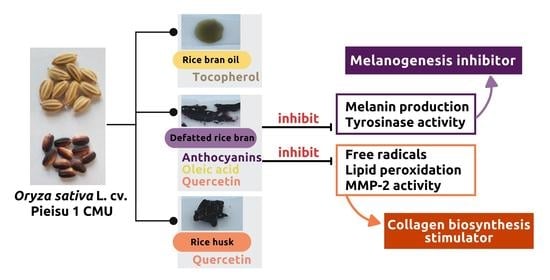
Natural Melanogenesis Inhibitor, Antioxidant, and Collagen Biosynthesis Stimulator of Phytochemicals in Rice Bran and Husk Extracts from Purple Glutinous Rice (Oryza sativa L. cv. Pieisu 1 CMU) for Cosmetic Application
Abstract
:1. Introduction
2. Results
2.1. Extract Preparations
2.2. Cytotoxicity Effect
2.3. Whitening Effects
2.3.1. Mushroom Tyrosinase Activity
2.3.2. Intracellular Melanin Content
2.3.3. Intracellular Tyrosinase Activity
2.4. Antioxidant Properties
2.4.1. Screening of Antioxidant Activities
2.4.2. Malondialdehyde Production
2.5. Collagen-Stimulating Effect via MMP-2 Inhibition
3. Discussion
4. Materials and Methods
4.1. Extraction Method
4.2. Cytotoxicity Assay
4.2.1. Cell Culture
4.2.2. SRB Assay
4.3. Determination of Whitening Effects
4.3.1. Cell-Free Tyrosinase Inhibition
4.3.2. Cellular Melanin Content Assay
4.3.3. Cellular Tyrosinase Assay
4.4. Determination of Antioxidant Properties
4.4.1. DPPH Scavenging Assay
4.4.2. ABTS Scavenging Assay
4.4.3. Iron Chelating Assay
4.4.4. Thiobarbituric Acid Reactive Substances (TBARS) Method
4.5. MMP-2 Inhibitory Activity by Gelatin Zymography
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bandumula, N. Rice production in Asia: Key to global food security. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2018, 88, 1323–1328. [Google Scholar] [CrossRef]
- Lemaire, A.; Limbourg, S. How can food loss and waste management achieve sustainable development goals? J. Clean Prod. 2019, 234, 1221–1234. [Google Scholar] [CrossRef]
- António, J.; Tadeu, A.; Marques, B.; Almeida, J.A.; Pinto, V. Application of rice husk in the development of new composite boards. Constr. Build. Mater. 2018, 176, 432–439. [Google Scholar] [CrossRef]
- Punia, S.; Kumar, M.; Siroha, A.K.; Purewal, S.S. Rice bran oil: Emerging trends in extraction, health benefit, and its industrial application. Rice Sci. 2021, 28, 217–232. [Google Scholar] [CrossRef]
- Ruksiriwanich, W.; Manosroi, J.; Abe, M.; Manosroi, W.; Manosroi, A. 5α-Reductase type 1 inhibition of Oryza sativa bran extract prepared by supercritical carbon dioxide fluid. J. Supercrit. Fluids 2011, 59, 61–71. [Google Scholar] [CrossRef]
- Khantham, C.; Linsaenkart, P.; Chaitep, T.; Jantrawut, P.; Chittasupho, C.; Rachtanapun, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Sommano, S.R.; Prom-U.-Thai, C. Antioxidation, anti-Inflammation, and regulation of SRD5A gene expression of Oryza sativa cv. Bue Bang 3 CMU husk and bran extracts as androgenetic alopecia molecular treatment substances. Plants 2022, 11, 330. [Google Scholar] [CrossRef]
- Wisetkomolmat, J.; Arjin, C.; Satsook, A.; Seel-Audom, M.; Ruksiriwanich, W.; Prom-u-Thai, C.; Sringarm, K. Comparative analysis of nutritional components and phytochemical attributes of selected Thai rice bran. Front. Nutr. 2022, 9, 833730. [Google Scholar] [CrossRef]
- Correia, P.; Araújo, P.; Ribeiro, C.; Oliveira, H.; Pereira, A.R.; Mateus, N.; de Freitas, V.; Brás, N.F.; Gameiro, P.; Coelho, P. Anthocyanin-related pigments: Natural allies for skin health maintenance and protection. Antioxidants 2021, 10, 1038. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, A.; Wang, J.; Huang, D.; Deng, Y.; Zhang, X.; Qu, Q.; Ma, W.; Xiong, R.; Zhu, M. Potential application of natural bioactive compounds as skin whitening agents: A review. J. Cosmet. Dermatol. 2022, 21, 6669–6687. [Google Scholar] [CrossRef]
