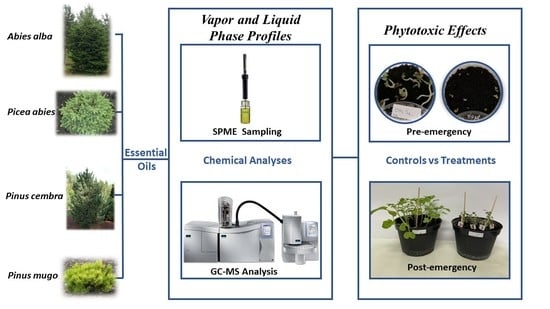
Vapor and Liquid Phase Profiles of Essential Oils from Abies, Picea and Pinus Species and Their Phytotoxic Interactions with Weed Growth in Pre- and Post-Emergence Conditions
Abstract
:1. Introduction
2. Results
2.1. Vapor Phase Chemical Composition of EOs
2.2. Liquid Phase Chemical Composition of EOs
2.3. Phytotoxicity of EOs in Vapor Phase
2.4. Phytotoxicity of EOs in Liquid Phase
2.5. Effectiveness of EOs in Post-Emergence Conditions
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Solid-Phase Microextraction (SPME)
4.3. Gas Chromatography/Mass Spectrometry (GC/MS)
4.4. Phytotoxicity
4.4.1. Pre-Emergence Test with EOs in Vapor Phase
4.4.2. Pre-Emergence Test with EOs in Liquid Phase
4.4.3. Post-Emergence Test with EOs in Liquid Phase
4.5. Data Processing
4.5.1. Phytotoxicity Indices
4.5.2. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verdeguer, M.; Sánchez-Moreiras, A.M.; Araniti, F. Phytotoxic Effects and Mechanism of Action of Essential Oils and Terpenoids. Plants 2020, 9, 1571. [Google Scholar] [CrossRef] [PubMed]
- Raffa, C.M.; Chiampo, F. Bioremediation of Agricultural Soils Polluted with Pesticides: A review. Bioengineering 2021, 8, 92. [Google Scholar] [CrossRef]
- Loddo, D.; McElroy, J.S.; Giannini, V. Problems and Perspectives in Weed Management. Ital. J. Agron. 2021, 16, 1854. [Google Scholar] [CrossRef]
- Scavo, A.; Mauromicale, G. Crop Allelopathy for Sustainable Weed Management in Agroecosystems: Knowing the Present with a View To the Future. Agronomy 2021, 11, 2104. [Google Scholar] [CrossRef]
- Patni, B.; Chandra, H.; Mishra, A.P.; Guru, S.K.; Vitalini, S.; Iriti, M. Rice Allelopathy in Weed Management: An Integrated Approach. Cell. Mol. Biol. 2018, 64, 84–93. [Google Scholar] [CrossRef] [Green Version]
- Hasan, M.; Ahmad-Hamdani, M.S.; Rosli, A.M.; Hamdan, H. Bioherbicides: An Eco-Friendly Tool for Sustainable Weed Management. Plants 2021, 10, 1212. [Google Scholar] [CrossRef]
- Maes, C.; Meersmans, J.; Lins, L.; Bouquillon, S.; Fauconnier, M.-L. Essential Oil-Based Bioherbicides: Human Health Risks Analysis. Int. J. Mol. Sci. 2021, 22, 9396. [Google Scholar] [CrossRef]
- International Herbicide-Resistant Weed Database. Available online: http://www.weedscience.org/Pages/Species.aspx (accessed on 25 February 2023).
- Viggiani, P. Le Dicotiledoni Nelle Colture Agrarie; Edagricole: Bologna, Italy, 2015. [Google Scholar]
- Hmamouchi, M.; Hamamouchi, J.; Zouhdi, M.; Bessiere, J.M. Chemical and Antimicrobial Properties of Essential Oils of Five Moroccan Pinaceae. J. Essent. Oil Res. 2001, 13, 298–302. [Google Scholar] [CrossRef]
- Gu, H.J.; Cheng, S.S.; Lin, C.Y.; Huang, C.G.; Chen, W.J.; Chang, S.T. Repellency of essential oils of Cryptomeria japonica (Pinaceae) against adults of the mosquitoes Aedes aegypti and Aedes albopictus (Diptera: Culicidae). J. Agric. Food Chem. 2009, 57, 11127–11133. [Google Scholar] [CrossRef]
- Koçak, A.; Kiliç, Ö. Identification of Essential Oil Composition of Four Picea Mill. (Pinaceae) species from Canada. J. Agric. Sci. Technol. B 2014, 4, 209–214. [Google Scholar]
- Lee, N.H.; Lee, S.M.; Lee, T.M.; Chung, N.; Lee, H.S. GC-MS analyses of the essential oils obtained from Pinaceae leaves in Korea. J. Essent. Oil Bear. Plants 2015, 18, 538–542. [Google Scholar] [CrossRef]
- Ham, Y.; Yang, J.; Choi, W.S.; Ahn, B.J.; Park, M.J. Antibacterial Activity of Essential Oils from Pinaceae Leaves Against Fish Pathogens. J. Korean Wood Sci. Technol. 2020, 48, 527–547. [Google Scholar] [CrossRef]
- Vitalini, S.; Iriti, M.; Vaglia, V.; Garzoli, S. Chemical Investigation and Dose-Response Phytotoxic Effect of Essential Oils from Two Gymnosperm Species (Juniperus communis var. saxatilis Pall. and Larix decidua Mill.). Plants 2022, 11, 1510. [Google Scholar] [CrossRef] [PubMed]
