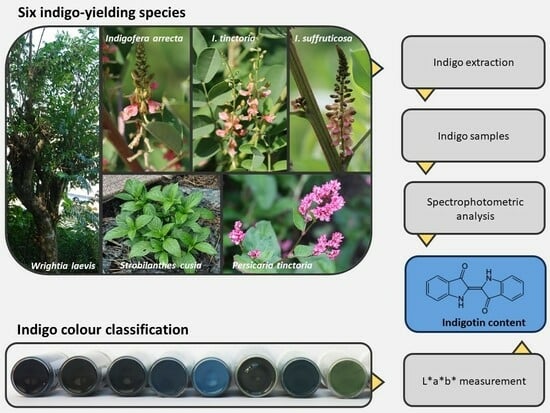
“Purplish Blue” or “Greenish Grey”? Indigo Qualities and Extraction Yields from Six Species
Abstract
:1. Introduction
1.1. New Interest in an Ancient Dye
1.2. A Cornucopia of Species and Manufacturing Methods
1.3. True Blue? (Too) Many Methods for Determining Indigo Quality
1.4. Aims of the Study
1.5. Definitions of Terms
- (a)
- The plants used;
- (b)
- The extract as a traded product;
- (c)
- The valuable component for dyeing contained in the extract;
- (d)
- The blue colour.
2. Results
2.1. Comparison of Species
2.1.1. Species Cultivated in Austria
2.1.2. Species Cultivated in China
2.2. Influence of Extraction Methods (MME, sLPE and Modifications) on Indigo Quality and Yield
2.3. Indigo Quality of Local Practice Samples
2.4. Is Indigo Colour an Indicator of Indigo Quality?
2.4.1. Visual Colour Classification of the Indigo Samples
2.4.2. Measured Colour Parameters (L*a*b*)
2.4.3. Correlation between Indigo Colour (L*a*b* Values) and Indigotin Content
2.4.4. Can Indigotin Content Be Predicted by Measuring Indigo Colour?
3. Discussion
3.1. Indigotin Content and Indigo(tin) Extraction Yield
3.2. The Evaluation of Indigo-Yielding Species Requires a Differentiated Approach
3.3. Indigofera arrecta—A “Forgotten Species” with a Promising Future?
3.4. The Ambivalence of Using Standardised Methods, and Potential Improvements
| Species | Indigotin Content [% in indigo] | Indigo Extraction Yield [g/kg plant material] | Indigotin Extraction Yield [g/kg plant material] | Parameters Tested and Extraction Method Applied | Analytical Method (in brackets: indigo processing state sampled/solvent used/nm measured) | Source |
| Indigofera tinctoria | n.d. | n.d. | 0.58–0.75 (per fw  ) ) | Test of agronomic factors (four ecotypes, three levels of deficit irrigation). Extraction method: boiling, cooling, adding Ca(OH)2 solution, aerating, settling, post-treatment with HCL, centrifuging suspension (laboratory scale). | Spectrophotometry (indigo suspension, centrifuged/H2SO4/611 nm) | [64] |
| n.d. | n.d. | 6.50–6.80 (per fw  ) ) | Test of agronomic factors (four ecotypes, three planting days). Extraction method: boiling, cooling, adding Ca(OH)2 solution, aerating, settling, post-treatment with HCL, centrifuging suspension (laboratory scale). | Spectrophotometry (indigo suspension, centrifuged/H2SO4/611 nm) | [65] | |
| n.d. | 268.301 (paste per fw ⌘) | 3.27 1 (per fw ⌘) | Comparison of I. tinctoria and Strobilanthes cusia. Extraction method: ambient temperature, adding Ca(OH)2 solution, aerating, washing precipitated indigo with Ca(OH)2 solution, centrifuging (laboratory scale). | Spectrophotometry (indigo paste, centrifuged/H2SO4/611 nm) | [59] | |
| 1.69–2.65 (in indigo paste) | 11.90–18.60 (indigo dry per fw ⌘) | n.d. | Test of four extraction methods without lime (conventional at ambient temperature and boiling the indigo paste, microbial with effective microorganisms, hot water at 60 °C subsequently mixed with cold water to obtain 38 °C and chemical with concentrated ammonia solution and calcium chloride) and six soaking durations (laboratory scale). | Spectrophotometry (indigo paste, centrifuged/H2SO4/611 nm) | [77] | |
| n.d. | 1.03–1.09 (indigo dry per fw  ) ) | n.d. | Test of four different I. tinctoria provenances. Comparison of I. tinctoria and Marsdenia tinctoria. Extraction method (without lime): extracting at ambient temperature, aerating, filtering off with vacuum pump, washing indigo with water, drying (laboratory scale). | No chemical analysis | [58] | |