- Burki, T. Skin-whitening creams: Worth the risk? Lancet Diabetes Endocrinol. 2021, 9, 10. [Google Scholar] [CrossRef]
- Boyajian, J.L.; Ghebretatios, M.; Schaly, S.; Islam, P.; Prakash, S. Microbiome and human aging: Probiotic and prebiotic potentials in longevity, skin health and cellular senescence. Nutrients 2021, 13, 4550. [Google Scholar] [CrossRef]
- Parrado, C.; Mercado-Saenz, S.; Perez-Davo, A.; Gilaberte, Y.; Gonzalez, S.; Juarranz, A. Environmental stressors on skin aging. Mechanistic insights. Front. Pharmacol. 2019, 10, 759. [Google Scholar] [CrossRef]
- Cao, C.; Xiao, Z.; Wu, Y.; Ge, C. Diet and skin aging—From the perspective of food nutrition. Nutrients 2020, 12, 870. [Google Scholar] [CrossRef] [Green Version]
- Pillaiyar, T.; Manickam, M.; Jung, S.-H. Downregulation of melanogenesis: Drug discovery and therapeutic options. Drug Discov. 2017, 22, 282–298. [Google Scholar] [CrossRef]
- McDaniel, D.; Farris, P.; Valacchi, G. Atmospheric skin aging—Contributors and inhibitors. J. Cosmet. Dermatol. 2018, 17, 124–137. [Google Scholar] [CrossRef]
- Ruksiriwanich, W.; Khantham, C.; Muangsanguan, A.; Chittasupho, C.; Rachtanapun, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Sommano, S.R.; Sringarm, K.; Ferrer, E. Phytochemical constitution, anti-inflammation, anti-androgen, and hair growth-promoting potential of shallot (Allium ascalonicum L.) extract. Plants 2022, 11, 1499. [Google Scholar] [CrossRef]
- Anh, L.H.; Quan, N.V.; Lam, V.Q.; Iuchi, Y.; Takami, A.; Teschke, R.; Xuan, T.D. Antioxidant, anti-tyrosinase, anti-α-amylase, and cytotoxic potentials of the invasive weed Andropogon virginicus. Plants 2020, 10, 69. [Google Scholar] [CrossRef]
- Chan, C.-F.; Wu, C.-T.; Huang, W.-Y.; Lin, W.-S.; Wu, H.-W.; Huang, T.-K.; Chang, M.-Y.; Lin, Y.-S. Antioxidation and melanogenesis inhibition of various Dendrobium tosaense extracts. Molecules 2018, 23, 1810. [Google Scholar] [CrossRef] [Green Version]
- Jang, D.K.; Pham, C.H.; Lee, I.S.; Jung, S.-H.; Jeong, J.H.; Shin, H.-S.; Yoo, H.M. Anti-melanogenesis activity of 6-O-isobutyrylbritannilactone from Inula britannica on B16F10 melanocytes and in vivo zebrafish models. Molecules 2020, 25, 3887. [Google Scholar] [CrossRef]
- Yeo, J.; Shahidi, F. Critical re-evaluation of DPPH assay: Presence of pigments affects the results. J. Agric. Food Chem. 2019, 67, 7526–7529. [Google Scholar] [CrossRef]
- Bibi Sadeer, N.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The versatility of antioxidant assays in food science and safety—Chemistry, applications, strengths, and limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ.; Alwasel, S.H. Metal ions, metal chelators and metal chelating assay as antioxidant method. Processes 2022, 10, 132. [Google Scholar] [CrossRef]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Cui, N.; Zhu, M.; Khalil, R.A. Placental growth factor reverses decreased vascular and uteroplacental MMP-2 and MMP-9 and increased MMP-1 and MMP-7 and collagen types I and IV in hypertensive pregnancy. Am. J. Physiol Heart Circ. Physiol. 2018, 315, H33–H47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Miao, Z.; Hu, Y.; Yang, X.; Tang, Y.; Zhu, D. Programmed microcapsule-type matrix metalloproteinase-2 (MMP-2)-responsive nanosensor for in situ monitoring of intracellular MMP-2. Sens. Actuators B Chem. 2018, 273, 511–518. [Google Scholar] [CrossRef]