- Dehsheikh, A.B.; Sourestani, M.M.; Dehsheikh, P.B.; Mottaghipisheh, J.; Vitalini, S.; Iriti, M. Monoterpenes: Essential Oil Components with Valuable Features. Mini Rev. Med. Chem. 2020, 20, 958–974. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.S.; Jiang, C.Y.; Liu, Y.; Zhang, X.Y.; Shao, H.; Zhang, C. Allelopathic Potential of Volatile Organic Compounds Released by Xanthium sibiricum Patrin ex Widder. Allelopath. J. 2019, 47, 233–242. [Google Scholar] [CrossRef]
- Pal Singh, H.; Kaur, S.; Mittal, S.; Batish, D.R.; Kohli, R.K. Phytotoxicity of major constituents of the volatile oil from leaves of Artemisia scoparia Waldst. & Kit. Z. Naturforsch. C 2008, 63, 663–666. [Google Scholar]
- Macías, F.A.; Mejías, F.J.; Molinillo, J.M. Recent Advances in Allelopathy for Weed Control: From Knowledge to Applications. Pest Manag. Sci. 2019, 75, 2413–2436. [Google Scholar] [CrossRef]
- Kordali, S.; Cakir, A.; Sutay, S. Inhibitory Effects of Monoterpenes on Seed Germination and Seedling Growth. Z. Naturforsch. C 2007, 62, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Vaid, S.; Batish, D.R.; Singh, H.P.; Kohli, R.K. Phytotoxicity of Limonene against Amaranthus viridis L. BioScan 2011, 6, 163–165. [Google Scholar]
- Zhao, J.; Yang, L.; Zhou, L.; Bai, Y.; Wang, B.; Hou, P.; Xu, Q.; Yang, W.; Zuo, Z. Inhibitory Effects of Eucalyptol and Limonene on the Photosynthetic Abilities in Chlorella vulgaris (Chlorophyceae). Phycologia 2016, 55, 696–702. [Google Scholar] [CrossRef]
- Jalaei, Z.; Fattahi, M.; Aramideh, S. Allelopathic and Insecticidal Activities of Essential Oil of Dracocephalum kotschyi Boiss. from Iran: A New Chemotype with Highest Limonene-10-al and Limonene. Ind. Crops Prod. 2015, 73, 109–117. [Google Scholar] [CrossRef]
- Raha, S.; Kohli, R.K. Analysis of Citrus Maxima (Burm.) Merr. Essential oil, Studies on Allelopathic Effect of Its Major Constituents and Morphological Observations of Its Encapsulates; Central University of Punjab: Punjab, India, 2020. [Google Scholar]
- Li, X.F.; Wang, J.; Huang, D.; Wang, L.X.; Wang, K. Allelopathic Potential of Artemisia frigida and Successional Changes of Plant Communities in the Northern China Steppe. Plant Soil 2011, 341, 383–398. [Google Scholar] [CrossRef]
- Chen, M.; Qiao, Y.; Quan, X.; Shi, H.; Duan, Z. Physiological, Biochemical and Phytohormone Responses of Elymus nutans to α-Pinene-Induced Allelopathy. Peer J. 2022, 10, e14100. [Google Scholar] [CrossRef]
- Areco, V.A.; Figueroa, S.; Cosa, M.T.; Dambolena, J.S.; Zygadlo, J.A.; Zunino, M.P. Effect of Pinene Isomers on Germination and Growth of Maize. Biochem. Syst. Ecol. 2014, 55, 27–33. [Google Scholar] [CrossRef]
- Nishida, N.; Tamotsu, S.; Nagata, N.; Saito, C.; Sakai, A. Allelopathic Effects of Volatile Monoterpenoids Produced by Salvia leucophylla: Inhibition of Cell Proliferation and DNA Synthesis in the Root Apical Meristem of Brassica campestris Seedlings. J. Chem. Ecol. 2005, 31, 1187–1203. [Google Scholar] [CrossRef]
- Razavi, S.M. Chemical and Allelopathic Analyses of Essential Oils of Prangos pabularia Lindl. from Iran. Nat. Prod. Res. 2012, 26, 2148–2151. [Google Scholar]
- Aidi Wannes, W.; Dakhlaoui, S.; Limam, H.; Tammar, S.; Msaada, K. Chemical Profile, Allelopathic, Antibacterial and Antioxidant Potential of the Essential Oil from Aleppo Pine (Pinus halepensis Miller) Needles. Arch. Phytopathol. Plant Prot. 2021, 54, 2483–2499. [Google Scholar] [CrossRef]
- Hamrouni, L.; Hanana, M.; Amri, I.; Romane, A.E.; Gargouri, S.; Jamoussi, B. Allelopathic effects of essential oils of Pinus halepensis Miller: Chemical composition and study of their antifungal and herbicidal activities. Arch. Phytopathol. Plant Prot. 2015, 48, 145–158. [Google Scholar] [CrossRef]
- Kennedy, J.E.; Dave, P.C.; Harbin, L.N.; Setzer, W.N. Allelopathic potential of Sassafras albidum and Pinus taeda essential oils. Allelopath. J. 2011, 27, 111–122. [Google Scholar]