| Indigofera suffruticosa | 17.31–43.40 (in dry indigo) | 20.00–34.00 (indigo dry per dw  ) ) | ? 2–11.20 (per dw  ) ) | Test of agronomic factors (documentation of growing cycle, effects of age at harvest time). Extraction method (without lime): extracting at 25 °C, aerating, cooling and settling, decanting, centrifuging suspension, drying, grinding (laboratory scale). | HPTLC-densitometry (indigo powder/3:2 mixture of toluene and ethyl acetate; N, N-dimethylformamide/610 nm) | [80] |
| Isatis tinctoria (incl. I. indigotica) | n.d. | n.d. | 0.30–0.80 (per fw  ) ) | Test of agronomic factors (sowing date, plant density, N fertilisation, seedling transplantation, irrigation rate). Extraction method: boiling, cooling, adding Ca(OH)2 solution, aerating, settling, post-treatment with HCl (laboratory scale). | Spectrophotometry (indigo suspension/ethyl acetate/600 nm) | [81] |
| 0.00–0.01 | n.d. | n.d. | Test of agronomic factors (N fertilisation, harvest time). Extraction method (without lime): hot extraction, pH control (NaOH), aerating, settling, washing indigo precipitant with water, filtering, drying, grinding (laboratory scale). | Spectrophotometry (indigo powder/glacial acetic acid/664 nm) | [82] | |
| n.d. (lab) 5.70–12.90 (pilot) 4.80–10.00 (prototype) (in dry indigo) | n.d. (lab) 0.90–3.90 (pilot) 0.60–2.60 (prototype) (indigo dry per fw  ) ) | 1.30–2.00 (lab) (per fw  ) ) 0.11–0.22 (pilot) 0.05–0.26 (prototype) (per fw  ) ) | Development of extraction protocols (laboratory scale) and upscaling (pilot and prototype). Test of extraction methods: leaf-to-water ratio, extraction temperature (hot and ambient; cooling), extraction duration, pH control (lime), aeration duration, post-treatment of indigo precipitate with acid, etc. | Spectrophotometry (indigo suspension/ethyl acetate/600 nm) and microgravimetric method (indigo powder) | [55] | |
| n.d. 20–40 (pilot) 3 | n.d. n.d. | 0.02–2.10 (lab) n.d. (per fw  ) ) | Development of extraction methods (laboratory scale) and test at pilot scale: test of different extraction temperatures, pH adjustment (HCl, sulphuric acid; ammonia solution), cooling, aerating (only at pilot scale: settling, filtering, drying). | Spectrophotometry (indigo suspension/ethyl acetate/600 nm) | [78] | |
| n.d. | n.d. | 0.74–6.52 (per fw  ) ) | Comparison of I. tinctoria and 14 other Isatis taxa. Extraction method: boiling, cooling, pH-adjustment 4, aerating, post-treatment with HCl (laboratory scale). | Spectrophotometry (indigo suspension/ethyl acetate/600 nm) | [35] | |
| n.d. | n.d. | 0.74–4.19 (per fw  ) ) | Comparison of I. tinctoria and three other Isatis sp. at different harvest times. Extraction method (without lime): boiling, cooling, pH adjustment (NaOH), aerating, settling, post-treatment with HCl (laboratory scale). | Spectrophotometry (indigo suspension/ethyl acetate/600 nm) | [34] | |
| Several other Isatis sp. | n.d. | n.d. | 0.01–10.00 (per fw  ) ) | Comparison of 14 Isatis taxa and I. tinctoria. Extraction method: boiling, cooling, pH-adjustment 4, aerating, post-treatment with HCl (laboratory scale). | Spectrophotometry (indigo suspension/ethyl acetate/600 nm) | [35] |
| n.d. | n.d. | not detected–2.53 (per fw  ) ) | Comparison of three Isatis sp. And I. tinctoria at different harvest times. Extraction method (without lime): boiling, cooling, pH adjustment (NaOH), aerating, settling, post-treatment with HCl (laboratory scale). | Spectrophotometry (indigo suspension/ethyl acetate/600 nm) | [34] | |
