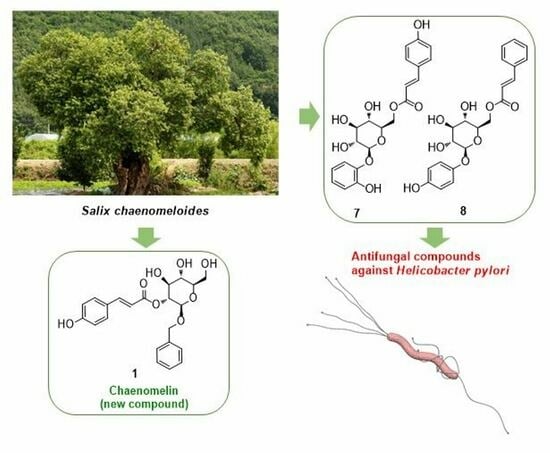
Chaenomelin, a New Phenolic Glycoside, and Anti-Helicobacter pylori Phenolic Compounds from the Leaves of Salix chaenomeloides
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation and Structural Elucidation of Compounds 1–13
2.2. Evaluation of Antibacterial Activity of the Isolated Compounds against Helicobacter pylori
3. Materials and Methods
3.1. Equipment Used for Analyses
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Acid Hydrolysis of 1
3.5. Anti-Helicobacter pylori Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Le, N.P.K.; Herz, C.; Gomes, J.V.D.; Förster, N.; Antoniadou, K.; Mittermeier-Kleßinger, V.K.; Mewis, I.; Dawid, C.; Ulrichs, C.; Lamy, E. Comparative anti-inflammatory effects of Salix cortex extracts and acetylsalicylic acid in SARS-CoV-2 peptide and LPS-activated human in vitro systems. Int. J. Mol. Sci. 2021, 22, 6766. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Jerz, G.; Shen, L.; Xiu, L.; Winterhalter, P. Isolation and structure determination of a lignan from the bark of Salix alba. Nat. Prod. Res. 2007, 21, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Bajraktari, D.; Bauer, B.; Zeneli, L. Antioxidant capacity of Salix alba (Fam. Salicaceae) and influence of heavy metal accumulation. Horticulturae 2022, 8, 642. [Google Scholar] [CrossRef]
- Tawfeek, N.; Mahmoud, M.F.; Hamdan, D.I.; Sobeh, M.; Farrag, N.; Wink, M.; El-Shazly, A.M. Phytochemistry, pharmacology and medicinal uses of plants of the genus Salix: An updated review. Front. Pharmacol. 2021, 12, 593856. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Z.; Zhang, X.-F.; Wang, L.-J.; Zheng, Y.-N.; Yuan, C.-C.; Sun, G.-Z. Isolation and characterization of phenolic compounds from the leaves of Salix matsudana. Molecules 2008, 13, 1530–1537. [Google Scholar] [CrossRef] [PubMed]
- Han, L.K.; Sumiyoshi, M.; Zhang, J.; Liu, M.X.; Zhang, X.F.; Zheng, Y.N.; Okuda, H.; Kimura, Y. Anti-obesity action of Salix matsudana leaves (Part 1). Anti-obesity action by polyphenols of Salix matsudana in high fat-diet treated rodent animals. Phytother. Res. 2003, 17, 1188–1194. [Google Scholar] [CrossRef]
- Evans, T.P.; Clausen, T.P.; Reichardt, P.B.; Chang, S. Structurally intriguing glucosides from Alaskan littletree willow (Salix arbusculoides). J. Nat. Prod. 1995, 58, 1897–1900. [Google Scholar] [CrossRef]
- Silva, I.D.R.; Ramos, U.F.; Cerqueira, L.E.; da Silva, A.S.B.I.; Junior, G.A.D.; Carollo, C.A.; dos Santos Bastos, I.; Orlandi, P.I.P.; Markus-Michalczyk, H.; Barbosa, W.L.R. Antiplasmodial activity and phenolic composition of Brazilian Salix humboldtiana Willd. extract and fractions. J. Med. Plants Res. 2022, 16, 35–43. [Google Scholar] [CrossRef]
- Yang, H.; Lee, S.H.; Sung, S.H.; Kim, J.; Kim, Y.C. Neuroprotective compounds from Salix pseudo-lasiogyne twigs and their anti-amnesic effects on scopolamine-induced memory deficit in mice. Planta Med. 2013, 79, 78–82. [Google Scholar] [CrossRef]
- Tantry, M.A.; Shah, S.; Dar, M.Y.; Mir, M.M.; Ghazanfar, K.; Sheikh, F.A.; Khuroo, M.A.; Akbar, S. 9,10-seco-9,19-cyclolanostane triterpene from Salix caprea L. (Goat Willow). Nat. Prod. Res. 2013, 27, 171–175. [Google Scholar] [CrossRef]