- Mirmostafaee, S.; Azizi, M.; Fujii, Y. Study of Allelopathic Interaction of Essential Oils From Medicinal and Aromatic Plants on Seed Germination and Seedling Growth of Lettuce. Agronomy 2020, 10, 163. [Google Scholar] [CrossRef] [Green Version]
- Vitalini, S.; Iriti, M.; Garzoli, S. GC-MS and SPME-GC/MS Analysis and Bioactive Potential Evaluation of Essential Oil from Two Viola Species Belonging to the V. calcarata Complex. Separations 2022, 9, 39. [Google Scholar] [CrossRef]
- Ranal, M.A.; Santana, D.G.; Ferreira, W.R.; Mendes-Rodrigues, C. Calculating Germination Measurements and Organizing Spreadsheets. Braz. J. Bot. 2009, 32, 849–855. [Google Scholar] [CrossRef] [Green Version]
- Al-Mudaris, M. Notes on Various Parameters Recording the Speed of Seed Germination. Der. Trop. 1998, 99, 147–154. [Google Scholar]
- Ellis, R.A.; Roberts, E.H. The Quantification of Ageing and Survival in Orthodox Seeds. Seed Sci. Technol. 1981, 9, 373–409. [Google Scholar]
- Nalini, S.; Parthasarathi, R. Optimization of Rhamnolipid Biosurfactant Production From Serratia rubidaea SNAU02 Under Solid-State Fermentation and Its Biocontrol Efficacy Against Fusarium Wilt of Eggplant. Ann. Agrar. Sci. 2018, 16, 108–115. [Google Scholar] [CrossRef]
| N° | Component 1 | Symbol 2 Class | LRI 3 | LRI 4 | A. alba (%) 5 | P. abies (%) 6 | P. cembra (%) 7 | P. mugo (%) 8 |
|---|---|---|---|---|---|---|---|---|
| 1 | santene | other | 880 | 887 | 2.9 ± 0.02 | 1.0 ± 0.01 | tr | - |
| 2 | cyclofenchene | m | 892 | 896 | tr | 2.8 ± 0.03 | tr | 2.3 ± 0.02 |
| 3 | tricyclene | m | 913 | 920 | 2.1 ± 0.02 | 0.7 ± 0.02 | 1.0 ± 0.01 | 1.2 ± 0.01 |
| 4 | α-pinene | m | 938 | 943 | 48.5 ± 0.04 | 24.7 ± 0.06 | 52.9 ± 0.07 | 28.0 ± 0.04 |
| 5 | camphene | m | 940 | 946 | 1.8 ± 0.01 | 8.6 ± 0.02 | 2.0 ± 0.02 | 2.9 ± 0.02 |
| 6 | sabinene | m | 975 | 972 | - | - | 0.1 ± 0.01 | 0.1 ± 0.01 |
| 7 | β-pinene | m | 981 | 978 | 9.0 ± 0.03 | 16.5 ± 0.03 | 3.0 ± 0.02 | - |
| 8 | β-myrcene | m | 983 | 987 | - | 24.8 ± 0.05 | 9.2 ± 0.03 | 27.8 ± 0.05 |
| 9 | α-phellandrene | m | 1000 | 1005 | - | - | 0.2 ± 0.02 | 0.5 ± 0.01 |
| 10 | limonene | m | 1025 | 1023 | 33.1 ± 0.05 | 17.2 ± 0.04 | 29.3 ± 0.04 | 33.1 ± 0.06 |
| 11 | trans-sabinene hydrate | m | 1045 | 1052 | - | - | tr | - |
| 12 | γ-terpinine | m | 1057 | 1054 | - | 0.1 ± 0.01 | tr | 0.3 ± 0.02 |
| 13 | terpinolene | m | 1083 | 1080 | 0.2 ± 0.01 | 0.4 ± 0.01 | 0.2 ± 0.02 | 1.8 ± 0.02 |
| 14 | p-cymenene | m | 1092 | 1091 | - | - | tr | - |
| 15 | fenchol | m | 1094 | 1098 | - | tr | - | - |
| 16 | L-pincarveol | m | 1121 | 1119 | - | - | 0.1 ± 0.02 | - |
| 17 | α-campholenal | m | 1124 | 1125 | - | - | tr | - |
| 18 | camphor | m | 1130 | 1126 | - | 0.6 ± 0.02 | - | - |
| 19 | sabina-ketone | m | 1133 | 1132 | - | - | 0.1 ± 0.01 | - |
| 20 | cis-sabinol | m | 1135 | 1133 | - | - | - | 0.1 ± 0.01 |
| 21 | pinocamphone | m | 1146 | 1141 | - | - | tr | - |
| 22 | camphene hydrate | m | 1154 | 1149 | - | 0.2 ± 0.01 | - | - |
| 23 | endo-borneol | m | 1160 | 1155 | 0.1 ± 0.01 | 0.5 ± 0.01 | 0.1 ± 0.01 | tr |
| 24 | terpinene-4-ol | m | 1162 | 1160 | - | 0.1 ± 0.01 | - | 0.2 ± 0.01 |
| 25 | α-terpineol | m | 1188 | 1183 | 0.1 ± 0.01 | 0.2 ± 0.01 | 0.1 ± 0.01 | 0.1 ± 0.01 |
| 26 | levoverbenone | m | 1184 | 1191 | - | tr | - | - |
| 27 | 2-pinen-10-ol | m | 1199 | 1194 | - | - | tr | - |
| 28 | myrtenol | m | 1200 | 1202 | - | tr | - | - |
| 29 | methyl thymyl ether | m | 1238 | 1234 | - | tr | tr | 0.2 ± 0.01 |
| 30 | piperitone | m | 1260 | 1254 | - | tr | tr | - |
| 31 | bornyl acetate | m | 1297 | 1290 | 1.3 ± 0.02 | 1.1 ± 0.03 | 0.2 ± 0.01 | 0.9 ± 0.02 |
| 32 | α-terpinyl acetate | m | 1350 | 1344 | tr | - | 0.1 ± 0.01 | tr |
| 33 | citronellol acetate | m | 1355 | 1348 | 0.1 ± 0.01 | - | - | - |
| 34 | nerol acetate | m | 1367 | 1363 | tr | - | - | - |
| 35 | α-copaene | s | 1377 | 1368 | tr | - | tr | - |
| 36 | α-cubebene | s | 1386 | 1381 | tr | - | - | - |
| 37 | longicyclene | s | 1400 | 1392 | - | tr | - | - |