| Marsdenia tinctoria | n.d. | 0.12–0.25 (indigo dry per fw  ) ) | n.d. | Comparison of M. tinctoria and Indigofera tinctoria. Test of extraction methods (without lime): different temperatures (ambient, hot) and steeping durations; aerating, filtering off with vacuum pump, washing indigo with water, drying (laboratory scale). | No chemical analysis | [58] |
| Persicaria tinctoria | <2.00–9.20 (in dry indigo) | 1.00–13.00 (indigo dry per fw ⌘) | ?5–0.40 (per fw ⌘) | Test of different storage conditions of harvested plant material. Extraction method (without lime): plant material was extracted twice (hot), storing extracts for 4–5 days with aerating once a day, adding CaCl2·2H2O to improve precipitation, filtering sediment, drying, grinding. Two analytical methods applied. | Spectrophotometry (indigo powder, measuring leuco-indigotin/reducing agent: aqueous alkaline solution of Fe(II)triethanolamine/410 nm) | [54] |
| 0.00–12.30 (in dry indigo) | <0.01–0.69 (per fw ⌘) | Standard exhaust dyeing experiments and spectrophotometry (indigo-dyed textile) | ||||
| n.d. (lab) 17.40–24.30 (pilot) 5.28–10.44 (prototype) (in dry indigo) | n.d. (lab) 3.20–7.80 (pilot) 1.80–10.00 (prototype) (indigo dry per fw ⌘) | 7.00–10.00 (lab) (per fw  ) ) 0.70–1.36 (pilot) 0.18–0.89 (prototype) (per fw ⌘) | Development of extraction protocols (laboratory scale) and upscaling (pilot and prototype). Test of extraction methods: leaf-to-water ratio, extraction temperature (hot and ambient; cooling), extraction duration, pH control (lime), aeration duration, post-treatment of indigo precipitate with acid, etc. | Spectrophotometry (indigo suspension/ethyl acetate/600 nm) and microgravimetric method (indigo powder) | [55] | |
| Strobilanthes cusia | 28.70–43.50 (lab) 45.20 (56.70 6) (pilot) (in dry indigo) | n.d. (lab) n.d. (pilot) | 4.00–4.50 (lab) n.d. (pilot) (per fw  ) ) | Development of extraction method at laboratory and pilot scales. Test of effect of dissolved oxygen concentration on indigotin and indirubin formation; evaluation of indigotin and indirubin production under anaerobic conditions in nitrogen gas environment compared with conditions with and without aeration; ambient temperature, pH adjustment with NaOH. | HPLC equipped with UV detector (indigo powder/dimethyl sulfoxide, A: water-acetic acid, B: acetonitrile/280 nm) | [50] |
| n.d. | n.d. | 0.85 (1.16 7) (per fw ⌘) | Development of two-step extraction process (laboratory scale): separate extraction of leaves (conventional extraction, pH adjustment) and stems (microwave drying, ethanol extraction, use of exogenous enzymes, pH adjustment). | HPLC equipped with UV detector (centrifuged indigo/A: water–acetic acid, B: acetonitrile/280 nm) | [49] | |
| n.d. | 98.60–156.90 (paste per fw ⌘) | 0.08–4.72 (per fw ⌘) | Comparison of Strobilanthes cusia and I. tinctoria. Only with Strobilanthes cusia: test of extraction methods: different plant material conditions (fresh, semi-dried, dried), different soaking durations; extraction at ambient temperature, adding Ca(OH)2 solution, aerating, washing precipitated indigo with Ca(OH)2 solution (lab scale). | Spectrophotometry (indigo paste, centrifuged/H2SO4/611 nm) | [59] |
 = leaves, ⌘ = leaves and stems, n.d. = not determined, HPLC = high-performance liquid chromatography, HPTLC-densitometry = high-performance thin-layer chromatography–densitometry.
