Cachrys L. Genus: A Comprehensive Review on Botany, Phytochemistry and Biological Properties
Abstract
:1. Introduction
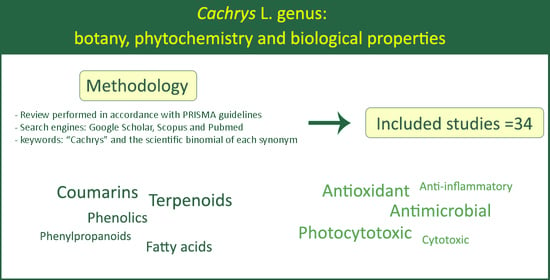
2. Methodology
3. Taxonomy of the Cachrys Genus
4. Botanical Description and Ethnobotanical Uses
5. Phytochemical Constituents
5.1. Coumarins
5.2. Terpenoids
5.3. Phenylpropanoids
5.4. Phenolic Compounds
5.5. Fatty Acids
5.6. Phytosterols and Others
6. Biological Properties of Cachrys Species
6.1. Antioxidant Activity
6.2. Antimicrobial Activity
6.3. Anti-Inflammatory Potential
6.4. Antiproliferative Activity
6.5. Photocytotoxic Activity
6.6. Acetylcholinesterase (AChE) and Butyrylcholinesterase (BChE) Inhibitory Activity
6.7. Xanthine Oxidoreductase (XOR) Inhibitory Activity
6.8. Insecticidal Activity
7. Phytotoxicity and Toxicological Profile
8. Other Cachrys Species
9. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- The International Plant Names Index. 2022. Available online: http://www.ipni.org (accessed on 2 February 2022).
- Ferrer-Gallego, P.P.; Fabado, J. (2822) Proposal to Conserve the Name Cachrys libanotis (Umbelliferae) with a Conserved Type. TAXON 2021, 70, 682–684. [Google Scholar] [CrossRef]
- WFO. 2022. Available online: http://www.worldfloraonline.org (accessed on 28 November 2022).
- Grande, M.; Aguado, M.T.; Mancheño, B.; Piera, F. Coumarins and Ferulol Esters from Cachrys sicula. Phytochemistry 1986, 25, 505–507. [Google Scholar] [CrossRef]
- Marrelli, M.; Perri, M.R.; Amodeo, V.; Giordano, F.; Statti, G.A.; Panno, M.L.; Conforti, F. Assessment of Photo-Induced Cytotoxic Activity of Cachrys sicula and Cachrys libanotis Enriched-Coumarin Extracts against Human Melanoma Cells. Plants 2021, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Menichini, G.; Alfano, C.; Provenzano, E.; Marrelli, M.; Statti, G.A.; Menichini, F.; Conforti, F. Cachrys pungens Jan Inhibits Human Melanoma Cell Proliferation through Photo-Induced Cytotoxic Activity. Cell Prolif. 2012, 45, 39–47. [Google Scholar] [CrossRef]
- Bouderdara, N.; Elomri, A.; Djarri, L.; Medjroubi, K.; Seguin, E.; Vérité, P. Chemical Composition of the Essential Oil of Cachrys libanotis from Algeria. Nat. Prod. Commun. 2011, 6, 115–117. [Google Scholar] [CrossRef] [Green Version]
- Khalilzadeh, M.A.; Tajbakhsh, M.; Gholami, F.A.; Hosaseinzadeh, M.; Dastoorani, P.; Norouzi, M.; Dabiri, H.A. Composition of the Essential Oils of Hippomarathrum microcarpum (M. Bieb.) B. Fedtsch. and Physospermum cornubiense (L.) DC. from Iran. J. Essent. Oil Res. 2007, 19, 567–568. [Google Scholar] [CrossRef]
- Perri, M.R.; Pellegrino, M.; Aquaro, S.; Cavaliere, F.; Lupia, C.; Uzunov, D.; Marrelli, M.; Conforti, F.; Statti, G. Cachrys spp. from Southern Italy: Phytochemical Characterization and JAK/STAT Signaling Pathway Inhibition. Plants 2022, 11, 2913. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Plants of the World Online. Available online: https://powo.science.kew.org/ (accessed on 28 November 2022).
- Duran, A.; Doğan, B.; Ay, H. Bilacunaria aksekiensis (Apiaceae), a New Species from South Anatolia, Turkey. Ann. Bot. Fenn. 2011, 48, 361–367. [Google Scholar] [CrossRef]
- Küçükboyacι, N.; Ayaz, F.; Adιgüzel, N.; Bani, B.; Gören, A.C. Fatty Acid Methyl Ester Composition of Some Turkish Apiaceae Seed Oils: New Sources for Petroselinic Acid. Nat. Prod. Commun. 2016, 11, 1697–1700. [Google Scholar] [CrossRef] [Green Version]
- Kürkçüoglu, M. Essential Oil Composition from Fruits and Aerial Parts of Bilacunaria anatolica A. Duran (Apiaceae) Endemic in Turkey. J. Essent. Oil Bear. Plants 2016, 19, 379–383. [Google Scholar] [CrossRef]
- Palá-Paúl, J.; Velasco-Negueruela, A.; Pérez-Alonso, M.J.; Sanz, J. Essential Oil Composition of the Aerial Parts of Cachrys Sicula L. Flavour Fragr. J. 2002, 17, 64–68. [Google Scholar] [CrossRef]
- Boissier, P.E. Flora Orientalis; Apud H. Georg, Bibliopolam: Lyon, France, 1872. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A. Flora Europaea; Cambridge University Press: London, UK, 1968. [Google Scholar]
- Mousavi, S.; Mozaffarian, V.; Mummenhoff, K.; Downie, S.R.; Zarre, S. An Updated Lineage-Based Tribal Classification of Apiaceae Subfamily Apioideae with Special Focus on Iranian Genera. Syst. Biodivers. 2021, 19, 89–109. [Google Scholar] [CrossRef]
- Ghazanfar, S.A.; Edmondson, J.R. (Eds.) Flora of Iraq; Volume 5, Part 2: Lythraceae to Campanulaceae; Ministry of Agriculture, Republic of Iraq: Baghdad, Iraq, 2013. [Google Scholar]
- Aouachria, S.; Boumerfeg, S.; Benslama, A.; Boussoualim, N.; Trabsa, H.; Baghiani, A. Phenolics Contents, Xanthine Oxidoreductase Inhibitory Potential, Antibacterial and Antioxidant Activities of Cachrys libanotis L. Root Extracts. J. Drug Deliv. Ther. 2020, 10, 71–79. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Demirci, B.; Akalin, E.; Özhatay, N. Composition of the Essential Oil of Cachrys alpine Bieb. J. Essent. Oil Res. 2004, 16, 167–168. [Google Scholar] [CrossRef]
- Tunçtürk, M.; Özgökçe, F. Chemical Composition of Some Apiaceae Plants Commonly Used in Herby Cheese in Eastern Anatolia. Turk. J. Agric. For. 2015, 39, 55–62. [Google Scholar] [CrossRef]
- Özek, G.; Özek, T.; Başer, K.H.C.; Hamzaoglu, E.; Duran, A. Composition of the Essential Oil of Hippomarathrum cristatum (DC.) Boiss. J. Essent. Oil Res. 2007, 19, 540–542. [Google Scholar] [CrossRef]
- Gül, S. Investigations into Feed Value of Hippomarathrum microcarpum (Bieb) Fedtsch Silages. ISPEC J. Agric. Sci. 2022, 6, 136–143. [Google Scholar] [CrossRef]
- Küpeli Akkol, E.; Genç, Y.; Karpuz, B.; Sobarzo-Sánchez, E.; Capasso, R. Coumarins and Coumarin-Related Compounds in Pharmacotherapy of Cancer. Cancers 2020, 12, 1959. [Google Scholar] [CrossRef]
- Conforti, F.; Marrelli, M.; Menichini, F.; Bonesi, M.; Statti, G.; Provenzano, E.; Menichini, F. Natural and Synthetic Furanocoumarins as Treatment for Vitiligo and Psoriasis. Curr. Drug Ther. 2009, 4, 38–58. [Google Scholar] [CrossRef]
- Bruni, R.; Barreca, D.; Protti, M.; Brighenti, V.; Righetti, L.; Anceschi, L.; Mercolini, L.; Benvenuti, S.; Gattuso, G.; Pellati, F. Botanical Sources, Chemistry, Analysis, and Biological Activity of Furanocoumarins of Pharmaceutical Interest. Molecules 2019, 24, 2163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abyshev, A.Z. The Coumarin Composition of the Roots of Hippomarathrum caspicum. Chem. Nat. Compd. 1973, 9, 514. [Google Scholar] [CrossRef]
