,766.5 | |
| 4-Hydroxybenzoic acid | C7H6O3 | 36.532 | 297,387,086.7 | 297,387,086.7 | |
| D-(+)-Tryptophan | C11H12N2O2 | 5.543 | 253,102,825.1 | 155,006,313.2 | 253,102,825.1 |
| D-(−)-Quinic acid | C7H12O6 | 3.174 | 247,235,220.9 | 247,235,220.9 | |
| 4-(tert-butyl)phenyl 3,5-dimethylisoxazole-4-carboxylate | C16H19NO3 | 3.226 | 240,995,401.5 | 240,995,401.5 | |
| Sedanolide | C12H18O2 | 38.527 | 183,054,018.2 | 183,054,018.2 | |
| Corchorifatty acid F | C18H32O5 | 31.371 | 178,994,397.1 | 178,994,397.1 | |
| 2,2,6,6-Tetramethyl-1-piperidinol (TEMPO) | C9H19NO | 29.349 | 164,033,305.7 | 164,033,305.7 | |
| α-Hydroxymidazolam | C18H13ClFN3O | 10.109 | 104,041,359.5 | 104,041,359.5 | |
| Ferulic acid | C10H10O4 | 11.449 | 101,224,790.3 | 101,224,790.3 | |
| Rhamnetin | C16H12O7 | 18.912 | 96,025,818.23 | 96,025,818.23 | |
| 4-Indolecarbaldehyde | C9H7NO | 16.502 | 79,507,735.41 | 79,507,735.41 | |
| 3-Methoxy-5,7,3’,4’-tetrahydroxy-flavone | C16H12O7 | 27.133 | 79,447,955.04 | 79,447,955.04 | 78,747,838.78 |
| Isoleucine | C6H13NO2 | 2.327 | 71,402,978.66 | 71,402,978.66 | |
| L-Pyroglutamic acid | C5H7NO3 | 2.204 | 61,948,424.66 | 61,948,424.66 | |
| L-Phenylalanine | C9H11NO2 | 2.135 | 61,341,499.61 | 61,341,499.61 | |
| Corchorifatty acid F | C18H32O5 | 26.367 | 60,847,215.25 | 60,847,215.25 | |
| ethyl 9H-beta-carboline-3-carboxylate | C14H12N2O2 | 19.841 | 56,707,514.56 | 56,707,514.56 | |
| Phomolide G | C12H20O5 | 19.341 | 54,163,834.9 | 54,163,834.9 | |
| Cinchophen | C16H11NO2 | 10.536 | 40,663,903.34 | 40,663,903.34 | |
| 9S,13R-12-Oxophytodienoic acid | C18H28O3 | 31.311 | 39,636,902.48 | 39,636,902.48 | |
| 4-Acetyl-3-hydroxy-5-methylphenyl β-D-glucopyranoside | C15H20O8 | 8.52 | 39,273,094.27 | 39,273,094.27 | |
| trans-Cinnamaldehyde | C9H8O | 11.449 | 38,739,571.25 | 38,739,571.25 | |
| Quercetin | C15H10O7 | 16.992 | 35,465,633.57 | 35,465,633.57 | |
| 3,4-Dihydroxybenzaldehyde | C7H6O3 | 8.401 | 33,254,282.78 | 33,254,282.78 | |
| Jasmonic acid | C12H18O3 | 24.837 | 31,224,896.2 | 31,224,896.2 | |
| Corchorifatty acid F | C18H32O5 | 36.331 | 29,858,068.04 | 29,858,068.04 | |
| Pyridoxal | C8H9NO3 | 15.696 | 29,711,710.82 | 29,711,710.82 | |
| 4-Indolecarbaldehyde | C9H7NO | 5.467 | 29,689,856.04 | 29,689,856.04 | |
| 2-benzyl-6-hydroxy-2-azabicyclo [2.2.2]octan-3-one | C14H17NO2 | 2.876 | 25,424,100.69 | 25,424,100.69 | |
| Sedanolide | C12H18O2 | 16.589 | 25,382,213.3 | 25,382,213.3 | |
| Azelaic acid | C9H16O4 | 18.216 | 23,684,670.17 | 23,684,670.17 | |
| L-Norleucine | C6H13NO2 | 2.884 | 17,292,410.45 | 17,292,410.45 | |
| Sedanolide | C12H18O2 | 27.958 | 15,283,130.43 | 15,283,130.43 | |
| trans-Anethole | C10H12O | 34.624 | 15,280,838.86 | 15,280,838.86 | |
| Tetradecanedioic acid | C14H26O4 | 29.245 | 14,533,711.98 | 14,533,711.98 | |
| Adenosine | C10H13N5O4 | 2.633 | 14,190,960.27 | 14,190,960.27 | |
| (±)-Abscisic acid | C15H20O4 | 21.622 | 12,847,644.97 | 12,847,644.97 | |
| Methyl cinnamate | C10H10O2 | 13.745 | 12,569,420.87 | 12,569,420.87 | |
| Fumaritine N-oxide | C20H21NO6 | 10.14 | 12,365,381.38 | 12,365,381.38 | |
| Melicopidine | C17H15NO5 | 18.576 | 12,285,467.72 | 12,285,467.72 | |
| Luteolin-3’,7-Diglucoside | C27H30O16 | 12.912 | 10,648,923.76 | 8,681,866.882 | 10,648,923.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Samarji, M.; Younes, M.; El Khoury, M.; Haykal, T.; Elias, N.; Gasilova, N.; Menin, L.; Houri, A.; Machaka-Houri, N.; Rizk, S. The Antioxidant and Proapoptotic Effects of Sternbergia clusiana Bulb Ethanolic Extract on Triple-Negative and Estrogen-Dependent Breast Cancer Cells In Vitro. Plants 2023, 12, 529. https://doi.org/10.3390/plants12030529
El Samarji M, Younes M, El Khoury M, Haykal T, Elias N, Gasilova N, Menin L, Houri A, Machaka-Houri N, Rizk S. The Antioxidant and Proapoptotic Effects of Sternbergia clusiana Bulb Ethanolic Extract on Triple-Negative and Estrogen-Dependent Breast Cancer Cells In Vitro. Plants. 2023; 12(3):529. https://doi.org/10.3390/plants12030529
Chicago/Turabian StyleEl Samarji, Mona, Maria Younes, Marianne El Khoury, Tony Haykal, Nazira Elias, Natalia Gasilova, Laure Menin, Ahmad Houri, Nisrine Machaka-Houri, and Sandra Rizk. 2023. "The Antioxidant and Proapoptotic Effects of Sternbergia clusiana Bulb Ethanolic Extract on Triple-Negative and Estrogen-Dependent Breast Cancer Cells In Vitro" Plants 12, no. 3: 529. https://doi.org/10.3390/plants12030529
APA StyleEl Samarji, M., Younes, M., El Khoury, M., Haykal, T., Elias, N., Gasilova, N., Menin, L., Houri, A., Machaka-Houri, N., & Rizk, S. (2023). The Antioxidant and Proapoptotic Effects of Sternbergia clusiana Bulb Ethanolic Extract on Triple-Negative and Estrogen-Dependent Breast Cancer Cells In Vitro. Plants, 12(3), 529. https://doi.org/10.3390/plants12030529