= leaves, ⌘ = leaves and stems, n.d. = not determined, HPLC = high-performance liquid chromatography, HPTLC-densitometry = high-performance thin-layer chromatography–densitometry.3.5. Colour Measurement as a Possible New Method to Determine Indigo Quality
4. Materials and Methods
4.1. Indigo Samples from Standardised Extraction
4.1.1. Plant Material for Extractions during Field Research in China
4.1.2. Plant Material of Species Cultivated in Vienna
4.1.3. Standardised Indigo Extraction Methods
MME (Mobile Mini Extraction)
sLPE (Simulated Local Practice Extraction)
4.2. Indigo Samples from Local Practices in China, Indonesia and India
4.3. Indigo Sample Preparation
4.4. Determination of Indigotin Content, Indigo Yield and Indigotin Yield
4.4.1. Spectrophotometric Analysis of Indigotin Content
4.4.2. Calculations
4.5. Determination of Indigo Sample Colour
4.5.1. Visual Classification of Colours
4.5.2. L*a*b* Colour Measurement
4.6. Statistical Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Terms/Context to Describe the Plant Extract | Terms/Context to Describe the Valuable Component for Dyeing |
|---|---|
| “crude dyestuff” [54] “crude indigo dye” [55] “crude indigo” [49,50,59,79] “indigo product” [32,78] “indigo paste” [77] “indigo powder” [79] “commercial indigo” [50] “indigo dye” [58,59,64] “indigo colour”, “indigo blue” [58] “dye”, “dye recovery” [77] “blue dye powder”, “blue colorant powder” [80] “woad powder” [82] “indigo” [25,30,51,63] | “indigo content” [49,54,55,64,65] “indigo percentage” [65] “indigo purity” [32,50] “pure indigo” [49,55,79] “indigo” [30,32,50,59,64,65,66,77,78,79,80,81] “colorant purity”, “dye purity” [80] “indigo dye concentration” [82] “indigotin” mentioned as a synonym for “indigo” [66,77,79,81] “indigotin” [25,51,63] |
| Species | Seed Source | Country of Origin, Collection Year | Accession Number/ Name of Provenance |
|---|---|---|---|
| Indigofera tinctoria | Millennium Seed Bank, Royal Botanic Gardens, Kew, UK | Burkina Faso, 1998 | 125549 |
| Millennium Seed Bank, Royal Botanic Gardens, Kew, UK | Maldives, 1986 | 68068 | |
| Indigofera suffruticosa | Millennium Seed Bank, Royal Botanic Gardens, Kew, UK | Brazil, 1983 | 47896 |
| Millennium Seed Bank, Royal Botanic Gardens, Kew, UK | Ecuador, 1980 | 17479 | |
| Millennium Seed Bank, Royal Botanic Gardens, Kew, UK | Venezuela, 1994 | 103897 | |
| Indigofera arrecta | Bonn University Botanic Gardens, Bonn, Germany | unknown | xx-0-BONN-19246 |
| Millennium Seed Bank, Royal Botanic Gardens, Kew, UK | Burkina Faso, 2007 | 444266 | |
| Millennium Seed Bank, Royal Botanic Gardens, Kew, UK | Mali, 2014 | 820888 | |
| Persicaria tinctoria | Bailiwick Blue, Guernsey, UK | unknown | “Maruba” |
| Bailiwick Blue, Guernsey, UK | unknown | “Senbon” | |
| Bailiwick Blue, Guernsey, UK | unknown | “Kojyoko” |
| Persicaria tinctoria | Indigofera tinctoria | Indigofera suffruticosa | Indigofera arrecta | |
|---|---|---|---|---|
| Cultivation in the greenhouse: | ||||
| Seed treatment | None | All Indigofera sp.: scarification to break seed dormancy | ||
| Sowing in seedling trays | 30/04 | 06/05 | 06/05 | 06/05 |
| Seeds per pot | 3 | 1 | 1 | 1 |
| Potting | 21/05 and 31/05 | 07/06 | 07/06 | 07/06 |
| Pruning | None | All Indigofera sp.: pruning of main shoot to enhance ramification and increase biomass yield, because only a few seeds were available per provenance | ||
| Watering | All species: as required | |||
| Treatment against Sciaridae | All species: yellow sticky traps and beneficial nematodes | |||
| Cultivation in the field: | ||||
| Transplanting | 17/06 | 28/06 | 28/06 | 22 + 28/06 |