- Doganca, S.; Ulubelen, A.; Ishikawa, T.; Ishii, H. (+)-Peucedanol Methyl Ether from Hippomarathrum cristatum, Umbelliferae. Chem. Pharm. Bull. 1979, 27, 1049–1050. [Google Scholar] [CrossRef] [Green Version]
- De Leo, M.; D’ambola, M.; Germanò, M.P.; Venturella, F. Coumarins from Cachrys sicula L. Pharmacologyonline 2017, 2, 213–217. [Google Scholar]
- Evergetis, E.; Haroutounian, S.A. Exploitation of Apiaceae Family Plants as Valuable Renewable Source of Essential Oils Containing Crops for the Production of Fine Chemicals. Ind. Crops Prod. 2014, 54, 70–77. [Google Scholar] [CrossRef]
- Tahar, S.; Hamdi, B.; Flamini, G.; Mehmet, Ö.; Duru, M.E.; Bruno, M.; Maggi, F. Chemical Composition, Antioxidant and Anticholinesterase Activity of the Essential Oil of Algerian Cachrys sicula L. Nat. Prod. Res. 2022, 36, 4094–4102. [Google Scholar] [CrossRef]
- Koutsaviti, A.; Georgiou, C.; Milenković, M.; Tzakou, O. Composition and Antimicrobial Activity of the Essential Oils from Different Parts of Cachrys cristata DC. from Greece. Rec. Nat. Prod. 2015, 9, 436. [Google Scholar]
- Özer, H.; Sökmen, M.; Güllüce, M.; Adigüzel, A.; Şahin, F.; Sökmen, A.; Kiliç, H.; Bariş, Ö. Chemical Composition and Antimicrobial and Antioxidant Activities of the Essential Oil and Methanol Extract of Hippomarathrum microcarpum (Bieb.) from Turkey. J. Agric. Food Chem. 2007, 55, 937–942. [Google Scholar] [CrossRef]
- Karakaya, S.; Göger, G.; Bostanlik, F.D.; Demirci, B.; Duman, H.; Kiliç, C.S. Comparison of the Essential Oils of Ferula orientalis L., Ferulago sandrasica Peşmen and Quézel, and Hippomarathrum microcarpum Petrov and Their Antimicrobial Activity. Turk. J. Pharm. Sci. 2019, 16, 69–75. [Google Scholar] [CrossRef]
- Sefidkon, F.; Shaabani, A. Analysis of the Oil of Hippomarathrum microcarpum (M. B.) B. Fedtsch. from Iran. J. Essent. Oil Res. 2003, 15, 261–262. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Özek, T.; Aytaç, Z. Essential Oil of Hippomarathrum boissieri Reuter et Hausskn. J. Essent. Oil Res. 2000, 12, 231–232. [Google Scholar] [CrossRef]
- Araniti, F.; Marrelli, M.; Lupini, A.; Mercati, F.; Statti, G.A.; Abenavoli, M.R. Phytotoxic Activity of Cachrys pungens Jan, a Mediterranean Species: Separation, Identification and Quantification of Potential Allelochemicals. Acta Physiol. Plant. 2014, 36, 1071–1083. [Google Scholar] [CrossRef]
- Pinar, M.; Alemany, A. N-N′-Di-o-Tolylethylendiamine from Cachrys sicula. Phytochemistry 1975, 14, 313–314. [Google Scholar] [CrossRef]
- Duran, A.; Uslu, N.; Doğan, B.; Özcan, M.M.; Çelik, M. Antioxidant Activity and Phenolic Contents of 30 Selected Medicinal Plants. J. Agroaliment. Process. Technol. 2015, 21, 136–141. [Google Scholar]
- Matejic, J.; Dzamic, A.; Mihajilov-Krstev, T.; Randjelovic, V.; Mileski, K.; Marin, P. Total Phenolic and Flavonoid Contents and Biological Activities of Cachrys cristata DC. Extracts. Arch. Biol. Sci. 2014, 66, 1117–1123. [Google Scholar] [CrossRef] [Green Version]
- Tavakolizadeh, M.; Andalib, S.; Ahmadi, M.H.; Khodaee, M. Anti-Inflammatory Effects of Ethanol Extracts of Hippomarathrum microcarpum (M. Bieb.) B. Fedtsch (Horse Fennel) in Laboratory Rats. Res. J. Pharmacogn. 2017, 4, 30. [Google Scholar]
- Karakaya, S.; Bakar, F.; KiliÇ, C.S. The Effect of Hippomarathrum microcarpum Petrov (Apiaceae) Growing in Turkey on PC3 Cancer Cell Proliferation. J. Fac. Pharm. Ankara 2016, 40, 19–25. [Google Scholar] [CrossRef]
- Marrelli, M.; Menichini, G.; Provenzano, E.; Conforti, F. Applications of Natural Compounds in the Photodynamic Therapy of Skin Cancer. Curr. Med. Chem. 2014, 21, 1371–1390. [Google Scholar] [CrossRef]
- Sumorek-Wiadro, J.; Zając, A.; Maciejczyk, A.; Jakubowicz-Gil, J. Furanocoumarins in Anticancer Therapy—For and Against. Fitoterapia 2020, 142, 104492. [Google Scholar] [CrossRef]
- Ahmed, S.; Khan, H.; Aschner, M.; Mirzae, H.; Küpeli Akkol, E.; Capasso, R. Anticancer Potential of Furanocoumarins: Mechanistic and Therapeutic Aspects. Int. J. Mol. Sci. 2020, 21, 5622. [Google Scholar] [CrossRef]
- Roelandts, R. The History of Phototherapy: Something New under the Sun? J. Am. Acad. Dermatol. 2002, 46, 926–930. [Google Scholar] [CrossRef]
- Carter, J.; Zug, K.A. Phototherapy for Cutaneous T-Cell Lymphoma: Online Survey and Literature Review. J. Am. Acad. Dermatol. 2009, 60, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Lindelöf, B.; Sigurgeirsson, B.; Tegner, E.; Larkö, O.; Johannesson, A.; Berne, B.; Christensen, O.B.; Andersson, T.; Törngren, M.; Molin, L.; et al. PUVA and Cancer: A Large-Scale Epidemiological Study. Lancet 1991, 338, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Helm, T.N.; Dijkstra, J.W.; Ferrara, R.J.; Glanz, S. PUVA Therapy. Am. Fam. Physician 1991, 43, 908–912. [Google Scholar] [PubMed]
- Gasparro, F.P. The Role of PUVA in the Treatment of Psoriasis. Am. J. Clin. Dermatol. 2000, 1, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Kumar, V.; Mal, M.; Houghton, P.J. Acetylcholinesterase inhibitors from plants. Phytomedicine 2007, 14, 289–300. [Google Scholar] [CrossRef]
- Greig, N.H.; Lahiri, D.K.; Sambamurti, K. Butyrylcholinesterase: An important new target in Alzheimer’s disease therapy. Int. Psychogeriatr. 2002, 14, 77–91. [Google Scholar] [CrossRef]
- Adewusi, E.A.; Moodley, N.; Steenkamp, V. Medicinal plants with cholinesterase inhibitory activity: A review. Afr. J. Biotechnol. 2010, 9, 8257–8276. [Google Scholar]
- Liu, N.; Xu, H.; Sun, Q.; Yu, X.; Chen, W.; Wei, H.; Jiang, J.; Xu, Y.; Lu, W. The Role of Oxidative Stress in Hyperuricemia and Xanthine Oxidoreductase (XOR) Inhibitors. Oxid. Med. Cell. Longev. 2021, 2021, e1470380. [Google Scholar] [CrossRef]
- Pascual-Villalobos, M.J.; Robledo, A. Screening for Anti-Insect Activity in Mediterranean Plants. Ind. Crops Prod. 1998, 8, 183–194. [Google Scholar] [CrossRef]
- Ena, P.; Dessi, G.; Chiarolini, F.; Fabbri, P. Phytophotodermatitis from a Cachrys Species. Contact Derm. 1989, 20, 144–145. [Google Scholar] [CrossRef]