- Manosroi, A.; Ruksiriwanich, W.; Abe, M.; Sakai, H.; Aburai, K.; Manosroi, W.; Manosroi, J. Physico-chemical properties of cationic niosomes loaded with fraction of rice (Oryza sativa) bran extract. J. Nanosci. Nanotechnol. 2012, 12, 7339–7345. [Google Scholar] [CrossRef]
- Manosroi, A.; Ruksiriwanich, W.; Manosroi, W.; Abe, M.; Manosroi, J. In vivo hair growth promotion activity of gel containing niosomes loaded with the Oryza sativa bran fraction (OSF3). Adv. Sci. Lett. 2012, 16, 222–228. [Google Scholar] [CrossRef]
- Wisetkomolmat, J.; Arjin, C.; Hongsibsong, S.; Ruksiriwanich, W.; Niwat, C.; Tiyayon, P.; Jamjod, S.; Yamuangmorn, S.; Prom, U.; Thai, C.; et al. Antioxidants contents and polyphenols characteristic of selected northern Thai rice husks: The relation with seed attributes. Rice Sci. 2023, 30, 148–159. [Google Scholar] [CrossRef]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling pathways in melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [Green Version]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [Green Version]
- Martins, L.S.; Lameira, J.; Kruger, H.G.; Alves, C.N.; Silva, J.R.A. Evaluating the performance of a non-bonded Cu2+ model including Jahn−Teller effect into the binding of tyrosinase inhibitors. Int. J. Mol. Sci. 2020, 21, 4783. [Google Scholar] [CrossRef]
- Promden, W.; Viriyabancha, W.; Monthakantirat, O.; Umehara, K.; Noguchi, H.; De-Eknamkul, W. Correlation between the potency of flavonoids on mushroom tyrosinase inhibitory activity and melanin synthesis in melanocytes. Molecules 2018, 23, 1403. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Gao, Y.; Wang, W.; Zhang, J.; Yin, J.; Le, T.; Xue, J.; Engelhardt, U.H.; Jiang, H. Kojic acid showed consistent inhibitory activity on tyrosinase from mushroom and in cultured B16F10 cells compared with arbutins. Antioxidants 2022, 11, 502. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Fukui, Y.; Izumi, R.; Numano, K.; Zeida, M. Effects of oligomeric proanthocyanidins (OPCs) of red wine to improve skin whitening and moisturizing in healthy women–a placebo-controlled randomized double-blind parallel group comparative study. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1571–1584. [Google Scholar]
- Karunarathne, W.A.H.M.; Molagoda, I.M.N.; Park, S.R.; Kim, J.W.; Lee, O.-K.; Kwon, H.Y.; Oren, M.; Choi, Y.H.; Ryu, H.W.; Oh, S.-R. Anthocyanins from Hibiscus syriacus L. inhibit melanogenesis by activating the ERK signaling pathway. Biomolecules 2019, 9, 645. [Google Scholar] [CrossRef] [Green Version]
- Smeriglio, A.; D’Angelo, V.; Denaro, M.; Trombetta, D.; Germanò, M.P. The hull of ripe pistachio nuts (Pistacia vera L.) as a source of new promising melanogenesis inhibitors. Plant Foods Hum. Nutr. 2021, 76, 111–117. [Google Scholar] [CrossRef]
- Correia, P.; Oliveira, H.; Araújo, P.; Brás, N.F.; Pereira, A.R.; Moreira, J.; de Freitas, V.; Mateus, N.; Oliveira, J.; Fernandes, I. The role of anthocyanins, deoxyanthocyanins and pyranoanthocyanins on the modulation of tyrosinase activity: An in vitro and in silico approach. Int. J. Mol. Sci. 2021, 22, 6192. [Google Scholar] [CrossRef]
- Lee, K.E.; Bharadwaj, S.; Sahoo, A.K.; Yadava, U.; Kang, S.G. Determination of tyrosinase-cyanidin-3-O-glucoside and (−/+)-catechin binding modes reveal mechanistic differences in tyrosinase inhibition. Sci. Rep. 2021, 11, 24494. [Google Scholar] [CrossRef]
- Murphy, E.C.; Friedman, A.J. Hydrogen peroxide and cutaneous biology: Translational applications, benefits, and risks. J. Am. Acad. Dermatol. 2019, 81, 1379–1386. [Google Scholar] [CrossRef]