- Jeon, S.H.; Chun, W.; Choi, Y.J.; Kwon, Y.S. Cytotoxic constituents from the bark of Salix hulteni. Arch. Pharm. Res. 2008, 31, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Kwon, O.W.; Kim, S.Y.; Choi, S.U.; Kim, J.Y.; Han, J.Y.; Choi, S.I.; Choi, J.G.; Kim, K.H.; Lee, K.R. Phenolic glycosides from the twigs of Salix glandulosa. J. Nat. Prod. 2014, 77, 1955–1961. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Subedi, L.; Park, K.J.; Kim, S.Y.; Choi, S.U.; Kim, K.H.; Lee, K.R. Salicin derivatives from Salix glandulosa and their biological activities. Fitoterapia 2015, 106, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, D.; Shen, Y. Discovery of novel bioactive natural products driven by genome mining. Drug Discov. Ther. 2018, 12, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug. Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Shen, B. A New Golden Age of Natural Products Drug Discovery. Cell 2015, 163, 1297–1300. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; Thuru, X.; Quesnel, B. Combined cytotoxic chemotherapy and immunotherapy of cancer: Modern times. NAR Cancer 2020, 2, zcaa002. [Google Scholar] [CrossRef] [PubMed]
- Nunez, C.V.; Vasconcellos, M.C.; Alaniz, L. Are natural products, used as antitumoral/antiangiogenic agents, less toxic than synthetic conventional chemotherapy? Front. Pharmacol. 2022, 13, 1055516. [Google Scholar] [CrossRef]
- Lee, B.S.; So, H.M.; Kim, S.; Kim, J.K.; Kim, J.-C.; Kang, D.-M.; Ahn, M.-J.; Ko, Y.-J.; Kim, K.H. Comparative evaluation of bioactive phytochemicals in Spinacia oleracea cultivated under greenhouse and open field conditions. Arch. Pharm. Res. 2022, 45, 795–805. [Google Scholar] [CrossRef]
- Cho, H.; Kim, K.H.; Han, S.H.; Kim, H.-J.; Cho, I.-H.; Lee, S. Structure determination of heishuixiecaoline A from Valeriana fauriei and its content from different cultivated regions by HPLC/PDA Analysis. Nat. Prod. Sci. 2022, 28, 181–186. [Google Scholar] [CrossRef]
- Yu, J.S.; Jeong, S.Y.; Li, C.; Oh, T.; Kwon, M.; Ahn, J.S.; Ko, S.-K.; Ko, Y.-J.; Cao, S.; Kim, K.H. New phenalenone derivatives from the Hawaiian volcanic soil-associated fungus Penicillium herquei FT729 and their inhibitory effects on indoleamine 2,3-dioxygenase 1 (IDO1). Arch. Pharm. Res. 2022, 45, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Lee, B.S.; Yu, J.S.; Kang, H.; Yoo, M.J.; Yi, S.A.; Han, J.-W.; Kim, S.; Kim, J.K.; Kim, J.-C. Identification of anti-adipogenic withanolides from the roots of Indian ginseng (Withania somnifera). J. Ginseng Res. 2022, 46, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.E.; Park, K.H.; Hong, J.H.; Kim, S.H.; Park, K.M.; Kim, K.H. Anti-osteoporosis effects of triterpenoids from the fruit of sea buckthorn (Hippophae rhamnoides) through the promotion of osteoblast differentiation in mesenchymal stem cells, C3H10T1/2. Arch. Pharm. Res. 2023, 46, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Rawat, P.; Khan, M.F.; Tamarkar, A.K.; Srivastava, A.K.; Arya, K.R.; Maurya, R. Phenolic glycosides from Dodecadenia grandiflora and their glucose-6-phosphatase inhibitory activity. Fitoterapia 2010, 81, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, N.; Ishihara, K.; Matsumura, S.; Hamada, H.; Nakamura, K.; Furuya, T. Lipase-catalyzed synthesis of arbutin cinnamate in an organic solvent and application of transesterification to stabilize plant pigments. Biosci. Biotechnol. Biochem. 1997, 61, 1926–1928. [Google Scholar] [CrossRef]