| Spacing | 30 cm × 28 cm | 30 cm × 20 cm | 30 cm × 20 cm | 30 cm × 20 cm |
| Application of horn shavings | All species: 17/07, ca. 25 kg/63 m2 | |||
| Weeding | All species: 22/06, 05/07, 12/07, 20/07, 12/08 | |||
| Watering | All species: as required | |||
| Harvested plant material | Stems with leaves * | Stems with leaves * + flowers/green pods | Stems with leaves * + flowers | Stems with leaves * + inflorescences/flowers/green pods |
| Country | Species | Colour Coordinates | |||||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | |||||
| Austria | Indigofera arrecta | 11.37 | bc | 3.39 | d | −9.76 | gh |
| Indigofera suffruticosa | 18.69 | a | −0.94 | f | −7.49 | g | |
| Indigofera tinctoria | 13.06 | b | 1.57 | e | −8.91 | gh | |
| Persicaria tinctoria | 9.76 | c | 2.75 | d | −10.25 | h | |
| China | Indigofera suffruticosa | 14.09 | s | 0.71 | w | 5.89 | x |
| Indigofera tinctoria | 12.75 | st | 0.49 | w | −7.17 | xy | |
| Strobilanthes cusia | 9.28 | tu | 2.77 | v | −12.06 | z | |
| Wrightia laevis (tree 1) 1 | 8.49 | u | 2.07 | v | −12.96 | z | |
| Wrightia laevis (tree 2) 2 | 11.9 | stu | 0.06 | w | −10.83 | yz | |
| Species | Provenance | Colour Coordinates | |||||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | |||||
| Indigofera arrecta | Burkina Faso 1 | 11.27 | a | 3.57 | b | −10.89 | d |
| Mali | 10.72 | a | 3.36 | b | −9.66 | cd | |
| unknown | 12.12 | a | 3.23 | b | −8.72 | c | |
| Indigofera suffruticosa | Brazil | 21.39 | e | −1.22 | f | −4.89 | g |
| Ecuador | 17.93 | e | −0.56 | f | −7.99 | g | |
| Venezuela 1 | 16.76 | e | −1.06 | f | −9.58 | g | |
| Indigofera tinctoria | Burkina Faso 2 1 | 13.56 | h | 1.15 | j | −8.00 | k |
| Maldives | 12.56 | h | 2.00 | i | −9.82 | k | |
| Persicaria tinctoria | “Kojyoko” | 9.20 | m | 2.80 | n | −10.26 | o |
| “Maruba” | 9.00 | m | 3.17 | n | −9.97 | o | |
| “Senbon” | 11.07 | l | 2.29 | n | −10.52 | o | |
References
- Splitstoser, J.C.; Dillehay, T.D.; Wouters, J.; Claro, A. Early Pre-Hispanic Use of Indigo Blue in Peru. Sci. Adv. 2016, 2, e1501623. [Google Scholar] [CrossRef] [PubMed]
- Balfour-Paul, J. Indigo—Egyptian Mummies to Blue Jeans; The British Museum Press: London, UK, 2011. [Google Scholar]
- Hofmann-de Keijzer, R.; van Bommel, M.R.; Joosten, I.; Hartl, A.; Proaño Gaibor, A.N.; Heiss, A.G.; Kralofsky, R.; Erlach, R.; de Groot, S. Die Farben und Färbetechniken der prähistorischen Textilien aus dem Salzbergbau Hallstatt/The Colours and Dyeing Techniques of Prehistoric Textiles from the Salt Mines of Hallstatt. In Textiles from Hallstatt—Weaving Culture from Bronze and Iron Age Salt Mines/Textilien aus Hallstatt—Gewebte Kultur aus Dem Bronze- und Eisenzeitlichen Salzbergwerk; Grömer, K., Kern, A., Reschreiter, H., Rösel-Mautendorfer, H., Meid, W., Eds.; Archaeolingua; Archaeolingua Alapitvány: Budapest, Hungary, 2013; Volume 29, pp. 135–162. ISBN 978-963-9911-46-8. HU-ISSN 1215-9239. [Google Scholar]
- Kramell, A.; Li, X.; Csuk, R.; Wagner, M.; Goslar, T.; Tarasov, P.E.; Kreusel, N.; Kluge, R.; Wunderlich, C.-H. Dyes of Late Bronze Age Textile Clothes and Accessories from the Yanghai Archaeological Site, Turfan, China: Determination of the Fibers, Color Analysis and Dating. Quat. Int. 2014, 348, 214–223. [Google Scholar] [CrossRef]
- Zhang, X.; Good, I.; Laursen, R. Characterization of Dyestuffs in Ancient Textiles from Xinjiang. J. Archaeol. Sci. 2008, 35, 1095–1103. [Google Scholar] [CrossRef]
- Kumar, P. Planters and Naturalists: Transnational Knowledge on Colonial Indigo Plantations in South Asia. Mod. Asian Stud. 2014, 48, 720–753. [Google Scholar] [CrossRef]