- Ena, P.; Cerri, R.; Dessi, G.; Manconi, P.M.; Atzei, D. Phototoxicity Due to Cachrys libanotis. Contact Derm. 1991, 24, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.; Ilardi, V.; Lupidi, G.; Quassinti, L.; Bramucci, M.; Fiorini, D.; Venditti, A.; Maggi, F. The Nonvolatile and Volatile Metabolites of Prangos Ferulacea and Their Biological Properties. Planta Med. 2019, 85, 815–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruno, M.; Ilardi, V.; Lupidi, G.; Quassinti, L.; Bramucci, M.; Fiorini, D.; Venditti, A.; Maggi, F. Composition and Biological Activities of the Essential Oil from a Sicilian Accession of Prangos ferulacea (L.) Lindl. Nat. Prod. Res. 2021, 35, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Ignat’eva, N.S.; Vandyshev, V.V.; Pimenov, M.G. Coumarins from the Roots of Cachrys pubescens. Chem. Nat. Compd. 1972, 8, 381. [Google Scholar] [CrossRef]
- Abad, M.J.; de las Heras, B.; Silván, A.M.; Pascual, R.; Bermejo, P.; Rodriguez, B.; Villar, A.M. Effects of Furocoumarins from Cachrys Trifida on Some Macrophage Functions. J. Pharm. Pharmacol. 2001, 53, 1163–1168. [Google Scholar] [CrossRef]
- Palá-Paül, J.; Velasco-Negueruela, A.; Pérez-Alonso, M.J.; Maqueda, J.; Sanz, J. Volatile Oil Constituents from Different Parts of Cachrys trifida L. J. Essent. Oil Res. 2004, 16, 347–349. [Google Scholar] [CrossRef]
- Bertoli, A.; Pistelli, L.; Morelli, I.; Spinelli, G.; Manunta, A. Constituents of Cachrys ferulacea Oils. J. Essent. Oil Res. 1998, 10, 533–536. [Google Scholar] [CrossRef]
- Camarda, L.; Mazzola, P.; Sprio, V. Coumarins from the fruits of Cachrys ferulacea. J. Nat. Prod. 1987, 50, 310. [Google Scholar] [CrossRef]
- Pistelli, L.; Catalano, S.; Manunta, A.; Marsili, A. Coumarins from Cachrys ferulacea Collected in Sardinia. Planta Med. 1989, 55, 203. [Google Scholar] [CrossRef]
- Pistelli, L.; Marsili, A.; Morelli, I.; Barili, P.L.; Pizza, C. Umbelliferose from Cachrys ferulacea Seeds: Determination of the Sugar Sequence by NMR 2D-COLOC Technique. Planta Med. 1990, 56, 230–231. [Google Scholar] [CrossRef] [PubMed]
- Badalamenti, N.; Maresca, V.; Di Napoli, M.; Bruno, M.; Basile, A.; Zanfardino, A. Chemical Composition and Biological Activities of Prangos ferulacea Essential Oils. Molecules 2022, 27, 7430. [Google Scholar] [CrossRef] [PubMed]
- Bagherifar, S.; Sourestani, M.M.; Zolfaghari, M.; Mottaghipisheh, J.; Zomborszki, Z.P.; Csupor, D. Chemodiversity of Volatile Oil Contents of Various Parts of 10 Iranian Prangos ferulacea Accessions, With Analysis of Antiradical Potential. Nat. Prod. Commun. 2019, 14, 1934578X19851985. [Google Scholar] [CrossRef] [Green Version]
- Đoković, D.D.; Bulatović, V.M.; Božić, B.Đ.; Kataranovski, M.V.; Zrakić, T.M.; Kovačević, N.N. 3,5-Nonadiyne Isolated from the Rhizome of Cachrys ferulacea Inhibits Endogenous Nitric Oxide Release by Rat Peritoneal Macrophages. Chem. Pharm. Bull. 2004, 52, 853–854. [Google Scholar] [CrossRef] [Green Version]
- Gajula, S.N.R.; Nanjappan, S. Metabolomics: A recent advanced omics technology in herbal medicine research. In Medicinal and Aromatic Plants; Aftab, T., Hakeem, K.R., Eds.; Academic Press: London, UK, 2021; pp. 97–117. [Google Scholar]
- Sumner, L.W.; Mendes, P.; Dixon, R.A. Plant metabolomics: Large-scale phytochemistry in the functional genomics era. Phytochemistry 2003, 62, 817–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witzel, K.; Neugart, S.; Ruppel, S.; Schreiner, M.; Wiesner, M.; Baldermann, S. Recent progress in the use of ‘omics technologies in brassicaceous vegetables. Front. Plant Sci. 2015, 6, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Species1 | Scientific Name Status | Synonym |
|---|---|---|
| C. aksekiensis (A.Duran & B.Doğan) Hand | Accepted | Bilacunaria aksekiensis A.Duran & B.Doğan |
| C. alpina M.Bieb. | Accepted | - |
| C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | Accepted | Bilacunaria boissieri (Reut. & Hausskn. ex Boiss.) Pimenov & V.N.Tikhom; Hippomarathrum boissieri Reut. & Hausskn. ex Boiss. |
| C. caspica (DC.) Menitsky | Accepted | Bilacunaria caspia (DC.) Pimenov & V.N.Tikhom; Echinophora caspica DC.; Hippomarathrum caspicum (DC.) Grossh. |
| C. crassiloba (Boiss.) Meikle | Accepted | Hippomarathrum crassilobum Boiss. & Heldr.; Peucedanum veneris Kotschy |
| C. cristata DC. | Accepted | Cachrys echinophora Guss. Hippomarathrum cristatum Boiss. Hippomarathrum cristatum var. pauciradiatum Heldr. & Halácsy Hippomarathrum libanotis var. cristatum (DC.) Maire Hippomarathrum pauciradiatum (Heldr. & Halácsy) Halácsy Trachymarathrum siculum Tausch |
| C. korolkowi Regel & Schmalh. | Ambiguous/ Doubtful | - |
| C. libanotis L. | Accepted | Cachrydium libanotis Link; Cachrys humilis Schousb.; Cachrys linearia Mill.; Cachrys peucedanoides Desf.; Cachrys pterochlaena var. leiocarpa Coss.; Cachrys sphaerocarpa Ten.; Crithmum libanotis Link; Hippomarathrum bocconei Boiss.; Hippomarathrum bocconei var. denticulatum Andr.; Hippomarathrum libanotis W.D.J.Koch ex DC.; Hippomarathrum libanotis subsp. bocconei (Boiss.) Maire; Hippomarathrum libanotis var. faurei Maire; Lophocachrys echinophora Bertol.; Smyrnium libanotis Crantz |
| C. microcarpos M.Bieb. | Accepted | Bilacunaria microcarpa (M.Bieb.) Pimenov & V.N.Tikhom; Cachrys amplifolia Ledeb.; Cachrys crispa Pers.; Cachrys libanotis Salzm. ex Ball; Cachrys longiloba DC.; Cachrys microcarpa M.Bieb.; Cachrys nudicaulis Godet ex DC.; Hippomarathrum amplifolium Ledeb. ex C.A.Mey.; Hippomarathrum crispum W.D.J.Koch; Hippomarathrum longilobum B.Fedtsch. ex Grossh.; Hippomarathrum microcarpum (M.Bieb.) Petrov |
| C. nematoloba Rech.f. & Riedl | Ambiguous/ Doubtful | - |
| C. pabularia (Lindl.) Herrnst. & Heyn | Unchecked | - |
| C. pungens Jan ex Guss | Accepted | Hippomarathrum libanotis var. pungens (Jan ex Guss.) Fiori; Hippomarathrum pungens Boiss. ex Pomel; Hippomarathrum pungens Lojac. |
| C. scabra (Fenzl) Meikle | Accepted | Bilacunaria scabra (Fenzl) Pimenov & V.N.Tikhom.; Ferula scabra Fenzl; Ferulago scabra Fenzl; Hippomarathrum scabrum Boiss. |
| C. sibirica Turcz. ex Ledeb. | Unchecked | - |
| C. sicula L. | Accepted | Cachrys crispata Pomel; Cachrys pterochlaena DC.; Hippomarathrum bocconei subsp. crispatum (Pomel) Batt.; Hippomarathrum bocconei var. brachylobum Batt.; Hippomarathrum brachylobum (Batt.) Sennen & Mauricio; Hippomarathrum crispatum Pomel; Hippomarathrum libanotis subsp. pterochlaenum (Boiss.) Maire; Hippomarathrum libanotis var. crispatum (Pomel) Maire; Hippomarathrum libanotis var. crispulum Maire; Hippomarathrum pterochlaenum Boiss.; Hippomarathrum siculum Hoffmanns. & Link; Seseli brachylobum (Batt.) M.Hiroe; Smyrnium hispidum Crantz; Ulospermum siculum Link |