- Mas Bargues, C.; Escrivá, C.; Dromant, M.; Borrás, C.; Viña, J. Lipid peroxidation as measured by chromatographic determination of malondialdehyde. Human plasma reference values in health and disease. Arch. Biochem. Biophys. 2021, 709, 108941. [Google Scholar] [CrossRef]
- Su, L.-J.; Zhang, J.-H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.-Y. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid. Med. Cell Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gęgotek, A.; Skrzydlewska, E. Biological effect of protein modifications by lipid peroxidation products. Chem. Phys. Lipids 2019, 221, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Ursini, F.; Maiorino, M. Lipid peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic. Biol. Med. 2020, 152, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.; Haider, K.; Jabeen, K.; Akash, M.S.H. Current perspectives of oleic acid: Regulation of molecular pathways in mitochondrial and endothelial functioning against insulin resistance and diabetes. Rev. Endocr. Metab. Disord. 2020, 21, 631–643. [Google Scholar] [CrossRef]
- Cotticelli, M.G.; Forestieri, R.; Xia, S.; Joyasawal, S.; Lee, T.; Xu, K.; Smith III, A.B.; Huryn, D.M.; Wilson, R.B. Identification of a novel oleic acid analog with protective effects in multiple cellular models of Friedreich ataxia. ACS Chem. Neurosci. 2020, 11, 2535–2542. [Google Scholar] [CrossRef]
- Bhattacharjee, B.; Pal, P.K.; Chattopadhyay, A.; Bandyopadhyay, D. Oleic acid protects against cadmium induced cardiac and hepatic tissue injury in male Wistar rats: A mechanistic study. Life Sci. 2020, 244, 117324. [Google Scholar] [CrossRef]
- Manosroi, A.; Chankhampan, C.; Kietthanakorn, B.O.; Ruksiriwanich, W.; Chaikul, P.; Boonpisuttinant, K.; Sainakham, M.; Manosroi, W.; Tangjai, T.; Manosroi, J. Pharmaceutical and cosmeceutical biological activities of hemp (Cannabis sativa L. var. sativa) leaf and seed extracts. Chiang Mai J. Sci. 2019, 46, 180–195. [Google Scholar]
- Soto-Alarcon, S.A.; Valenzuela, R.; Valenzuela, A.; Videla, L.A. Liver protective effects of extra virgin olive oil: Interaction between its chemical composition and the cell-signaling pathways involved in protection. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 75–84. [Google Scholar] [CrossRef]
- Gholampour, F.; Saki, N. Hepatic and renal protective effects of quercetin in ferrous sulfate-induced toxicity. Gen. Physiol. Biophys. 2019, 38, 27–38. [Google Scholar] [CrossRef]
- Smith, M.J.; Fowler, M.; Naftalin, R.J.; Siow, R.C. UVA irradiation increases ferrous iron release from human skin fibroblast and endothelial cell ferritin: Consequences for cell senescence and aging. Free Radic. Biol. Med. 2020, 155, 49–57. [Google Scholar] [CrossRef]
- Dong, W.; Chen, D.; Chen, Z.; Sun, H.; Xu, Z. Antioxidant capacity differences between the major flavonoids in cherry (Prunus pseudocerasus) in vitro and in vivo models. LWT 2021, 141, 110938. [Google Scholar] [CrossRef]
- Sulastri, E.; Zubair, M.S.; Anas, N.I.; Abidin, S.; Hardani, R.; Yulianti, R. Total phenolic, total flavonoid, quercetin content and antioxidant activity of standardized extract of Moringa oleifera leaf from regions with different elevation. Pharmacogn. J. 2018, 10, s104–s108. [Google Scholar] [CrossRef] [Green Version]