- Darrac, P.-P.; van Schendel, W. Global Blue: Indigo and Espionage in Colonial Bengal; The University Press Limited: Dhaka, Bangladesh, 2006. [Google Scholar]
- Kumar, P. Plantation Science: Improving Natural Indigo in Colonial India, 1860–1913. Br. J. Hist. Sci. 2007, 40, 537–565. [Google Scholar] [CrossRef]
- Hu, R.; Li, T.; Qin, Y.; Liu, Y.; Huang, Y. Ethnobotanical Study on Plans Used to Dye Traditional Costumes by the Baiku Yao Nationality of China. J. Ethnobiol. Ethnomed. 2022, 18, 2. [Google Scholar] [CrossRef]
- Li, S.; Cunningham, A.B.; Fan, R.; Wang, Y. Identity Blues: The Ethnobotany of the Indigo Dyeing by Landian Yao (Iu Mien) in Yunnan, Southwest China. J. Ethnobiol. Ethnomed. 2019, 15, 13. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ahmed, S.; Liu, B.; Guo, Z.; Huang, W.; Wu, X.; Li, S.; Zhou, J.; Lei, Q.; Long, C. Ethnobotany of Dye Plants in Dong Communities of China. J. Ethnobiol. Ethnomed. 2014, 10, 23. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Cunningham, A.B.; Shi, Y.; Wang, Y. Island Blues: Indigenous Knowledge of Indigo-Yielding Plant Species Used by Hainan Miao and Li Dyers on Hainan Island, China. J. Ethnobiol. Ethnomed. 2019, 15, 31. [Google Scholar] [CrossRef]
- Teron, R.; Borthakur, S.K. Traditional Knowledge of Herbal Dyes and Cultural Significance of Colors among the Karbis Ethnic Tribe in Northeast India. Ethnobot. Res. Appl. 2012, 10, 593–603. [Google Scholar]
- Sutradhar, B.; Deb, D.; Majumdar, K.; Datta, B.K. Short Communication: Traditional Dye Yielding Plants of Tripura, Northeast India. Biodiversitas 2015, 16, 121–127. [Google Scholar] [CrossRef]
- Alawa, K.S.; Ray, S.; Dubey, A. Dye Yielding Plants Used by Tribals of Dhar District, Madhya Pradesh, India. Sci. Res. Rep. 2013, 3, 30–32. [Google Scholar]
- Rashid, A. Dye Yielding Plant Diversity of District Rajouri, Jammu and Kashmir State, India. Int. J. Pharma Bio Sci. 2013, 4, 263–266. [Google Scholar]
- Singh, N.R.; Yaiphaba, N.; David, T.; Babita, R.K.; Bino Devi, C.; Rajmuhon Singh, N. Traditional Knowledge and Natural Dyeing System of Manipur—With Special Reference to Kum Dye. Indian J. Tradit. Knowl. 2009, 8, 84–88. [Google Scholar]
- Ningombam, D.S.; Ningthoujam, S.S.; Singh, P.K.; Singh, O.B. Legacy of Kum Dye: A Case Study with the Meitei Community in Manipur, Northeast India. Ethnobot. Res. Appl. 2012, 10, 561–570. [Google Scholar]
- Kar, A.; Borthakur, S.K. Dye Yielding Plants of Assam for Dyeing Handloom Textile Products. Indian J. Tradit. Knowl. 2008, 7, 166–171. [Google Scholar]
- Cunningham, A.B.; Kadati, W.D.; Ximenes, J.; Howe, J.; Maduarta, I.M.; Ingram, W. Plants as the Pivot: The Ethnobotany of Timorese Textiles. In Textiles of Timor, Island in the Woven Sea; Hamilton, R.W., Barrkman, J., Eds.; Fowler Museum at UCLA: Los Angeles, CA, USA, 2014; pp. 88–103. [Google Scholar]
- Cunningham, A.B.; Ingram, W.; Kadati, W.; Maduarta, I.M. Opportunities, Barriers and Support Needs: Microenterprise and Small Enterprise Development Based on Non-Timber Products in Eastern Indonesia. Aust. For. 2017, 80, 161–177. [Google Scholar] [CrossRef]
- UNESCO; CCI (Eds.) International Symposium/Workshop on Natural Dyes/Atelier-Symposium International Sur Les Teintures Naturelles/Simposio-Taller Internacional Sobre Tintes Naturales, Hyderabad, India, 5–12 November 2006; Interventions (Full Texts): Hyderabad, India, 2007. [Google Scholar]
- ISEND Committee. International Symposium and Exhibition on Natural Dyes (ISEND). From Tradition to Innovation; UNESCO, Ministry of Culture Sports and Tourism: Daegu Metropolitan City, Republic of Korea, 2008.