| Compound Name | Species | Plant Part | Extract | Analysis | Ref. |
|---|---|---|---|---|---|
| Coumarins | |||||
| Bergapten | C. libanotis L. | aerial parts | MeOH extracts (maceration and Naviglio®) | GC-MS 1 | [5] |
| C. pungens Jan ex Guss | aerial parts | MeOH extract | GC-MS, GC 2 | [6] | |
| C. sicula L. | aerial parts | MeOH extracts (maceration and Naviglio®) | GC-MS | [5] | |
| umbels | benzene extract | NMR 3, IR 4, MS 5 | [4] | ||
| Columbianetin | C. pungens Jan ex Guss | aerial parts | MeOH extract | GC-MS | [6] |
| Heraclenin | C. caspica (DC.) Menitsky | root | CHCl3 extract | NMR | [28] |
| 8-Hydroxymethylpsoralen | C. sicula L. | leaves | EtOAc extract | NMR | [30] |
| Isogeijerin | C. libanotis L. | aerial parts | MeOH extracts (maceration and Naviglio®) | GC-MS | [5] |
| Imperatorin | C. sicula. L. | umbels, root | benzene extract | NMR, IR, MS | [4] |
| Isoimperatorin | C. caspica (DC.) Menitsky | root | CHCl3 extract | IR | [28] |
| C. sicula. L. | umbels | benzene extract | NMR, IR, MS | [4] | |
| Isooxypeucedanin | C. pungens Jan ex Guss | aerial parts | MeOH extract | GC-MS, GC | [6] |
| Isopimpinellin | C. libanotis L. | aerial parts | MeOH extracts (maceration and Naviglio®) | GC-MS | [5] |
| C. pungens Jan ex Guss | aerial parts | MeOH extract | GC-MS, GC | [6] | |
| C. sicula L. | aerial parts | MeOH extracts (maceration and Naviglio®) | GC-MS | [5] | |
| umbels | benzene extract | NMR, IR, MS | [4] | ||
| Jatamansin | C. pungens Jan ex Guss | aerial parts | MeOH extract | GC-MS | [6] |
| tert-O-methylheraclenol | C. sicula. L. | umbels | benzene extract | NMR, IR, MS | [4] |
| 3-Methylsuberosine | C. pungens Jan ex Guss | aerial parts | MeOH extract | GC-MS, GC | [10] |
| Osthole | C. caspica (DC.) Menitsky | root | CHCl3 extract | IR | [28] |
| C. libanotis L. | aerial parts | MeOH extracts | GC-MS | [5] | |
| Oxypeucedanin | C. caspica (DC.) Menitsky | root | CHCl3 extract | IR | [28] |
| C. sicula L. | leaves | EtOAc extract | NMR | [30] | |
| root | benzene extract | NMR, IR, MS | [4] | ||
| Pabulenol | C. sicula. L. | root | benzene extract | NMR, IR, MS | [4] |
| Pangelin | C. caspica (DC.) Menitsky | root | CHCl3 extract | NMR | [28] |
| Psoralen | C. pungens Jan ex Guss | aerial parts | MeOH extract | GC-MS, GC | [6] |
| Prantschimgin | C. sicula. L. | umbels, root | benzene extract | NMR, IR, MS | [4] |
| Salaxin | C. sicula. L. | root | benzene extract | NMR, IR, MS | [4] |
| Seselin | C. libanotis L. | aerial parts | MeOH extract | GC-MS | [5] |
| Sprengelianin | C. sicula L. | leaf | EtOAc extract | NMR | [30] |
| umbels, root | benzene extract | NMR, IR, MS | [4] | ||
| Suberosin | C. libanotis L. | aerial parts | MeOH extract | GC-MS | [5] |
| Ulopterol | C. cristata DC. | - | CHCl3 extract | IR, NMR | [29] |
| C. sicula. L. | umbels | benzene extract | NMR, IR, MS | [4] | |
| Umbelliferone | C. caspica (DC.) Menitsky | root | CHCl3 extract | IR | [28] |
| Xanthotoxin | C. libanotis L. | aerial parts | MeOH extracts (maceration and Naviglio®) | GC-MS | [5] |
| C. pungens Jan ex Guss | aerial parts | MeOH extract | GC-MS, GC | [6] | |
| C. sicula L. | aerial parts | MeOH extracts (maceration and Naviglio®) | GC-MS | [5] | |
| umbels | Benzene extract | NMR, IR, MS | [4] | ||
| 2-Methyl-2-butenoic acid 9,10-dihydro-8,8-dimethyl-2-oxo-2H,8Hbenzo[1,2- b:3,4-b0]dipyran-9-yl ester | C. libanotis L. | aerial parts | MeOH extract (Naviglio®) | GC-MS | [5] |
| C. sicula L. | aerial parts | MeOH extract (Naviglio®) | GC-MS | [5] | |
| Terpenoids | |||||
| Anethole | C. cristata DC. | fruit | EO 6 | GC-MS | [23] |
| C. sicula L. | aerial parts | MeOH extract (Naviglio®) | GC-MS | [5] | |
| Aromadendrene | C. cristata DC. | leaf | EO | GC-MS | [33] |
| C. sicula L. | aerial parts | EO | GC-MS | [15] | |
| Bicycloelemene | C. libanotis L. | aerial parts | EO | GC-MS | [7] |
| bicyclogermacrene | C. alpina M.Bieb. | fruit | EO | GC-MS | [21] |
| C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] | |
| C. cristata DC. | leaf, stem, fruit | EO | GC-MS | [23,33] | |
| whole plant | EO | GC-MS | [31] | ||
| C. sicula L. | leaf | EO | GC-MS | [32] | |
| β-bisabolene | C. cristata DC. | leaf, root, fruit | EO | GC-MS | [23,33] |
| C. microcarpos M.Bieb. | flower, leaf, aerial parts | EO | GC-MS | [8,36] | |
| C. pungens Jan ex Guss | aerial parts | MeOH extract | GC-MS | [6] | |
| γ-bisabolene | C. microcarpos M.Bieb. | leaf | EO | GC-MS | [34] |
| α-bisabolol | C. cristata DC. | leaf, root, fruit | EO | GC-MS | [23,33] |
| epi-α-bisabolol | C. sicula L. | leaf | EO | GC-MS | [32] |
| borneol | C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] |
| C. microcarpos M.Bieb. | leaf | EO | GC-MS | [34] | |
| aerial parts | EO | GC-MS | [8] | ||
| C. sicula L. | aerial parts | EO | GC-MS | [15] | |
| bornyl acetate | C. alpina M.Bieb. | fruit | EO | GC-MS | [21] |
| C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] | |
| C. cristata DC. | leaf, stem, root, fruit | EO | GC-MS | [23,33] | |
| C. microcarpos M.Bieb. | flower, leaf, aerial parts | EO | GC-MS | [8,34,36] | |
| C. sicula L. | aerial parts, leaf | EO | GC-MS | [15,32] | |
| α-bourbonene | C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] |
| C. cristata DC. | fruit | EO | GC-MS | [23] | |
| β-bourbonene | C. alpina M.Bieb. | fruit | EO | GC-MS | [21] |
| C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] | |
| C. cristata DC. | fruit | EO | GC-MS | [23] | |
| C. microcarpos M.Bieb. | flower, leaf, aerial parts | EO | GC-MS | [8,34,36] | |
| C. sicula L. | aerial parts | EO | GC-MS | [15] | |
| γ-cadinene | C. alpina M.Bieb. | fruit | EO | GC-MS | [21] |
| C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] | |
| C. cristata DC. | leaf, fruit | EO | GC-MS | [23,33] | |
| C. microcarpos M.Bieb. | leaf | EO | GC-MS | [34] | |
| C. pungens Jan ex Guss | aerial parts | MeOH extract | GC-MS | [6] | |
| δ-cadinene | C. alpina M.Bieb. | fruit | EO | GC-MS | [21] |
| C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] | |
| C. cristata DC. | leaf, stem, fruit | EO | GC-MS | [23,33] | |
| whole plant | EO | GC-MS | [31] | ||
| C. libanotis L. | aerial parts | EO | GC-MS | [7] | |
| C. microcarpos M.Bieb. | flower, leaf, aerial parts | EO | GC-MS | [34,35,36] | |