- Chen, J.-F.; Tan, L.; Ju, F.; Kuang, Q.-X.; Yang, T.-L.; Deng, F.; Gu, Y.-C.; Jiang, L.-S.; Deng, Y.; Guo, D.-L. Phenolic glycosides from Sanguisorba officinalis and their anti-inflammatory effects. Nat. Prod. Res. 2022, 36, 2097–2104. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.G.; Cho, J.-Y.; Kim, C.-M.; Lee, S.-H.; Kim, W.-S.; Jeon, T.-I.; Park, K.-H.; Moon, J.-H. Coumaroyl quinic acid derivatives and flavonoids from immature pear (Pyrus pyrifolia Nakai) fruit. Food Sci. Biotechnol. 2013, 22, 803–810. [Google Scholar] [CrossRef]
- Ishikawa, T.; Nishigaya, K.; Takami, K.; Uchikoshi, H.; Chen, I.-S.; Tsai, I.-L. Isolation of salicin derivatives from Homalium cochinchinensis and their antiviral activities. J. Nat. Prod. 2004, 67, 659–663. [Google Scholar] [CrossRef]
- Kim, M.-R.; Lee, S.-K.; Kim, C.-S.; Kim, K.-S.; Moon, D.-C. Phytochemical constituents of Carpesium macrocephalum F R-et S AV. Arch. Pharm. Res. 2004, 27, 1029–1033. [Google Scholar] [CrossRef]
- Zeng, J.; Ma, R.-J.; Wang, L.; Zhang, S.-N.; Song, H.-Z.; Yang, Y.; Tan, Q.-G. Chemical constituents from the leaves of Melia azedarach. Nat. Prod. Res. 2019, 33, 2860–2863. [Google Scholar] [CrossRef]
- Xu, L.; Liu, H.; Ma, Y.; Wu, C.; Li, R.; Chao, Z. Isomeric phenolic glycosides from Populus tomentosa. Rec. Nat. Prod. 2018, 13, 97–103. [Google Scholar] [CrossRef]
- Sang, D.; Tu, X.; Tian, J.; He, Z.; Yao, M. Anchimerically Assisted Cleavage of Aryl Methyl Ethers by Aluminum Chloride-Sodium Iodide in Acetonitrile. ChemistrySelect 2018, 3, 10103–10107. [Google Scholar] [CrossRef]
- Zhang, J.; Mohamad, H.; Wong, J.H.; Bilal, M.; Ismail, A.H.B.; Lloyd, A.J.; Yusoff, A.A.M.; Osman, H.; Wong, K.T.; Idris, Z. The Effect of 4-hydroxybenzaldehyde on the γ-aminobutyric Acid Type A Receptor. Malays. J. Med. Sci. MJMS 2017, 24, 94. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Z.; Choi, S.U.; Lee, K.R. Phytochemical constituents of the aerial parts from Solidago virga-aurea var. gigantea. Arch. Pharm. Res. 2004, 27, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Kim, H.J.; Song, Y.S.; Jin, C.; Lee, K.-T.; Cho, J.; Lee, Y.S. Constituents of the stems and fruits of Opuntia ficus-indica var. saboten. Arch. Pharm. Res. 2003, 26, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- da Cunha, B.R.; Fonseca, L.P.; Calado, C.R.C. Antibiotic Discovery: Where Have We Come from, Where Do We Go? Antibiotics 2019, 8, 45. [Google Scholar] [CrossRef]
- Smith, P.W.; Watkins, K.; Hewlett, A. Infection control through the ages. Am. J. Infect. Control 2012, 40, 35–42. [Google Scholar] [CrossRef]