| 38 | α-longipinene | s | 1406 | 1400 | 0.1 ± 0.01 | tr | - | - |
| 39 | longifolene | s | 1413 | 1408 | 0.1 ± 0.01 | 0.1 ± 0.01 | - | - |
| 40 | β-caryophyllene | s | 1429 | 1424 | 0.4 ± 0.02 | 0.1 ± 0.01 | 0.1 ± 0.01 | 0.3 ± 0.02 |
| 41 | aromadendrene | s | 1458 | 1460 | - | - | tr | - |
| 42 | cis-muurola-4(15), 5-diene | s | 1465 | 1461 | - | tr | tr | tr |
| 43 | humulene | s | 1471 | 1465 | 0.1 ± 0.01 | tr | - | tr |
| 44 | cis-muurola-4(14), 5-diene | s | 1483 | 1478 | - | tr | - | tr |
| 45 | γ-muurolene | s | 1494 | 1487 | - | - | 0.1 ± 0.01 | - |
| 46 | α-franesene | s | 1501 | 1496 | tr | - | - | - |
| 47 | β-himachalene | s | 1505 | 1495 | tr | - | - | - |
| 48 | germacrene D | s | 1509 | 1500 | - | - | 0.1 ± 0.01 | - |
| 49 | β-bisabolene | s | 1512 | 1501 | - | - | 0.1 ± 0.01 | - |
| 50 | δ-cadinene | s | 1524 | 1530 | - | - | 0.3 ± 0.02 | tr |
| 51 | α-muurolene | s | 1530 | 1534 | - | - | 0.1 ± 0.01 | - |
| 52 | spathulenol | s | 1612 | 1601 | - | - | tr | - |
| 53 | α-bisabolol | s | 1672 | 1668 | - | - | tr | - |
| 54 | α-cadinol | s | 1680 | 1676 | - | - | tr | - |
| SUM | 99.9 | 99.7 | 99.4 | 99.8 | ||||
| Monoterpenes | 96.3 | 95.5 | 98.6 | 99.5 | ||||
| Sesquiterpenes | 0.7 | 0.2 | 0.8 | 0.3 | ||||
| Other | 2.9 | 1.0 | - | - |
| N° | Component 1 | Symbol 2 Class | LRI 3 | LRI 4 | A. alba (%) 5 | P. abies (%) 6 | P. cembra (%) 7 | P. mugo (%) 8 |
|---|---|---|---|---|---|---|---|---|
| 1 | santene | other | 880 | 887 | 0.8 ± 0.02 | 0.3 ± 0.02 | - | - |
| 2 | cyclofenchene | m | 892 | 896 | 0.4 ± 0.02 | 2.5 ± 0.02 | 1.0 ± 0.02 | 4.4 ± 0.03 |
| 3 | tricyclene | m | 913 | 920 | 1.0 ± 0.03 | 0.4 ± 0.02 | 0.1 ± 0.02 | - |
| 4 | α-pinene | m | 938 | 943 | 20.6 ± 0.06 | 12.4 ± 0.03 | 36.2 ± 0.06 | 3.5 ± 0.02 |
| 5 | camphene | m | 940 | 946 | 8.1 ± 0.02 | 4.9 ± 0.02 | 1.5 ± 0.02 | 0.4 ± 0.02 |
| 6 | sabinene | m | 975 | 972 | - | 0.2 ± 0.01 | 0.1 ± 0.01 | - |
| 7 | β-pinene | m | 981 | 978 | 4.9 ± 0.02 | 17.6 ± 0.03 | 1.3 ± 0.02 | 0.2 ± 0.02 |
| 8 | β-myrcene | m | 983 | 987 | tr | 11.3 ± 0.03 | 9.4 ± 0.04 | 5.1 ± 0.03 |
| 9 | α-phellandrene | m | 1000 | 1005 | - | - | - | 0.1 ± 0.02 |
| 10 | p-cymene | m | 1010 | 1016 | - | 0.3 ± 0.02 | - | - |
| 11 | limonene | m | 1025 | 1023 | 30.4 ± 0.04 | 20.3 ± 0.05 | 34.3 ± 0.05 | 78.5 ± 0.08 |
| 12 | γ-terpinene | m | 1057 | 1054 | - | 0.2 ± 0.02 | 0.1 ± 0.01 | 0.1 ± 0.01 |
| 13 | terpinolene | m | 1083 | 1080 | 0.3 ± 0.02 | 0.8 ± 0.02 | 0.4 ± 0.02 | 0.6 ± 0.02 |
| 14 | linalool | m | 1087 | 1095 | 0.2 ± 0.02 | - | - | - |
| 15 | p-cymenene | m | 1092 | 1091 | - | - | - | 0.1 ± 0.01 |
| 16 | fenchol | m | 1094 | 1098 | - | 0.1 ± 0.01 | - | - |
| 17 | L-pincarveol | m | 1121 | 1119 | - | - | 2.3 ± 0.02 | - |
| 18 | α-campholenal | m | 1124 | 1125 | 0.3 ± 0.02 | - | tr | - |
| 19 | camphor | m | 1130 | 1126 | - | 1.6 ± 0.03 | - | 0.1 ± 0.02 |
| 20 | cis-sabinol | m | 1135 | 1133 | tr | - | - | - |
| 21 | camphene hydrate | m | 1154 | 1149 | - | 0.6 ± 0.02 | - | - |
| 22 | endo-borneol | m | 1160 | 1155 | tr | 1.9 ± 0.02 | 0.1 ± 0.01 | 0.1 ± 0.01 |
| 23 | terpinene-4-ol | m | 1162 | 1160 | - | 0.4 ± 0.02 | 0.3 ± 0.02 | |
| 24 | isothujol | m | 1169 | 1165 | 0.3 ± 0.02 | - | ||
| 25 | α-terpineol | m | 1188 | 1183 | 0.1 ± 0.01 | 0.9 ± 0.02 | 0.5 ± 0.02 | 0.1 ± 0.02 |
| 26 | crypton | m | 1192 | 1188 | - | - | 0.4 ± 0.02 | - |
| 27 | 2-pinen-10-ol | m | 1199 | 1194 | - | 0.1 ± 0.02 | - | - |
| 28 | linalyl formate | m | 1205 | 1206 | 0.3 ± 0.02 | - | - | - |
| 29 | citronellol | m | 1219 | 1212 | - | 0.1 ± 0.01 | - | 0.1 ± 0.01 |
| 30 | methyl thymyl ether | m | 1238 | 1234 | - | 0.2 ± 0.02 | 0.6 ± 0.02 | 0.2 ± 0.02 |
| 31 | isobornyl formate | m | 1240 | 1237 | - | 0.1 ± 0.01 | - | - |
| 32 | α-ocimene | m | 1244 | 1239 | - | 0.1 ± 0.01 | - | - |
| 33 | piperitone | m | 1260 | 1254 | - | 0.1 ± 0.01 | - | - |
| 34 | 2-undecanone | m | 1281 | 1276 | - | 0.1 ± 0.01 | - | - |