| C. sicula L. | aerial parts, leaf | EO | GC-MS | [15,32] | |
| α-cadinol | C. alpina M.Bieb | fruit | EO | GC-MS | [21] |
| C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] | |
| C. cristata DC. | fruit | EO | GC-MS | [23] | |
| C. microcarpos M.Bieb. | aerial parts | EO | GC-MS | [8] | |
| C. sicula L. | aerial parts, leaf | EO | GC-MS | [15,32] | |
| Τ-cadinol | C. alpina M.Bieb | fruit | EO | GC-MS | [21] |
| C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] | |
| C. sicula L. | leaf | EO | GC-MS | [32] | |
| α-calacorene | C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] |
| C. libanotis L. | aerial parts | EO | GC-MS | [7] | |
| β-calacorene | C. microcarpos M.Bieb. | leaf | EO | GC-MS | [34] |
| cis-calamenene | C. libanotis L. | aerial parts | EO | GC-MS | [7] |
| calarene | C. libanotis L. | aerial parts | EO | GC-MS | [7] |
| camphene | C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] |
| C. cristata DC. | leaf, stem, root, fruit | EO | GC-MS | [23,33] | |
| C. microcarpos M.Bieb. | aerial parts, leaf, flower | EO | GC-MS | [8,35,36] | |
| C. sicula L. | aerial parts, leaf | EO | GC-MS | [15,32] | |
| camphor | C. microcarpos M.Bieb. | leaf | EO | GC-MS | [34] |
| δ-3-carene | C. cristata DC. | leaf, stem, root | EO | GC-MS | [33] |
| C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] | |
| C. sicula L. | aerial parts | EO | GC-MS | [15] | |
| carvacrol | C. alpina M.Bieb. | fruit | EO | GC-MS | [21] |
| C. cristata DC. | fruit | EO | GC-MS | [23] | |
| C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] | |
| C. libanotis L. | aerial parts | EO | GC-MS | [7] | |
| C. microcarpos M.Bieb. | flower, leaf | EO | GC-MS | [36] | |
| C. pungens Jan ex Guss | aerial parts | MeOH extract | GC-MS | [6] | |
| C. sicula L. | aerial parts | EO | GC-MS | [15] | |
| carvacrol methyl ether | C. cristata DC. | leaf, stem | EO | GC-MS | [33] |
| trans-carveol | C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] |
| C. microcarpos M.Bieb. | leaf | EO | GC-MS | [34] | |
| carvenone | C. cristata DC. | leaf | EO | GC-MS | [33] |
| carvone | C. microcarpos M.Bieb. | leaf | EO | GC-MS | [34] |
| β-caryophyllene | C. alpina M.Bieb | fruit | EO | GC-MS | [21] |
| C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] | |
| C. cristata DC. | leaf, stem, fruit | EO | GC-MS | [23,33] | |
| whole plant | EO | GC-MS | [31] | ||
| C. microcarpos M.Bieb. | flower, leaf, aerial parts | EO | GC-MS | [8,34,35,36] | |
| C. pungens Jan ex Guss | aerial parts | MeOH extract | GC-MS | [6] | |
| C. sicula L. | leaf | EO | GC-MS | [32] | |
| caryophyllene oxide | C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] |
| C. cristata DC. | leaf, stem, fruit | EO | GC-MS | [23,24,25,26,27,28,29,30,31,32,33] | |
| C. libanotis L. | aerial parts | EO | GC-MS | [7] | |
| C. microcarpos M.Bieb. | flower, leaf, aerial parts | EO | GC-MS | [8,34,35,36] | |
| C. sicula L. | leaf | EO | GC-MS | [32] | |
| 1,8-cineole | C. microcarpos M.Bieb. | leaf | EO | GC-MS | [34] |
| clovene | C. pungens Jan ex Guss | aerial parts | MeOH extract | GC-MS | [6] |
| α-copaene | C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] |
| C. cristata DC. | leaf, stem, fruit | EO | GC-MS | [23,24,25,26,27,28,29,30,31,32,33] | |
| C. libanotis L. | aerial parts | EO | GC-MS | [7] | |
| C. microcarpos M.Bieb. | flower, leaf, aerial parts | EO | GC-MS | [34,35,36] | |
| C. pungens Jan ex Guss | aerial parts | MeOH extract | GC-MS | [6] | |
| C. sicula L. | leaf | EO | GC-MS | [32] | |
| β-copaene | C. cristata DC. | fruit | EO | GC-MS | [23] |
| cryptone | C. cristata DC. | root | EO | GC-MS | [33] |
| p-cymene | C. alpina M.Bieb | fruit | EO | GC-MS | [21] |
| C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] | |
| C. cristata DC. | leaf, stem, root, fruit | EO | GC-MS | [23,33] | |
| C. microcarpos M.Bieb. | flower, leaf, aerial parts | EO | GC-MS | [8,34,35,36] | |
| C. libanotis L. | aerial parts | EO | GC-MS | [7] | |
| C. sicula L. | aerial parts, leaf | EO | GC-MS | [15,32] | |
| p-cymenene | C. cristata DC. | root | EO | GC-MS | [33] |
| p-cymen-8-ol | C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] |
| C. cristata DC. | leaf, stem | EO | GC-MS | [33] | |
| C. libanotis L. | aerial parts | EO | GC-MS | [7] | |
| C. microcarpos M.Bieb. | leaf | EO | GC-MS | [34] | |
| C. sicula L. | aerial parts | EO | GC-MS | [15] | |
| α-cubebene | C. libanotis L. | aerial parts | EO | GC-MS | [7] |
| C. microcarpos M.Bieb. | flower | EO | GC-MS | [36] | |
| C. pungens Jan ex Guss | aerial parts | MeOH extract | GC-MS | [6] | |
| C. sicula L. | aerial parts | EO | GC-MS | [15] | |
| β-cubebene | C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] |
| C. cristata DC. | leaf, stem | EO | GC-MS | [33] | |
| C. libanotis L. | aerial parts | EO | GC-MS | [7] | |
| C. microcarpos M.Bieb. | flower, leaf, aerial parts | EO | GC-MS | [8,36] | |
| C. sicula L. | leaf | EO | GC-MS | [32] | |
| epi-cubebol | C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] |
| C. sicula L. | aerial parts | EO | GC-MS | [15] | |
| cubebol | C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] |
| C. cristata DC. | fruit | EO | GC-MS | [23] | |
| α-cuprenene | C. cristata DC. | leaf | EO | GC-MS | [33] |
| daucene | C. cristata DC. | leaf, stem | EO | GC-MS | [33] |
| β-elemene | C. alpina M.Bieb. | fruit | EO | GC-MS | [21] |
| C. cristata DC. | leaf, stem, fruit | EO | GC-MS | [23,24,25,26,27,28,29,30,31,32,33] | |
| C. microcarpos M.Bieb. | leaf | EO | GC-MS | [34] | |
| γ-elemene | C. cristata DC. | leaf, stem | EO | GC-MS | [33] |
| C. sicula L. | aerial parts | EO | GC-MS | [15] | |
| δ-elemene | C. cristata DC. | leaf | EO | GC-MS | [33] |
| elemol | C. cristata DC. | root | EO | GC-MS | [33] |
| eremophilene | C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] |
| estragole | C. sicula L. | aerial parts | MeOH extracts (maceration and Naviglio®) | GC-MS | [5] |
| C. libanotis L. | aerial parts | MeOH extracts (maceration and Naviglio®) | GC-MS | [5] | |
| 7-epi-dehydrosesquicineole | C. cristata DC. | leaf, stem | EO | GC-MS | [33] |
| 4-epi-cis-dihydro-agarofuran | C. cristata DC. | root | EO | GC-MS | [33] |
| 2,5-dimethoxy-p-cymene | C. cristata DC. | root | EO | GC-MS | [33] |
| α-farnesene | C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] |
| C. cristata DC. | leaf | EO | GC-MS | [33] | |