- Cardon, D.; de la Sayette, A. (Eds.) International Symposium and Exhibition on Natural Dyes. In Proceedings of the International Society for Horticultural Science, Angers, France, 14–20 August 2020. [Google Scholar]
- Cardon, D. Natural Dyes—Sources, Tradition, Technology and Science; Archetype Publications: London, UK, 2007. [Google Scholar]
- Yu, H.; Li, T.; Ran, Q.; Huang, Q.; Wang, J. Strobilanthes cusia (Nees) Kuntze, a Multifunctional Traditional Chinese Medicinal Plant, and its Herbal Medicines: A Comprehensive Review. J. Ethnopharmacol. 2021, 265, 113325. [Google Scholar] [CrossRef]
- Meijer, L.; Guyard, N.; Skaltsounis, L.; Eisenbrand, G. (Eds.) Indirubin, the Red Shade of Indigo; Life in Progress Editions: Roscoff, France, 2006. [Google Scholar]
- Sun, Q.; Leng, J.; Tang, L.; Wang, L.; Fu, C. A Comprehensive Review of the Chemistry, Pharmacokinetics, Pharmacology, Clinical Applications, Adverse Events, and Quality Control of Indigo Naturalis. Front. Pharmacol. 2021, 12, 664022. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, T.; He, Y.; Huang, S.; Deng, X.; Han, L.; Xie, C. From Natural Dye to Herbal Medicine: A Systematic Review of Chemical Constituents, Pharmacological Effects and Clinical Applications of Indigo Naturalis. Chin. Med. 2020, 15, 127. [Google Scholar]
- Schweppe, H. Handbuch der Naturfarbstoffe—Vorkommen, Verwendung, Nachweis; Nikol Verlagsgesellschaft: Hamburg, Germany, 1993. [Google Scholar]
- Schweppe, H. Indigo and Woad. In Artists’ Pigments. A Handbook of Their History and Characteristics; West Fitzhugh, E., Ed.; Oxford University Press: New York, NY, USA; Oxford, UK, 1997; Volume 3, pp. 81–107. [Google Scholar]
- Garcia-Macias, P.; John, P. Formation of Natural Indigo Derived from Woad (Isatis tinctoria L.) in Relation to Product Purity. J. Agric. Food Chem. 2004, 2004, 7891–7896. [Google Scholar] [CrossRef] [PubMed]
- John, P. Indigo—Extraction. In Handbook of Natural Colorants; Bechtold, T., Mussak, R., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2009; pp. 105–133. [Google Scholar]
- Comlekcioglu, N.; Efe, L.; Karaman, S. Extraction of Indigo from Some Isatis Species and Dyeing Standardization Using Low-Technology Methods. Braz. Arch. Biol. Technol. 2015, 58, 96–102. [Google Scholar] [CrossRef]
- Karaman, Ş.; Diraz, E.; Çömlekçioğlu, N.; İçim, A.; Durdu, H.; Tansi, S. High Yielding Indigo Sources in Native Isatis (Brassicaceae) Taxa from Turkey. Genet. Resour. Crop Evol. 2016, 63, 531–543. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, H.; Long, W.; Lei, Y.; Cai, Q.; Lan, M.; Zhong, L. Survey on Agricultural Biological Resources and Traditional Cultural Knowledge of Hani People in Yunnan. Asian Agric. Res. 2015, 7, 60–65. [Google Scholar]
- Mahanta, D.; Tiwari, S.C. Natural Dye-Yielding Plants and Indigenous Knowledge on Dye Preparation in Arunachal Pradesh, Northeast India. Curr. Sci. 2005, 88, 1474–1480. [Google Scholar]
- Tiwari, S.C.; Bharat, A. Natural Dye-Yielding Plants and Indigenous Knowledge of Dye Preparation in Achanakmar-Amarkantak Biosphere Reserve, Central India. Nat. Prod. Radiance 2008, 7, 82–87. [Google Scholar]
- Joseph, H. Les Couleures de la Guadeloupe: Renouveau, Innovation, Production. Presented at the Forum International de la Couleur Vegetale, Lauris, France, 10–11 October 2015.
- WFO World Flora Online. Published on the Internet. Available online: https://www.worldfloraonline.org (accessed on 10 November 2022).