| 35 | bornyl acetate | m | 1297 | 1290 | 7.3 ± 0.03 | 7.1 ± 0.03 | 1.5 ± 0.02 | 1.7 ± 0.02 |
| 36 | α-terpinyl acetate | m | 1350 | 1344 | - | 0.5 ± 0.02 | 0.5 ± 0.02 | 0.3 ± 0.02 |
| 37 | citronellol acetate | m | 1355 | 1348 | 0.8 ± 0.02 | 0.1 ± 0.02 | - | - |
| 38 | nerol acetate | m | 1367 | 1363 | 0.6 ± 0.02 | - | - | - |
| 39 | α-copaene | s | 1377 | 1368 | - | - | 0.1 ± 0.01 | - |
| 40 | α-cubebene | s | 1386 | 1381 | 0.2 ± 0.02 | - | 0.1 ± 0.00 | - |
| 41 | longicyclene | s | 1400 | 1392 | - | 0.4 ± 0.02 | - | - |
| 42 | α-longipinene | s | 1406 | 1400 | 1.8 ± 0.02 | - | - | - |
| 43 | longifolene | s | 1413 | 1408 | 1.1 ± 0.02 | 2.7 ± 0.03 | - | - |
| 44 | β-caryophyllene | s | 1429 | 1424 | 9.6 ± 0.04 | 3.7 ± 0.03 | 0.9 ± 0.03 | 2.0 ± 0.02 |
| 45 | aromadendrene | s | 1458 | 1460 | 1.5 ± 0.02 | - | 0.2 ± 0.02 | - |
| 46 | humulene | s | 1471 | 1465 | 3.4 ± 0.02 | 1.4 ± 0.02 | 0.5 ± 0.02 | 0.3 ± 0.02 |
| 47 | β-eudesmene | s | 1490 | 1483 | 0.4 ± 0.02 | - | - | - |
| 48 | γ-muurolene | s | 1494 | 1487 | - | 0.2 ± 0.02 | 0.9 ± 0.02 | 0.1 ± 0.01 |
| 49 | β-bisabolene | s | 1499 | 1494 | 0.9 ± 0.02 | - | - | - |
| 50 | α-franesene | s | 1501 | 1496 | - | 0.3 ± 0.02 | - | - |
| 51 | β-himachalene | s | 1505 | 1495 | 0.9 ± 0.02 | - | - | - |
| 52 | germacrene D | s | 1509 | 1500 | - | 0.5 ± 0.02 | 1.5 ± 0.02 | 0.2 ± 0.02 |
| 53 | β-bisabolene | s | 1512 | 1501 | - | 0.3 ± 0.02 | 0.7 ± 0.02 | - |
| 54 | γ-cadinene | s | 1515 | 1508 | - | - | 0.9 ± 0.02 | - |
| 55 | δ-cadinene | s | 1524 | 1530 | 1.8 ± 0.02 | 1.9 ± 0.03 | 2.4 ± 0.02 | 0.5 ± 0.02 |
| 56 | α-muurolene | s | 1530 | 1534 | - | 0.5 ± 0.02 | 0.2 ± 0.02 | 0.3 ± 0.02 |
| 57 | α-calacorene | s | 1543 | 1539 | 0.2 ± 0.02 | - | - | - |
| 58 | β-calacorene | s | 1554 | 1548 | tr | - | - | - |
| 59 | spathulenol | s | 1612 | 1601 | - | - | 0.4 ± 0.02 | 0.1 ± 0.02 |
| 60 | caryophyllene oxide | s | 1617 | 1613 | 1.0 ± 0.01 | 0.2 ± 0.02 | 0.1 ± 0.00 | 0.2 ± 0.02 |
| 61 | epicubenol | s | 1622 | 1618 | 0.4 ± 0.02 | 0.2 ± 0.02 | - | - |
| 62 | β-himachalol | s | 1642 | 1637 | 0.7 ± 0.02 | - | - | - |
| 63 | τ-muurolol | s | 1653 | 1647 | - | 0.6 ± 0.02 | - | - |
| 64 | α-bisabolol | s | 1672 | 1668 | - | tr | 0.1 ± 0.01 | - |
| 65 | α-cadinol | s | 1680 | 1676 | - | 0.3 ± 0.02 | 0.2 ± 0.02 | 0.1 ± 0.00 |
| 66 | cembrene | s | 1955 | 1948 | - | 1.1 ± 0.02 | - | - |
| 67 | α-camphorene | s | 1976 | 1970 | - | 0.2 ± 0.02 | - | - |
| 68 | geranyllinalool | s | 2025 | 2020 | - | 0.2 ± 0.02 | - | - |
| SUM | 99.4 | 99.9 | 96.4 | 99.7 | ||||
| Monoterpenes | 74.7 | 83.8 | 87.2 | 95.9 | ||||
| Sesquiterpenes | 23.9 | 15.7 | 9.2 | 3.8 | ||||
| Other | 0.8 | 0.4 | - | - |
| Target Species | EO (μL) | G (%) | GI | CVG | MGT | Shoot (mm) |
|---|---|---|---|---|---|---|
| A. alba | ||||||
| L. multiflorum | 0 | 85 ± 8.5 a | 158 ± 15.9 a | 82 ± 11.3 a | 5.2 ± 0.0 a | 67 ± 2.4 a |
| 2 | 80 ± 5.7 a | 130 ± 11.0 b | 64 ± 7.6 b | 5.2 ± 0.1 a | 64 ± 3.5 a | |
| 20 | 75 ± 10.0 a | 114 ± 15.6 bc | 62 ± 7.7 b | 5.4 ± 0.1 ab | 61 ± 4.2 a | |
| 50 | 70 ± 11.5 a | 87 ± 20.2 c | 48 ± 13.5 b | 5.6 ± 0.1 bc | 50 ± 1.6 b | |
| 100 | 53 ± 5.3 b | 56 ± 8.8 d | 29 ± 2.7 c | 5.8 ± 0.2 c | 26 ± 3.9 c | |
| p-value | 0.001 * | 0.000 * | 0.000 * | 0.007 * | 0.000 * | |
| S. alba | 0 | 90 ± 11.5 a | 247 ± 24.6 a | 106 ± 15.6 a | 4.3 ± 0.3 a | 28 ± 1.1 a |
| 2 | 77 ± 4.0 b | 209 ± 11.2 b | 84 ± 5.9 b | 4.6 ± 0.1 ab | 25 ± 2.6 b | |
| 20 | 77 ± 4.0 b | 196 ± 10.4 b | 82 ± 7.0 b | 4.7 ± 0.1 b | 24 ± 0.5 b | |
| 50 | 67 ± 5.3 b | 149 ± 23.6 c | 57 ± 11.3 c | 4.7 ± 0.1 b | 19 ± 2.1 c | |
| 100 | 53 ± 5.3 c | 123 ± 19.8 c | 46 ± 8.8 c | 4.9 ± 0.1 b | 18 ± 1.3 c | |
| p-value | 0.000 * | 0.000 * | 0.000 * | 0.007 * | 0.000 * | |
| P. abies | ||||||
| L. multiflorum | 0 | 92 ± 6.2 a | 179 ± 22.5 a | 89 ± 5.7 a | 5.0 ± 0.2 a | 71 ± 3.1 a |
| 2 | 82 ± 6.7 ab | 138 ± 9.5 b | 70 ± 6.6 b | 5.2 ± 0.0 ab | 67 ± 5.7 a | |
| 20 | 77 ± 11.6 ab | 132 ± 13.5 b | 66 ± 7.1 b | 5.2 ± 0.0 ab | 63 ± 4.6 a | |