| β-farnesene | C. cristata DC. | fruit | EO | GC-MS | [23] |
| C. pungens Jan ex Guss | aerial parts | MeOH extract | GC-MS | [6] | |
| C. sicula L. | aerial parts | EO | GC-MS | [15] | |
| fenchyl alcohol | C. microcarpos M.Bieb. | aerial parts | EO | GC-MS | [35] |
| C. cristata DC. | leaf, stem, root | EO | GC-MS | [33] | |
| fenchone | C. sicula L. | aerial parts | MeOH extract (Naviglio®) | GC-MS | [5] |
| β-funebrene | C. cristata DC. | fruit | EO | GC-MS | [23] |
| geranyl acetone | C. cristata DC. | fruit | EO | GC-MS | [23] |
| geraniol | C. microcarpos M.Bieb. | leaf | EO | GC-MS | [36] |
| germacrene B | C. alpina M.Bieb. | fruit | EO | GC-MS | [21] |
| C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] | |
| C. cristata DC. | leaf, fruit | EO | GC-MS | [23,24,25,26,27,28,29,30,31,32,33] | |
| C. microcarpos M.Bieb. | aerial parts | EO | GC-MS | [8] | |
| C. sicula L. | aerial parts | EO | GC-MS | [15] | |
| germacrene D | C. alpina M.Bieb. | fruit | EO | GC-MS | [21] |
| C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] | |
| C. cristata DC. | leaf, stem, fruit | EO | GC-MS | [23,24,25,26,27,28,29,30,31,32,33] | |
| whole plant | EO | GC-MS | [31] | ||
| C. libanotis L. | aerial parts | EO | GC-MS | [7] | |
| C. microcarpos M.Bieb. | flower, leaf, aerial parts | EO | GC-MS | [8,34,35,36] | |
| C. sicula L. | aerial parts, leaf | EO | GC-MS | [15,32] | |
| β-gurjunene | C. sicula L. | aerial parts | EO | GC-MS | [15] |
| C. microcarpos M.Bieb. | flower, leaf | EO | GC-MS | [36] | |
| γ-gurjunene | C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] |
| α-humulene | C. alpina M.Bieb. | fruit | EO | GC-MS | [21] |
| C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] | |
| C. cristata DC. | leaf, stem, fruit | EO | GC-MS | [23,24,25,26,27,28,29,30,31,32,33] | |
| C. microcarpos M.Bieb. | flower, leaf, aerial parts | EO | GC-MS | [8,34,35,36] | |
| C. sicula L. | aerial parts, leaf | EO | GC-MS | [15,32] | |
| humulene epoxide | C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] |
| C. cristata DC. | fruit | EO | GC-MS | [23] | |
| C. microcarpos M.Bieb. | aerial parts | EO | GC-MS | [35] | |
| kessane | C. cristata DC. | root, fruit | EO | GC-MS | [23,24,25,26,27,28,29,30,31,32,33] |
| limonene | C. alpina M.Bieb. | fruit | EO | GC-MS | [21] |
| C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] | |
| C. cristata DC. | fruit | EO | GC-MS | [23] | |
| C. libanotis L. | aerial parts | EO | GC-MS | [7] | |
| C. microcarpos M.Bieb. | Leaf, aerial parts | EO | GC-MS | [34,35] | |
| C. sicula L. | aerial parts | MeOH extracts (maceration and Naviglio®) | GC-MS | [5] | |
| aerial parts, leaf | EO | GC-MS | [15,32] | ||
| linalool | C. microcarpos M.Bieb. | flower, leaf | EO | GC-MS | [34,36] |
| C. sicula L. | aerial parts, leaf | EO | GC-MS | [15,32] | |
| α-longipinene | C. pungens Jan ex Guss | aerial parts | MeOH extract | GC-MS | [6] |
| 1,3,8-p-menthatriene | C. cristata DC. | leaf, root | EO | GC-MS | [33] |
| C. sicula L. | aerial parts | EO | GC-MS | [15] | |
| α-muurolene | C. alpina M.Bieb. | fruit | EO | GC-MS | [21] |
| C. cristata DC. | root | EO | GC-MS | [33] | |
| γ-muurolene | C. alpina M.Bieb. | fruit | EO | GC-MS | [21] |
| C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] | |
| C. cristata DC. | stem, fruit | EO | GC-MS | [23,24,25,26,27,28,29,30,31,32,33] | |
| C. microcarpos M.Bieb. | leaf | EO | GC-MS | [36] | |
| α-muurolol | C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] |
| T-muurolol | C. alpina M.Bieb. | fruit | EO | GC-MS | [21] |
| C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] | |
| β-myrcene | C. cristata DC. | leaf, stem, root, fruit | EO | GC-MS | [23,24,25,26,27,28,29,30,31,32,33] |
| whole plant | EO | GC-MS | [31] | ||
| C. libanotis L. | aerial parts | EO | GC-MS | [7] | |
| C. microcarpos M.Bieb. | aerial parts | EO | GC-MS | [8] | |
| leaf, flower | EO | GC-MS | [34,36] | ||
| C. pungens Jan ex Guss | aerial parts | MeOH extract | GC-MS | [6] | |
| C. sicula L. | aerial parts, leaf | EO | GC-MS | [15,32] | |
| myrtenol | C. libanotis L. | aerial parts | EO | GC-MS | [7] |
| myrtenyl acetate | C. microcarpos M.Bieb. | leaf, flower | EO | GC-MS | [36] |
| neophytadiene | C. sicula L. | aerial parts | MeOH extract | GC-MS | [5] |
| C. libanotis L. | aerial parts | EO | GC-MS | [7] | |
| C. pungens Jan ex Guss | aerial parts | MeOH extract | GC-MS | [6] | |
| β-ocimene | C. cristata DC. | leaf, stem, root, fruit | EO | GC-MS | [23,24,25,26,27,28,29,30,31,32,33] |
| C. microcarpos M.Bieb. | leaf, aerial parts | EO | GC-MS | [8,34,35] | |
| C. pungens Jan ex Guss | aerial parts | MeOH extract | GC-MS | [6] | |
| C. sicula L. | aerial parts, leaf | EO | GC-MS | [15,32] | |
| allo-ocimene | C. cristata DC. | leaf, stem | EO | GC-MS | [33] |
| C. libanotis L. | aerial parts | EO | GC-MS | [7] | |
| epoxy-ocimene | C. cristata DC. | leaf, stem | EO | GC-MS | [33] |
| α-phellandrene | C. alpina M.Bieb. | fruit | EO | GC-MS | [21] |
| C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] | |
| C. cristata DC. | leaf, stem, root | EO | GC-MS | [33] | |
| C. microcarpos M.Bieb. | aerial parts | EO | GC-MS | [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35] | |
| C. pungens Jan ex Guss | aerial parts | MeOH extract | GC-MS | [6] | |
| C. sicula L. | aerial parts | EO | GC-MS | [15] | |
| β-phellandrene | C. alpina M.Bieb. | fruit | EO | GC-MS | [21] |
| C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] | |
| C. cristata DC. | whole plant, fruit | EO | GC-MS | [23,24,25,26,27,28,29,30,31] | |
| C. pungens Jan ex Guss | aerial parts | MeOH extract | GC-MS | [6] | |
| C. microcarpos M.Bieb. | flower, leaf, aerial parts | EO | GC-MS | [8,34,35,36] | |
| phytol | C. cristata DC. | whole plant | EO | GC-MS | [31] |
| C. microcarpos M.Bieb. | aerial parts | EO | GC-MS | [8] | |
| α-pinene | C. alpina M.Bieb. | fruit | EO | GC-MS | [21] |
| C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] | |
| C. cristata DC. | leaf, stem, root, fruit | EO | GC-MS | [23,24,25,26,27,28,29,30,31,32,33] | |
| C. microcarpos M.Bieb. | leaf, flower, aerial parts | EO | GC-MS | [8,34,35,36] | |
| C. libanotis L. | aerial parts | EO | GC-MS | [7] | |
| C. sicula L. | aerial parts, leaf | EO | GC-MS | [15,32] | |
| β-pinene | C. alpina M.Bieb. | fruit | EO | GC-MS | [21] |