- McGee, D.J.; George, A.E.; Trainor, E.A.; Horton, K.E.; Hildebrandt, E.; Testerman, T.L. Cholesterol enhances Helicobacter pylori resistance to antibiotics and LL-37. Antimicrob. Agents Chemother. 2011, 55, 2897–2904. [Google Scholar] [CrossRef]
- Chey, W.D.; Wong, B.C.; Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am. J. Gastroenterol. 2007, 102, 1808–1825. [Google Scholar] [CrossRef]
- Ramos, P.A.; Moreirinha, C.; Silva, S.; Costa, E.M.; Veiga, M.; Coscueta, E.; Santos, S.A.; Almeida, A.; Pintado, M.M.; Freire, C.S. The health-promoting potential of Salix spp. bark polar extracts: Key insights on phenolic composition and in vitro bioactivity and biocompatibility. Antioxidants 2019, 8, 609. [Google Scholar] [CrossRef]
- Wahab, G.A.; Sallam, A.; Elgaml, A.; Lahloub, M.F.; Afifi, M.S. Antioxidant and antimicrobial activities of Salix babylonica extracts. World. J. Pharm. Sci. 2018, 6, 1–6. [Google Scholar]
- Zeng, X.; Wang, H.; Gong, Z.; Huang, J.; Pei, W.; Wang, X.; Zhang, J.; Tang, X. Antimicrobial and cytotoxic phenolics and phenolic glycosides from Sargentodoxa cuneata. Fitoterapia 2015, 101, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Thi Ngoc, N.; Hong Quang, T.; Thi Hong Hanh, T.; Xuan Cuong, N.; The Cuong, N.; Hoang Ha, C.; Hoai Nam, N.; Van Minh, C. Phenolic Glycosides from the Leaves of Iodes cirrhosa Turcz. with Cytotoxic and Antimicrobial Effects. Chem. Biodivers. 2022, 19, e202200182. [Google Scholar] [CrossRef]
- Brown, J.; Wang, J.; Kasman, L.; Jiang, X.; Haley-Zitlin, V. Activities of muscadine grape skin and quercetin against Helicobacter pylori infection in mice. J. Appl. Microbiol. 2011, 110, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, W.X.; Zheng, M.F.; Xu, Q.L.; Wan, F.H.; Wang, J.; Lei, T.; Zhou, Z.Y.; Tan, J.W. Bioactive quinic acid derivatives from Ageratina adenophora. Molecules 2013, 18, 14096–14104. [Google Scholar] [CrossRef] [PubMed]
- Kyriakopoulou, I.; Magiatis, P.; Skaltsounis, A.L.; Aligiannis, N.; Harvala, C. Samioside, a new phenylethanoid glycoside with free-radical scavenging and antimicrobial activities from Phlomis samia. J. Nat. Prod. 2001, 64, 1095–1097. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Beemelmanns, C.; Clardy, J.; Cao, S. A new antibacterial octaketide and cytotoxic phenylethanoid glycosides from Pogostemon cablin (Blanco) Benth. Bioorg. Med. Chem. Lett. 2015, 25, 2834–2836. [Google Scholar] [CrossRef]
- Kuang, H.X.; Xia, Y.G.; Liang, J.; Yang, B.Y.; Wang, Q.H. Lianqiaoxinoside B, a novel caffeoyl phenylethanoid glycoside from Forsythia suspensa. Molecules 2011, 16, 5674–5681. [Google Scholar] [CrossRef]
- Qu, H.; Zhang, Y.; Chai, X.; Sun, W. Isoforsythiaside, an antioxidant and antibacterial phenylethanoid glycoside isolated from Forsythia suspensa. Bioorg. Chem. 2012, 40, 87–91. [Google Scholar] [CrossRef]
- Khalil, A.A.K.; Park, W.S.; Lee, J.; Kim, H.J.; Akter, K.M.; Goo, Y.M.; Bae, J.Y.; Chun, M.S.; Kim, J.H.; Ahn, M.J. A new anti-Helicobacter pylori juglone from Reynoutria japonica. Arch. Pharm. Res. 2019, 42, 505–511. [Google Scholar] [CrossRef]

 ) and HMBC (
) and HMBC ( ) correlations for compound 1.
) correlations for compound 1.