| 50 | 69 ± 8.3 b | 103 ± 11.9 c | 50 ± 3.9 c | 5.3 ± 0.0 b | 32 ± 3.5 b | |
| 100 | 50 ± 3.5 c | 33 ± 4.5 d | 18 ± 3.8 d | 6.1 ± 0.1 c | 29 ± 2.6 b | |
| p-value | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | |
| S. alba | 0 | 88 ± 9.9 a | 234 ± 18.8 a | 101 ± 14.1 a | 4.6 ± 0.1 a | 33 ± 0.9 a |
| 2 | 78 ± 6.7 ab | 205 ± 18.5 b | 78 ± 7.4 b | 4.8 ± 0.2 bc | 29 ± 2.1 b | |
| 20 | 67 ± 9.4 b | 138 ± 17.6 c | 60 ± 7.4 c | 4.8 ± 0.1 bc | 25 ± 2.8 c | |
| 50 | 40 ± 13.5 c | 84 ± 5.0 d | 29 ± 7.5 d | 5.0 ± 0.1 c | 19 ± 2.5 d | |
| 100 | 35 ± 4.4 c | 82 ± 13.3 d | 28 ± 1.6 d | 5.0 ± 0.1 c | 12 ± 1.4 e | |
| p-value | 0.000 * | 0.000 * | 0.000 * | 0.012 * | 0.000 * | |
| P. cembra | ||||||
| L. multiflorum | 0 | 87 ± 7.5 a | 173 ± 14.6 a | 85 ± 10.2 a | 5.1 ± 0.0 a | 67 ± 3.9 a |
| 2 | 77 ± 4.0 ab | 155 ± 7.5 a | 73 ± 5.8 a | 5.1 ± 0.0 a | 58 ± 4.7 ab | |
| 20 | 62 ± 15.6 bc | 95 ± 20.6 b | 44 ± 13.5 b | 5.3 ± 0.1 b | 50 ± 11.2 bc | |
| 50 | 55 ± 10.0 bc | 81 ± 12.8 b | 36 ± 8.6 b | 5.4 ± 0.1 b | 45 ± 2.3 c | |
| 100 | 47 ± 5.3 c | 65 ± 16.2 b | 29 ± 7.6 b | 5.6 ± 0.1 c | 39 ± 4.9 c | |
| p-value | 0.000 * | 0.000 * | 0.000 * | 0.012 * | 0.000 * | |
| S. alba | 0 | 92 ± 8.3 a | 257 ± 8.5 a | 112 ± 14.1 a | 4.6 ± 0.1 a | 26 ± 0.7 a |
| 2 | 82 ± 8.3 ab | 163 ± 20.1 b | 77 ± 9.5 b | 4.8 ± 0.1 b | 21 ± 1.7 b | |
| 20 | 70 ± 11.5 b | 140 ± 12.8 b | 65 ± 11.8 b | 5.0 ± 0.1 b | 17 ± 2.5 c | |
| 50 | 27 ± 5.3 c | 40 ± 8.1 c | 14 ± 3.5 c | 5.0 ± 0.1 b | 16 ± 2.2 c | |
| 100 | 17 ± 8.7 c | 38 ± 14.1 c | 12 ± 5.7 c | 5.3 ± 0.1 c | 9 ± 0.9 d | |
| p-value | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | |
| P. mugo | ||||||
| L. multiflorum | 0 | 82 ± 3.5 a | 140 ± 7.4 a | 72 ± 1.9 a | 5.3 ± 0.1 a | 69 ± 5.8 a |
| 2 | 75 ± 16.1 a | 124 ± 11.4 ab | 61 ± 12.0 ab | 5.3 ± 0.0 a | 62 ± 6.0 ab | |
| 20 | 73 ± 5.3 a | 120 ± 11.8 ab | 59 ± 5.4 ab | 5.3 ± 0.1 a | 58 ± 4.1 bc | |
| 50 | 64 ± 7.0 ab | 102 ± 13.6 b | 48 ± 8.2 b | 5.4 ± 0.1 a | 58 ± 2.7 bc | |
| 100 | 50 ± 12.9 b | 66 ± 10.5 c | 31 ± 7.5 c | 5.6 ± 0.1 b | 50 ± 1.5 c | |
| p-value | 0.005 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | |
| S. alba | 0 | 92 ± 8.3 a | 252 ± 17.1 a | 111 ± 12.2 a | 4.6 ± 0.1 a | 27 ± 1.8 a |
| 2 | 38 ± 6.7 b | 67 ± 8.6 b | 26 ± 4.4 b | 4.8 ± 0.2 b | 25 ± 2.3 ab | |
| 20 | 33 ± 5.3 b | 58 ± 11.7 b | 22 ± 5.0 b | 5.2 ± 0.1 c | 21 ± 2.8 bc | |
| 50 | 30 ± 6.5 b | 54 ± 12.1 b | 20 ± 5.7 b | 5.3 ± 0.1 c | 21 ± 0.3 c | |
| 100 | 17 ± 4.0 c | 42 ± 11.7 b | 12 ± 3.5 b | 5.3 ± 0.1 c | 14 ± 2.7 d | |
| p-value | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | |
| Target Species | EO (μL/mL) | G (%) | GI | CVG | MGT | Shoot (mm) |
|---|---|---|---|---|---|---|
| A. alba | ||||||
| L. multiflorum | 0 | 87 ± 0.0 a | 161 ± 13.7 a | 80 ± 5.6 a | 5.1 ± 0.1 a | 65 ± 3.5 a |
| 2 | 82 ± 6.7 ab | 116 ± 27.5 b | 62 ± 11.7 b | 5.4 ± 0.1 b | 69 ± 5.1 a | |
| 5 | 75 ± 11.5 ab | 98 ± 17.7 bc | 48 ± 12.3 b | 5.5 ± 0.2 b | 62 ± 12.6 a | |
| 10 | 70 ± 11.6 b | 80 ± 12.5 c | 47 ± 9.9 b | 5.8 ± 0.1 c | 58 ± 5.7 a | |
| 20 | 30 ± 3.5 c | 26 ± 2.9 d | 12 ± 1.9 c | 5.9 ± 0.1 d | 17 ± 2.2 b | |
| p-value | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | |
| S. alba | 0 | 85 ± 6.3 a | 214 ± 30.5 a | 94 ± 9.6 a | 4.7 ± 0.1 a | 29 ± 1.5 a |
| 2 | 80 ± 9.4 a | 183 ± 11.4 b | 82 ± 3.5 b | 4.9 ± 0.1 b | 18 ± 2.6 b | |
| 5 | 80 ± 5.7 a | 141 ± 4.9 c | 67 ± 3.6 c | 5.1 ± 0.1 c | 18 ± 0.9 b | |
| 10 | 78 ± 3.5 a | 120 ± 9.0 c | 58 ± 2.5 cd | 5.2 ± 0.1 c | 16 ± 0.9 b | |
| 20 | 72 ± 8.3 a | 84 ± 7.2 d | 50 ± 4.2 d | 5.7 ± 0.1 d | 15 ± 1.5 b | |
| p-value | 0.152 | 0.000 * | 0.000 * | 0.000 * | 0.000 * | |
| P. abies | ||||||
| L. multiflorum | 0 | 90 ± 6.5 a | 192 ± 22.6 a | 92 ± 14.1 a | 5.0 ± 0.0 a | 64 ± 3.1 a |
| 2 | 78 ± 3.5 ab | 149 ± 18.8 b | 69 ± 9.6 b | 5.0 ± 0.0 a | 56 ± 1.4 b | |
| 5 | 69 ± 8.3 b | 121 ± 11.2 b | 54 ± 7.6 b | 5.1 ± 0.0 a | 48 ± 4.6 c | |