- Park, S. Biochemical, structural and physical changes in aging human skin, and their relationship. Biogerontology 2022, 23, 275–288. [Google Scholar] [CrossRef]
- Liu, T.; Li, N.; Yan, Y.q.; Liu, Y.; Xiong, K.; Liu, Y.; Xia, Q.m.; Zhang, H.; Liu, Z.d. Recent advances in the anti-aging effects of phytoestrogens on collagen, water content, and oxidative stress. Phytother. Res. 2020, 34, 435–447. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Zhang, X.; Liu, D. Collagen peptides and the related synthetic peptides: A review on improving skin health. J. Funct. Foods 2021, 86, 104680. [Google Scholar] [CrossRef]
- Reilly, D.M.; Lozano, J. Skin collagen through the lifestages: Importance for skin health and beauty. Plast Aesthet. Res. 2021, 8, 2. [Google Scholar] [CrossRef]
- Rappu, P.; Salo, A.M.; Myllyharju, J.; Heino, J. Role of prolyl hydroxylation in the molecular interactions of collagens. Essays Biochem. 2019, 63, 325–335. [Google Scholar]
- Jhawar, N.; Wang, J.V.; Saedi, N. Oral collagen supplementation for skin aging: A fad or the future? J. Cosmet. Dermatol. 2020, 19, 910–912. [Google Scholar] [CrossRef]
- Rabelo, A.C.S.; de Pádua Lúcio, K.; Araújo, C.M.; de Araújo, G.R.; de Amorim Miranda, P.H.; Carneiro, A.C.A.; de Castro Ribeiro, É.M.; de Melo Silva, B.; de Lima, W.G.; Costa, D.C. Baccharis trimera protects against ethanol induced hepatotoxicity in vitro and in vivo. J. Ethnopharmacol. 2018, 215, 1–13. [Google Scholar] [CrossRef]
- Shin, E.J.; Lee, J.S.; Hong, S.; Lim, T.-G.; Byun, S. Quercetin directly targets JAK2 and PKCδ and prevents UV-induced photoaging in human skin. Int. J. Mol. Sci. 2019, 20, 5262. [Google Scholar] [CrossRef] [Green Version]
- Ozkan, E.; Bakar-Ates, F. Potentiation of the effect of lonidamine by quercetin in MCF-7 human breast cancer cells through downregulation of MMP-2/9 mRNA expression. An. Acad. Bras. Cienc. 2020, 92, e20200548. [Google Scholar] [CrossRef] [PubMed]
- Boťanská, B.; Barteková, M.; Ferenczyová, K.; Fogarassyová, M.; Kindernay, L.; Barančík, M. Matrix metalloproteinases and their role in mechanisms underlying effects of quercetin on heart function in aged zucker diabetic fatty rats. Int. J. Mol. Sci. 2021, 22, 4457. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, W.J.; Park, S.Y.; Kim, H.; Chung, D.K. In vitro anti-wrinkle and skin-moisturizing effects of evening primrose (Oenothera biennis) sprout and identification of its active components. Processes 2021, 9, 145. [Google Scholar] [CrossRef]
- Wu, L.; Chen, C.; Cheng, C.; Dai, H.; Ai, Y.; Lin, C.; Chung, Y. Evaluation of tyrosinase inhibitory, antioxidant, antimicrobial, and antiaging activities of Magnolia officinalis extracts after Aspergillus niger fermentation. Biomed. Res. Int. 2018, 2018, 5201786. [Google Scholar] [CrossRef] [Green Version]
- Im, K.H.; Baek, S.A.; Choi, J.; Lee, T.S. Antioxidant, anti-melanogenic and anti-wrinkle effects of Phellinus vaninii. Mycobiology 2019, 47, 494–505. [Google Scholar] [CrossRef] [Green Version]
- Agalou, A.; Thrapsianiotis, M.; Angelis, A.; Papakyriakou, A.; Skaltsounis, A.-L.; Aligiannis, N.; Beis, D. Identification of novel melanin synthesis inhibitors from Crataegus pycnoloba using an in vivo zebrafish phenotypic assay. Front. Pharmacol. 2018, 9, 265. [Google Scholar] [CrossRef]
- Teng, H.; Fan, X.; Lv, Q.; Zhang, Q.; Xiao, J.; Qian, Y.; Zheng, B.; Gao, H.; Gao, S.; Chen, L. Folium nelumbinis (Lotus leaf) volatile-rich fraction and its mechanisms of action against melanogenesis in B16 cells. Food Chem. 2020, 330, 127030. [Google Scholar] [CrossRef]