| C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] | |
| C. cristata DC. | whole plant, fruit | EO | GC-MS | [23,24,25,26,27,28,29,30,31] | |
| C. libanotis L. | aerial parts | EO | GC-MS | [7] | |
| C. microcarpos M.Bieb. | flower, leaf, aerial parts | EO | GC-MS | [8,35,36] | |
| C. sicula L. | aerial parts, leaf | EO | GC-MS | [15,32] | |
| pinocamphone | C. microcarpos M.Bieb. | leaf | EO | GC-MS | [34] |
| pinocarvone | C. libanotis L. | aerial parts | EO | GC-MS | [7] |
| C. microcarpos M.Bieb. | leaf | EO | GC-MS | [34] | |
| piperitenone | C. microcarpos M.Bieb. | leaf | EO | GC-MS | [34] |
| piperitenone oxide | C. microcarpos M.Bieb. | leaf | EO | GC-MS | [34] |
| piperitone | C. microcarpos M.Bieb. | leaf | EO | GC-MS | [34] |
| pregeijerene | C. cristata DC. | fruit | EO | GC-MS | [23] |
| pulegone | C. microcarpos M.Bieb. | leaf | EO | GC-MS | [34] |
| sabinene | C. alpina M.Bieb. | fruit | EO | GC-MS | [21] |
| C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] | |
| C. cristata DC. | leaf, stem, root, fruit | EO | GC-MS | [23,24,25,26,27,28,29,30,31,32,33] | |
| C. libanotis L. | aerial parts | EO | GC-MS | [7] | |
| C. microcarpos M.Bieb. | flower, leaf, aerial parts | EO | GC-MS | [8,35,36] | |
| C. sicula L. | aerial parts, leaf | EO | GC-MS | [15,32] | |
| sabinyl acetate | C. cristata DC. | root | EO | GC-MS | [33] |
| C. microcarpos M.Bieb. | leaf | EO | GC-MS | [34] | |
| C. sicula L. | aerial parts | EO | GC-MS | [15] | |
| safranal | C. microcarpos M.Bieb. | leaf | EO | GC-MS | [34] |
| selina 3,7(11)-diene | C. cristata DC. | leaf, stem | EO | GC-MS | [33] |
| α-selinene | C. cristata DC. | root, fruit | EO | GC-MS | [23,24,25,26,27,28,29,30,31,32,33] |
| β-selinene | C. cristata DC. | leaf, stem | EO | GC-MS | [33] |
| C. pungens Jan ex Guss | aerial parts | MeOH extract | GC-MS | [6] | |
| β-sesquiphellandrene | C. cristata DC. | leaf | EO | GC-MS | [33] |
| C. microcarpos M.Bieb. | leaf, aerial parts | EO | GC-MS | [8,34,36] | |
| spathulenol | C. alpina M.Bieb. | fruit | EO | GC-MS | [21] |
| C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] | |
| C. cristata DC. | leaf, stem, fruit | EO | GC-MS | [23,24,25,26,27,28,29,30,31,32,33] | |
| C. libanotis L. | aerial parts | EO | GC-MS | [7] | |
| C. microcarpos M.Bieb. | flower, leaf | EO | GC-MS | [34,36] | |
| C. sicula L. | aerial parts | EO | GC-MS | [15] | |
| α-terpinene | C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] |
| C. libanotis L. | aerial parts | EO | GC-MS | [7] | |
| C. microcarpos M.Bieb. | aerial parts | EO | GC-MS | [8] | |
| C. sicula L. | aerial parts, leaf | EO | GC-MS | [15,32] | |
| γ-terpinene | C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] |
| C. cristata DC. | leaf, stem, root, fruit | EO | GC-MS | [23,24,25,26,27,28,29,30,31,32,33] | |
| whole plant | EO | GC-MS | [31] | ||
| C. libanotis L. | aerial parts | EO | GC-MS | [7] | |
| C. microcarpos M.Bieb. | aerial parts, leaf | EO | GC-MS | [8,35,36] | |
| C. sicula L. | aerial parts, leaf | EO | GC-MS | [15,32] | |
| terpinen-4-ol | C. cristata DC. | leaf, stem | EO | GC-MS | [33] |
| C. libanotis L. | aerial parts | EO | GC-MS | [7] | |
| C. microcarpos M.Bieb. | aerial parts | EO | GC-MS | [8] | |
| C. sicula L. | aerial parts | EO | GC-MS | [15] | |
| α-terpineol | C. microcarpos M.Bieb. | leaf | EO | GC-MS | [34] |
| C. sicula L. | aerial parts, leaf | EO | GC-MS | [15,32] | |
| terpinolene | C. alpina M.Bieb. | fruit | EO | GC-MS | [21] |
| C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] | |
| C. cristata DC. | leaf, stem, root | EO | GC-MS | [33] | |
| C. libanotis L. | aerial parts | EO | GC-MS | [7] | |
| C. microcarpos M.Bieb. | aerial parts | EO | GC-MS | [8,35] | |
| C. sicula L. | aerial parts, leaf | EO | GC-MS | [15,32] | |
| thymol | C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] |
| C. libanotis L. | aerial parts | EO | GC-MS | [7] | |
| C. microcarpos M.Bieb. | flower, leaf | EO | GC-MS | [34,36] | |
| C. sicula L. | aerial parts | EO | GC-MS | [15] | |
| thymol methyl ether | C. cristata DC. | leaf, stem | EO | GC-MS | [33] |
| C. libanotis L. | aerial parts | EO | GC-MS | [7] | |
| 2,4(10)-thujadiene | C. microcarpos M.Bieb. | leaf | EO | GC-MS | [34] |
| α-thujene | C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] |
| C. cristata DC. | leaf, stem, root | EO | GC-MS | [33] | |
| C. microcarpos M.Bieb. | aerial parts | EO | GC-MS | [8] | |
| C. sicula L. | aerial parts, leaf | EO | GC-MS | [15,32] | |
| valencene | C. microcarpos M.Bieb. | leaf | EO | GC-MS | [36] |
| verbenene | C. libanotis L. | aerial parts | EO | GC-MS | [7] |
| verbenol | C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] |
| C. microcarpos M.Bieb. | leaf | EO | GC-MS | [34] | |
| verbenone | C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] |
| C. microcarpos M.Bieb. | leaf | EO | GC-MS | [34] | |
| β-vetivone | C. cristata DC. | leaf, stem | EO | GC-MS | [33] |
| zingiberene | C. cristata DC. | fruit | EO | GC-MS | [23] |
| C. microcarpos M.Bieb. | flower, leaf, aerial parts | EO | GC-MS | [8,36] | |
| zonarene | C. cristata DC. | leaf, stem | EO | GC-MS | [33] |
| Phenylpropanoids | |||||
| eugenol | C. boissieri (Reut. & Hausskn. ex Boiss.) Hand | aerial parts | EO | GC-MS | [37] |
| methyl eugenol | C. cristata DC. | fruit | EO | GC-MS | [23] |
| myristicin | C. cristata DC. | fruit | EO | GC-MS | [23] |
| Phenolic compounds | |||||
| caffeic acid | C. pungens Jan ex Guss | aerial parts | MeOH extract | HPTLC | [38] |
| catechin | C. pungens Jan ex Guss | aerial parts | MeOH extract | HPTLC | [38] |
| ferulic acid | C. pungens Jan ex Guss | aerial parts | MeOH extract | HPTLC | [38] |
| gallic acid | C. pungens Jan ex Guss | aerial parts | MeOH extract | HPTLC | [38] |
| naringin | C. pungens Jan ex Guss | aerial parts | MeOH extract | HPTLC | [38] |
| quercitrin | C. pungens Jan ex Guss | aerial parts | MeOH extract | HPTLC | [38] |
| Fatty acids | |||||
| behenic acid methyl ester | C. pungens Jan ex Guss | aerial parts | MeOH extract | GC-MS | [6] |
| ethyl linoleate | C. cristata DC. | fruit | EO | GC-MS | [23] |
| ethyl palmitate | C. cristata DC. | fruit | EO | GC-MS | [23] |
| ethyl oleate | C. cristata DC. | fruit | EO | GC-MS | [23] |
| lauric acid | C. pungens Jan ex Guss | aerial parts | MeOH extract | GC-MS | [6] |