| 10 | 47 ± 7.4 c | 67 ± 23.1 c | 31 ± 10.0 c | 5.5 ± 0.2 b | 39 ± 3.9 d | |
| 20 | 44 ± 4.0 c | 51 ± 2.2 c | 25 ± 1.3 c | 5.8 ± 0.1 c | 30 ± 2.6 e | |
| p-value | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | |
| S. alba | 0 | 82 ± 6.7 a | 223 ± 11.6 a | 94 ± 8.5 a | 4.7 ± 0.1 a | 27 ± 0.5 a |
| 2 | 63 ± 6.5 b | 119 ± 17.4 b | 69 ± 8.4 b | 5.0 ± 0.1 b | 19 ± 3.7 b | |
| 5 | 55 ± 9.9 b | 87 ± 12.2 c | 48 ± 10.1 c | 5.0 ± 0.1 b | 16 ± 2.0 bc | |
| 10 | 42 ± 3.5 c | 69 ± 8.4 cd | 33 ± 4.6 d | 5.3 ± 0.1 c | 13 ± 1.2 d | |
| 20 | 29 ± 3.0 d | 58 ± 10.1 d | 20 ± 3.3 d | 5.8 ± 0.1 d | 12 ± 3.2 d | |
| p-value | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | |
| P. cembra | ||||||
| L. multiflorum | 0 | 100 ± 0.0 a | 190 ± 14.7 a | 99 ± 7.2 a | 5.1 ± 0.1 a | 72 ± 3.9 a |
| 2 | 75 ± 14.6 b | 90 ± 13.8 b | 45 ± 9.3 b | 5.5 ± 0.0 b | 63 ± 2.1 b | |
| 5 | 67 ± 9.4 bc | 84 ± 12.0 b | 42 ± 8.8 bc | 5.5 ± 0.1 b | 53 ± 2.7 c | |
| 10 | 58 ± 3.5 c | 82 ± 5.8 b | 38 ± 2.3 bc | 5.5 ± 0.1 b | 35 ± 3.7 d | |
| 20 | 53 ± 0.0 c | 55 ± 2.6 c | 29 ± 1.7 d | 5.8 ± 0.1 c | 24 ± 2.2 e | |
| p-value | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | |
| S. alba | 0 | 84 ± 4.0 a | 218 ± 18.9 a | 92 ± 8.5 a | 4.7 ± 0.0 a | 27 ± 1.8 a |
| 2 | 77 ± 15.8 a | 184 ± 27.1 ab | 80 ± 19.7 ab | 4.8 ± 0.1 ab | 27 ± 1.3 a | |
| 5 | 69 ± 3.0 ab | 151 ± 16.9 b | 63 ± 8.2 bc | 4.9 ± 0.1 ab | 19 ± 1.2 b | |
| 10 | 60 ± 0.0 bc | 106 ± 21.0 c | 44 ± 6.7 cd | 5.1 ± 0.2 b | 16 ± 2.1 c | |
| 20 | 48 ± 6.2 c | 97 ± 6.8 c | 38 ± 3.4 d | 5.1 ± 0.1 b | 9 ± 1.4 d | |
| p-value | 0.000 * | 0.000 * | 0.000 * | 0.002 * | 0.000 * | |
| P. mugo | ||||||
| L. multiflorum | 0 | 89 ± 3.0 a | 190 ± 10.5 a | 89 ± 7.1 a | 4.9 ± 0.1 a | 67 ± 2.3 a |
| 2 | 82 ± 8.0 a | 153 ± 24.0 b | 70 ± 13.0 b | 5.0 ± 0.1 a | 59 ± 4.0 b | |
| 5 | 64 ± 4.0 b | 118 ± 9.0 c | 51 ± 4.0 c | 5.1 ± 0.1 a | 56 ± 2.0 b | |
| 10 | 62 ± 3.5 b | 95 ± 17.0 c | 46 ± 5.0 c | 5.2 ± 0.2 a | 49 ± 4.0 c | |
| 20 | 52 ± 6.0 c | 91 ± 5.0 c | 38 ± 5.0 c | 5.6 ± 0.1 b | 45 ± 3.0 c | |
| p-value | 0.000 * | 0.000 * | 0.000 * | 0.002 * | 0.000 * | |
| S. alba | 0 | 89 ± 3.0 a | 243 ± 10.6 a | 105 ± 4.8 a | 4.7 ± 0.1 a | 28 ± 1.2 a |
| 2 | 72 ± 11.0 b | 158 ± 23.0 b | 68 ± 12.0 b | 5.0 ± 0.0 b | 24 ± 1.0 b | |
| 5 | 65 ± 6.0 bc | 145 ± 13.0 bc | 60 ± 7.0 bc | 5.0 ± 0.1 b | 19 ± 1.0 c | |
| 10 | 53 ± 5.0 c | 121 ± 8.0 c | 48 ± 3.0 c | 5.5 ± 0.0 c | 17 ± 1.0 d | |
| 20 | 40 ± 6.0 d | 78 ± 6.0 d | 30 ± 4.0 d | 5.5 ± 0.1 c | 14 ± 2.0 e | |
| p-value | 0.000 * | 0.000 * | 0.000 * | 0.002 * | 0.000 * | |
| Target Species | EO (μL/mL) | Damaged Seedlings (%) | Damaged Leaves (%) | Damaged Leaf Surface (%) | Phytotoxicity (0–10) |
|---|---|---|---|---|---|
| A. alba | |||||
| L. multiflorum | 0 | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0 ± 0.0 a |
| 10 | 23.3 ± 3.0 b | 17.8 ± 2.2 b | 14.8 ± 2.0 b | 2 ± 0.0 b | |
| 20 | 42.5 ± 5.6 c | 40.7 ± 2.2 c | 33.1 ± 1.9 c | 4 ± 1.0 c | |
| 50 | 100.0 ± 0.0 d | 93.3 ± 0.0 c | 68.8 ± 7.1 d | 6 ± 2.8 d | |
| 100 | 100.0 ± 0.0 d | 96.0 ± 3.8 c | 74.4 ± 8.4 d | 8 ± 2.1 d | |
| p-value | 0.000 * | 0.000 * | 0.000 * | 0.000 * | |
| S. alba | 0 | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0 ± 0.0 a |
| 10 | 26.7 ± 3.9 b | 22.2 ± 3.0 b | 20.6 ± 2.5 b | 2 ± 0.8 b | |
| 20 | 69.4 ± 7.8 c | 60.5 ± 6.3 c | 52.2 ± 3.0 c | 6 ± 1.1 c | |
| 50 | 100.0 ± 0.0 d | 100.0 ± 0.0 d | 85.6 ± 5.9 d | 9 ± 1.3 d | |
| 100 | 100.0 ± 0.0 d | 100.0 ± 0.0 d | 98.4 ± 7.6 e | 10 ± 1.2 d | |
| p-value | 0.000 * | 0.000 * | 0.000 * | 0.000 * | |
| P. abies | |||||
| L. multiflorum | 0 | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0 ± 0.0 a |
| 10 | 17.8 ± 2.0 b | 15.6 ± 2.4 b | 10.0 ± 2.8 b | 1 ± 0.4 b | |
| 20 | 42.7 ± 6.8 c | 33.6 ± 3.2 c | 25.9 ± 4.4 c | 3 ± 1.0 c | |
| 50 | 100 ± 0.0 d | 96.7 ± 0.0 d | 54.7 ± 6.3 d | 5 ± 0.5 d | |
| 100 | 100 ± 0.0 d | 97.0 ± 3.3 d | 76.2 ± 6.9 e | 7 ± 1.4 e | |