| Position | 1 | |
|---|---|---|
| δH (J in Hz) | δC | |
| 1 | 127.5 C | |
| 2 | 7.47 d (8.5) | 131.3 CH |
| 3 | 6.83 d (8.5) | 116.9 CH |
| 4 | 161.5 C | |
| 5 | 6.83 d (8.5) | 116.9 CH |
| 6 | 7.47 d (8.5) | 131.3 CH |
| 7 | 7.61 d (16.0) | 146.9 CH |
| 8 | 6.33 d (16.0) | 115.4 CH |
| 9 | 168.6 C | |
| 1′ | 4.56 d (8.0) | 101.4 CH |
| 2′ | 4.89 dd (9.5, 8.0) | 75.2 CH |
| 3′ | 3.56 m | 76.2 CH2 |
| 4′ | 3.41 m | 71.8 CH |
| 5′ | 3.33 m | 78.3 CH |
| 6′ | 3.93 dd (12.0, 2.0); 3.73 dd (12.0, 6.0) | 62.8 CH2 |
| 1″ | 139.2 C | |
| 2″ | 7.27 d (7.0) | 129.0 CH |
| 3″ | 7.22 m | 129.3 CH |
| 4″ | 7.22 m | 129.1 CH |
| 5″ | 7.22 m | 129.3 CH |
| 6″ | 7.27 d (7.0) | 129.0 CH |
| 7″ | 4.87 d (12.5); 4.64 d (12.5) | 71.6 CH2 |
| Compound | Inhibition (%) B |
|---|---|
| 1 | 21.6 ± 4.4 c |
| 2 | 11.9 ± 0.9 d |
| 3 | 13.6 ± 1.8 d |
| 4 | 19.0 ± 2.8 cd |
| 5 | 16.3 ± 6.3 cd |
| 6 | 13.0 ± 4.4 d |
| 7 | 31.4 ± 3.9 b |
| 8 | 33.9 ± 4.4 b |
| 9 | 1.2 ± 0.7 e |
| 10 | 18.9 ± 0.3 cd |
| 11 | 19.4 ± 1.7 cd |
| 12 | 15.5 ± 0.7 cd |
| 13 | 19.1 ± 3.0 cd |
| Quercetin A | 38.4 ± 2.3 b |
| Metronidazole A | 96.6 ± 0.5 a |
| Experimental Procedure | Equipment |
|---|---|
| Optical rotations | JASCO P-2000 polarimeter (JASCO, Easton, MD, USA) |
| Ultraviolet (UV) spectra | Agilent 8453 UV-visible spectrophotometer (Agilent Technologies, Santa Clara, CA, USA) |
| Infrared (IR) spectra | Bruker IFS-66/S FT-IR spectrometer (Bruker, Karlsruhe, Germany) |
| Nuclear magnetic resonance (NMR) spectra | Bruker AVANCE III HD 850 NMR spectrometer with a 5 mm TCI CryoProbe operating at 850 MHz (1H) and 212.5 MHz (13C) |
| HR-ESIMS |
|
| Preparative HPLC | Waters 1525 Binary HPLC pump with a Waters 996 Photodiode Array Detector (Waters Corporation, Milford, MA, USA) and an Agilent Eclipse C18 column (250 × 21.2 mm, 5 μm; flow rate: 5 mL/min; Agilent Technologies) |
| Semi-preparative HPLC | Waters 1525 Binary HPLC pump with a Waters 996 Photodiode Array Detector (Waters Corporation, Milford, CT, USA)
|
| LC/MS analysis | Agilent 1200 Series HPLC system equipped with a diode array detector and 6130 Series ESI mass spectrometer using an analytical Kinetex C18 100 Å column (100 × 2.1 mm, 5 μm; flow rate: 0.3 mL/min; Phenomenex). |
| Column chromatography |
|
| Thin-layer chromatography (TLC) | Pre-coated silica gel F254 plates and RP-C18 F254s plates (Merck) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.A.; Kang, D.-M.; Ko, Y.-J.; Ra, M.-J.; Jung, S.-M.; Yu, J.-N.; Ahn, M.-J.; Kim, K.H. Chaenomelin, a New Phenolic Glycoside, and Anti-Helicobacter pylori Phenolic Compounds from the Leaves of Salix chaenomeloides. Plants 2024, 13, 701. https://doi.org/10.3390/plants13050701
Kim KA, Kang D-M, Ko Y-J, Ra M-J, Jung S-M, Yu J-N, Ahn M-J, Kim KH. Chaenomelin, a New Phenolic Glycoside, and Anti-Helicobacter pylori Phenolic Compounds from the Leaves of Salix chaenomeloides. Plants. 2024; 13(5):701. https://doi.org/10.3390/plants13050701
Chicago/Turabian StyleKim, Kyung Ah, Dong-Min Kang, Yoon-Joo Ko, Moon-Jin Ra, Sang-Mi Jung, Jeong-Nam Yu, Mi-Jeong Ahn, and Ki Hyun Kim. 2024. "Chaenomelin, a New Phenolic Glycoside, and Anti-Helicobacter pylori Phenolic Compounds from the Leaves of Salix chaenomeloides" Plants 13, no. 5: 701. https://doi.org/10.3390/plants13050701
APA StyleKim, K. A., Kang, D. -M., Ko, Y. -J., Ra, M. -J., Jung, S. -M., Yu, J. -N., Ahn, M. -J., & Kim, K. H. (2024). Chaenomelin, a New Phenolic Glycoside, and Anti-Helicobacter pylori Phenolic Compounds from the Leaves of Salix chaenomeloides. Plants, 13(5), 701. https://doi.org/10.3390/plants13050701