- Atalay, S.; Dobrzyńska, I.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol protects keratinocyte cell membranes following exposure to UVB and hydrogen peroxide. Redox Biol. 2020, 36, 101613. [Google Scholar] [CrossRef]
- Xiang, J.; Wan, C.; Guo, R.; Guo, D. Is hydrogen peroxide a suitable apoptosis inducer for all cell types? Biomed. Res. Int. 2016, 2016, 7343965. [Google Scholar] [CrossRef] [Green Version]
- Nazir, Y.; Linsaenkart, P.; Khantham, C.; Chaitep, T.; Jantrawut, P.; Chittasupho, C.; Rachtanapun, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Sommano, S.R.; et al. High efficiency in vitro wound healing of Dictyophora indusiata extracts via anti-Inflammatory and collagen stimulating (MMP-2 inhibition) mechanisms. J. Fungi 2021, 7, 1100. [Google Scholar] [CrossRef]






| Samples | IC50 (mg/mL) Monophenolase Activity | IC50 (mg/mL) Diphenolase Activity |
|---|---|---|
| PES1CMU−RBO | 12.54 ± 0.48 a | 23.14 ± 3.60 a |
| PES1CMU−DFRB | 0.99 ± 0.30 b | 1.92 ± 0.71 b |
| PES1CMU−H | 2.90 ± 0.04 c | 3.46 ± 0.00 b |
| Standard arbutin | 0.51 ± 0.03 b | 3.44 ± 0.04 b |
| Samples | DPPH-TEAC (mg/g) | ABTS-TEAC (mg/g) | Iron Chelation-EECC (mg/g) |
|---|---|---|---|
| PES1CMU−RBO | 112.98 ± 1.57 a | 11.36 ± 0.58 a | 90.56 ± 17.70 ab |
| PES1CMU−DFRB | 648.39 ± 8.99 b | 377.49 ± 19.43 b | 65.61 ± 12.82 a |
| PES1CMU−H | 214.13 ± 2.97 c | 192.20 ± 9.89 c | 131.55 ± 25.71 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linsaenkart, P.; Ruksiriwanich, W.; Jantrawut, P.; Chittasupho, C.; Rachtanapun, P.; Jantanasakulwong, K.; Sommano, S.R.; Prom-u-thai, C.; Jamjod, S.; Arjin, C.; et al. Natural Melanogenesis Inhibitor, Antioxidant, and Collagen Biosynthesis Stimulator of Phytochemicals in Rice Bran and Husk Extracts from Purple Glutinous Rice (Oryza sativa L. cv. Pieisu 1 CMU) for Cosmetic Application. Plants 2023, 12, 970. https://doi.org/10.3390/plants12040970
Linsaenkart P, Ruksiriwanich W, Jantrawut P, Chittasupho C, Rachtanapun P, Jantanasakulwong K, Sommano SR, Prom-u-thai C, Jamjod S, Arjin C, et al. Natural Melanogenesis Inhibitor, Antioxidant, and Collagen Biosynthesis Stimulator of Phytochemicals in Rice Bran and Husk Extracts from Purple Glutinous Rice (Oryza sativa L. cv. Pieisu 1 CMU) for Cosmetic Application. Plants. 2023; 12(4):970. https://doi.org/10.3390/plants12040970
Chicago/Turabian StyleLinsaenkart, Pichchapa, Warintorn Ruksiriwanich, Pensak Jantrawut, Chuda Chittasupho, Pornchai Rachtanapun, Kittisak Jantanasakulwong, Sarana Rose Sommano, Chanakan Prom-u-thai, Sansanee Jamjod, Chaiwat Arjin, and et al. 2023. "Natural Melanogenesis Inhibitor, Antioxidant, and Collagen Biosynthesis Stimulator of Phytochemicals in Rice Bran and Husk Extracts from Purple Glutinous Rice (Oryza sativa L. cv. Pieisu 1 CMU) for Cosmetic Application" Plants 12, no. 4: 970. https://doi.org/10.3390/plants12040970
APA StyleLinsaenkart, P., Ruksiriwanich, W., Jantrawut, P., Chittasupho, C., Rachtanapun, P., Jantanasakulwong, K., Sommano, S. R., Prom-u-thai, C., Jamjod, S., Arjin, C., Sringarm, K., & Barba, F. J. (2023). Natural Melanogenesis Inhibitor, Antioxidant, and Collagen Biosynthesis Stimulator of Phytochemicals in Rice Bran and Husk Extracts from Purple Glutinous Rice (Oryza sativa L. cv. Pieisu 1 CMU) for Cosmetic Application. Plants, 12(4), 970. https://doi.org/10.3390/plants12040970