| p-value | 0.000 * | 0.000 * | 0.000 * | 0.000 * | |
| S. alba | 0 | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0 ± 0.0 a |
| 10 | 13.3 ± 3.2 b | 12.2 ± 1.0 b | 12.5 ± 2.2 b | 1 ± 1.0 b | |
| 20 | 51.1 ± 8.8 c | 44.4 ± 6.7 c | 27.7 ± 4.3 c | 3 ± 0.6 c | |
| 50 | 100 ± 0.0 d | 83.3 ± 5.0 d | 48.8 ± 5.3 d | 5 ± 0.8 d | |
| 100 | 100 ± 0.0 d | 99.0 ± 1.9 e | 67.2 ± 1.6 e | 7 ± 2.6 e | |
| p-value | 0.000 * | 0.000 * | 0.000 * | 0.000 * | |
| P. cembra | |||||
| L. multiflorum | 0 | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0 ± 0.0 a |
| 10 | 28.9 ± 6.5 b | 18.9 ± 3.5 b | 13.3 ± 4.7 b | 1 ± 0.4 b | |
| 20 | 46.7 ± 6.6 c | 41.1 ± 5.1 c | 24.8 ± 4.1 c | 2 ± 0.9 c | |
| 50 | 86.7 ± 8.0 d | 76.7 ± 9.0 d | 59.1 ± 6.7 d | 3 ± 1.3 c | |
| 100 | 100.0 ± 0.0 e | 91.0 ± 5.1 e | 66.1 ± 12.7 d | 6 ± 1.8 d | |
| p-value | 0.000 * | 0.000 * | 0.000 * | 0.000 * | |
| S. alba | 0 | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0 ± 0.0 a |
| 10 | 28.9 ± 5.4 b | 21.1 ± 5.7 b | 18.5 ± 6.2 b | 2 ± 0.7 b | |
| 20 | 55.6 ± 5.8 c | 54.4 ± 7.5 c | 30.5 ± 4.0 c | 3 ± 0.5 b | |
| 50 | 100.0 ± 0.0 d | 83.3 ± 4.8 d | 52.0 ± 6.8 d | 5 ± 1.1 c | |
| 100 | 100.0 ± 0.0 d | 97.0 ± 5.8 e | 70.6 ± 12.7 e | 7 ± 1.6 d | |
| p-value | 0.000 * | 0.000 * | 0.000 * | 0.000 * | |
| P. mugo | |||||
| L. multiflorum | 0 | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0 ± 0.0 a |
| 10 | 20.0 ± 4.1 b | 18.9 ± 2.9 b | 14.9 ± 3.5 b | 1 ± 0.0 b | |
| 20 | 55.6 ± 7.8 c | 41.1 ± 6.9 c | 32.7 ± 8.9 c | 3 ± 1.0 c | |
| 50 | 100.0 ± 0.0 d | 86.7 ± 0.0 d | 58.8 ± 9.8 d | 5 ± 2.2 d | |
| 100 | 100.0 ± 0.0 e | 100.0 ± 0.0 e | 80.7 ± 12.8 e | 8 ± 1.1 e | |
| p-value | 0.000 * | 0.000 * | 0.000 * | 0.000 * | |
| S. alba | 0 | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0 ± 0.0 a |
| 10 | 26.7 ± 7.0 b | 20.0 ± 6.1 b | 18.6 ± 5.6 b | 2 ± 1.0 b | |
| 20 | 57.7 ± 8.5 c | 47.7 ± 5.5 c | 39.5 ± 9.5 c | 4 ± 1.0 c | |
| 50 | 75.5 ± 6.7 d | 71.1 ± 9.3 d | 60.5 ± 8.4 d | 7 ± 1.2 d | |
| 100 | 100.0 ± 0.0 e | 100.0 ± 0.0 e | 86.3 ± 8.8 e | 9 ± 0.9 e | |
| p-value | 0.000 * | 0.000 * | 0.000 * | 0.000 * | |
| Rating | Target Species Responses/Injury | Description |
|---|---|---|
| 0 | 0 | no symptoms |
| 1 | 1–10 | very slight symptoms |
| 2 | 11–20 | more severe, but not lasting symptoms |
| 3 | 21–30 | moderate and more lasting symptoms |
| 4 | 31–40 | medium and lasting symptoms |
| 5 | 41–50 | moderately heavy symptoms |
| 6 | 51–60 | heavy symptoms |
| 7 | 61–70 | very heavy symptoms |
| 8 | 71–80 | nearly destroyed leaves/seedlings |
| 9 | 81–90 | destroyed leaves/seedlings |
| 10 | 91–100 | completely destroyed leaves/seedlings |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garzoli, S.; Vaglia, V.; Iriti, M.; Vitalini, S. Vapor and Liquid Phase Profiles of Essential Oils from Abies, Picea and Pinus Species and Their Phytotoxic Interactions with Weed Growth in Pre- and Post-Emergence Conditions. Plants 2023, 12, 1172. https://doi.org/10.3390/plants12051172
Garzoli S, Vaglia V, Iriti M, Vitalini S. Vapor and Liquid Phase Profiles of Essential Oils from Abies, Picea and Pinus Species and Their Phytotoxic Interactions with Weed Growth in Pre- and Post-Emergence Conditions. Plants. 2023; 12(5):1172. https://doi.org/10.3390/plants12051172
Chicago/Turabian StyleGarzoli, Stefania, Valentina Vaglia, Marcello Iriti, and Sara Vitalini. 2023. "Vapor and Liquid Phase Profiles of Essential Oils from Abies, Picea and Pinus Species and Their Phytotoxic Interactions with Weed Growth in Pre- and Post-Emergence Conditions" Plants 12, no. 5: 1172. https://doi.org/10.3390/plants12051172
APA StyleGarzoli, S., Vaglia, V., Iriti, M., & Vitalini, S. (2023). Vapor and Liquid Phase Profiles of Essential Oils from Abies, Picea and Pinus Species and Their Phytotoxic Interactions with Weed Growth in Pre- and Post-Emergence Conditions. Plants, 12(5), 1172. https://doi.org/10.3390/plants12051172