| linoleic acid | C. cristata DC. | seed | light petroleum extract | GC-MS | [13] |
| C. pungens Jan ex Guss | aerial parts | MeOH extract | GC-MS | [6] | |
| α-linolenic acid | C. libanotis L. | aerial parts | MeOH extract | GC-MS | [5] |
| margaric acid | C. pungens Jan ex Guss | aerial parts | MeOH extract | GC-MS | [6] |
| methyl linoleate | C. cristata DC. | fruit | EO | GC-MS | [23] |
| methyl oleate | C. cristata DC. | fruit | EO | GC-MS | [23] |
| methyl palmitate | C. cristata DC. | fruit | EO | GC-MS | [23] |
| myristic acid | C. cristata DC. | fruit | EO | GC-MS | [23] |
| C. libanotis L. | aerial parts | MeOH extract (Naviglio®) | GC-MS | [5] | |
| myristic acid methyl ester | C. pungens Jan ex Guss | aerial parts | MeOH extract | GC-MS | [6] |
| 8,11-octadecadienoic acid, methyl ester | C. pungens Jan ex Guss | aerial parts | MeOH extract | GC-MS | [6] |
| palmitic acid | C. cristata DC. | fruit | EO | GC-MS | [23] |
| C. libanotis L. | aerial parts | MeOH extracts (maceration and Naviglio®) | GC-MS | [5] | |
| C. microcarpos M.Bieb. | aerial parts | EO | GC-MS | [8] | |
| C. pungens Jan ex Guss | aerial parts | MeOH extract | GC-MS | [6] | |
| C. sicula L. | aerial parts | MeOH extracts (maceration and Naviglio®) | GC-MS | [5] | |
| pentadecanoic acid methyl ester | C. pungens Jan ex Guss | aerial parts | MeOH extract | GC-MS | [6] |
| petroselinic acid | C. cristata DC. | seed | light petroleum extract | GC-MS | [13] |
| Phytosterols | |||||
| campesterol | C. pungens Jan ex Guss | aerial parts | MeOH extract | GC-MS | [6] |
| 9,19-cyclolanostan-3-ol,24-methylene | C. pungens Jan ex Guss | aerial parts | MeOH extract | GC-MS | [6] |
| cycloartenol | C. pungens Jan ex Guss | aerial parts | MeOH extract | GC-MS | [6] |
| γ-sitosterol | C. pungens Jan ex Guss | aerial parts | MeOH extract | GC-MS | [6] |
| stigmasta-5,22-dien-3-ol | C. pungens Jan ex Guss | aerial parts | MeOH extract | GC-MS | [6] |
| stigmast-7-en-3-ol | C. pungens Jan ex Guss | aerial parts | MeOH extract | GC-MS | [6] |
| Others | |||||
| N-N’-di-o-tolylethylendiamine | C. sicula L. | whole plant | MeOH extract | IR, NMR | [39] |
| Biological Activity | Species | Plant Part | Extract | Model | Ref. |
|---|---|---|---|---|---|
| Antioxidant | C. crassiloba (Boiss.) Meikle | aerial parts | Hydroalcoholic extract | DPPH | [40] |
| C. cristata DC. | aerial parts and fruits | MeOH, ethyl acetate, acetone and water extracts | DPPH and ABTS assays | [41] | |
| C. libanotis L. | root | hydroalcoholic extracts and fractions | DPPH, FRAP, ferrous iron chelation and β-carotene bleaching assays | [20] | |
| aerial parts | MeOH extracts (maceration and Naviglio®) | DPPH and β-carotene bleaching assays | [5] | ||
| C. microcarpos M.Bieb. | leaf | EO and MeOH extract | DPPH assay | [34] | |
| C. pungens Jan ex Guss | aerial parts | MeOH extract and its fractions | DPPH and β-carotene bleaching assays | [6] | |
| C. sicula L. | aerial parts | MeOH extracts (maceration and Naviglio®) | DPPH and β-carotene bleaching assays | [5] | |
| leaf | EO | ABTS, DPPH and metal chelating assays | [32] | ||
| Antibacterial | C. cristata DC. | aerial parts and fruits | MeOH, ethyl acetate, acetone and water extracts | micro-well dilution assay | [41] |
| leaf, steam | EO | broth microdilution method | [33] | ||
| C. libanotis L. | root | hydroalcoholic extracts and fractions | Agar disc diffusion method | [20] | |
| C. microcarpos M.Bieb. | leaf | EO and MeOH extract | disk diffusion assay, microwell dilution Assay | [34] | |
| aerial parts | EO | TLC-bioautography assay | [35] | ||
| Antifungal | C. cristata DC. | steam | EO | broth microdilution method | [33] |
| C. microcarpos M.Bieb. | leaf | EO | disk diffusion assay, MIC agar dilution assay | [34] | |
| aerial parts | EO | TLC-bioautography assay | [35] | ||
| Anti-inflammatory | C. libanotis L. | aerial parts | MeOH extracts (maceration and Naviglio®) | TNF-α and IL-6 inhibition; NO production inhibition; inhibited the phosphorylation of JAK2 and STAT3 proteins | [9] |
| C. microcarpos M.Bieb. | - | EtOH extract | carrageenan-induced oedema in rats | [42] | |
| C. pungens Jan ex Guss | aerial parts | MeOH extracts (maceration and Naviglio®) | TNF-α and IL-6 inhibition; NO production inhibition; induced release of IL-10; inhibited the phosphorylation of JAK2 and STAT3 proteins | [9] | |
| Antiproliferative | C. microcarpos M.Bieb. | aerial parts | EtOH and aqueous extracts | MTT test on human prostate cancer cells (PC-3) | [43] |
| Photocytotoxic | C. libanotis L. | aerial parts | MeOH extracts (maceration and Naviglio®) | UVA- irradiated C32 melanoma cells | [5] |
| C. pungens Jan ex Guss | aerial parts | MeOH extract and its fractions | UVA- irradiated A375 melanoma cells | [6] | |
| C. sicula L. | aerial parts | MeOH extracts (maceration and Naviglio®) | UVA- irradiated C32 melanoma cells | [5] | |
| AChE and BChE inhibitory activity | C. sicula L. | leaf | EO | ABTS, DPPH and metal chelating assays | [32] |
| XOR inhibitory activity | C. libanotis L. | root | hydroalcoholic extracts and fractions | xanthine/XOR system | [20] |
| Insecticidal | C. sicula L. | stems | hexane extract | insect (Tribolium castaneum) growth and/or feeding inhibition | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musolino, V.; Perri, M.R.; Conforti, F.; Gliozzi, M.; Marrelli, M.; Mollace, V. Cachrys L. Genus: A Comprehensive Review on Botany, Phytochemistry and Biological Properties. Plants 2023, 12, 565. https://doi.org/10.3390/plants12030565
Musolino V, Perri MR, Conforti F, Gliozzi M, Marrelli M, Mollace V. Cachrys L. Genus: A Comprehensive Review on Botany, Phytochemistry and Biological Properties. Plants. 2023; 12(3):565. https://doi.org/10.3390/plants12030565
Chicago/Turabian StyleMusolino, Vincenzo, Maria Rosaria Perri, Filomena Conforti, Micaela Gliozzi, Mariangela Marrelli, and Vincenzo Mollace. 2023. "Cachrys L. Genus: A Comprehensive Review on Botany, Phytochemistry and Biological Properties" Plants 12, no. 3: 565. https://doi.org/10.3390/plants12030565
APA StyleMusolino, V., Perri, M. R., Conforti, F., Gliozzi, M., Marrelli, M., & Mollace, V. (2023). Cachrys L. Genus: A Comprehensive Review on Botany, Phytochemistry and Biological Properties. Plants, 12(3), 565. https://doi.org/10.3390/plants12030565