- John, P.; Angelini, L.G. Indigo—Agricultural Aspects. In Handbook of Natural Colorants; Bechtold, T., Musiak, R., Eds.; John Wiley and Sons Ltd.: Chichester, UK, 2009; pp. 75–104. [Google Scholar]
- Ricketts, R. Polygonum tinctorium: Contemporary Indigo Farming and Processing in Japan. In Indirubin, the Red Shade of Indigo; Meijer, L., Guyard, N., Skaltsounis, L., Eisenbrand, G., Eds.; Life in Progress Editions: Roscoff, France, 2006; pp. 7–22. [Google Scholar]
- Klingelfuss-Schneider, U. Processing of Indigo Plants and Dyeing with Indigo in Japan: Impressions of a Visit in Autumn 2000. In Dyes in History and Archaeology 20; Kirby, J., Ed.; Archetype Publications: London, UK, 2005; pp. 121–126. [Google Scholar]
- Akimpou, G.; Rongmei, K.; Yadava, P.S. Traditional Dye Yielding Plants of Manipur, North East India. Indian J. Tradit. Knowl. 2005, 4, 33–38. [Google Scholar]
- Potsangbam, L.; Ningombam, S.; Laitonjam, W.S. Natural Dye Yielding Plants and Indigenous Knowledge of Dyeing in Manipur, Northeast India. Indian J. Tradit. Knowl. 2008, 7, 141–147. [Google Scholar]
- Medhi, P.; Borthakur, S.K. Phytoresources from North Cachar Hills of Assam, India-VII: Semi-Domesticated and Protected Plants. Pleione 2012, 6, 66–79. [Google Scholar]
- Fu, Y.; Chen, J.; Guo, H.; Chen, A.; Cui, J. Utilisation and Conservation Strategies for Plant Resources in Tropical Montane Agroecosystems: A Case Study from Xishuangbanna, SW China. Int. J. Biodivers. Sci. Manag. 2008, 4, 32–43. [Google Scholar] [CrossRef]
- Fan, Y.; Zhao, Y.; Liu, A.; Hamilton, A.; Wang, C.; Li, L.; Yang, Y.; Yang, L. Indigenous Knowledge of Dye-Yielding Plants among Bai Communities in Dali, Northwest Yunnan, China. J. Ethnobiol. Ethnomed. 2018, 14, 74. [Google Scholar] [CrossRef]
- Jiang, Y.; Xiao, Y.; Wang, Z.; Yu, H.; Hu, K.; Zhang, T.-A.; Hu, J.; Gao, M.-T. A Two-Step Process for Indigo Production from Baphicacanthus cusia Stem. J. Clean. Prod. 2022, 374, 133935. [Google Scholar] [CrossRef]
- Jin, Z.; Hu, K.; Yang, F.; Nawaz, M.; Wei, H.; Jiang, Y.; Xiao, Y.; Li, J.; Hu, J.; Gao, M.-T. Integrated Process for the Production of Indigo and Indirubin in an Anaerobic Environment at Laboratory and Pilot Level. J. Clean. Prod. 2022, 364, 132610. [Google Scholar] [CrossRef]
- Perkin, A.G.; Everest, A.E. The Natural Organic Colouring Matters; Monographs on industrial chemistry; Longmans, Green and Co.: London, UK, 1918. [Google Scholar]
- von Georgievics, G. Der Indigo vom Praktischen und Theoretischen Standpunkt Dargestellt; Franz Deuticke: Leipzig, Germany; Vienna, Austria, 1892. [Google Scholar]
- Shi, Y.; Zhang, L.; Wang, L.; Li, S.; Qiu, Z.; Ding, X.; Wang, Y. Quality Blues: Traditional Knowledge Used for Natural Indigo Identification in Southern China. J. Ethnobiol. Ethnomed. 2021, 17, 25. [Google Scholar] [CrossRef] [PubMed]
- Bechtold, T.; Turcanu, A.; Geissler, S.; Ganglberger, E. Process Balance and Product Quality in the Production of Natural Indigo from Polygonum tinctorium Ait. Applying Low-Technology Methods. Bioresour. Technol. 2002, 81, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Angelini, L.G.; John, P.; Tozzi, S.; Vandenburg, H. Extraction of Indigo from Isatis tinctoria L. and Polygonum tinctorium Ait. as a Basis for Large-Scale Production. In Proceedings of the 2005 Annual Meeting of the Association for the Advancement of Industrial Crops: International Conference on Industrial Crops and Rural Development, Murcia, Spain, 17–21 September 2005; Pascual-Villalobos, M.J., Nakayama, F.S., Bailey, C.A., Correal, E., Schloman, W.W., Jr., Eds.; IMIDA (Istituto Marciano de Investigation y Desarollo Agrario y Alimentario) Selegrafica, S.L. Murcia: Murcia, Spain, 2005; pp. 521–534. [Google Scholar]
- Campeol, E.; Angelini, L.G.; Tozzi, S.; Bertolacci, M. Seasonal Variation of Indigo Precursors in Isatis tinctoria L. and Polygonum tinctorium Ait. as Affected by Water Deficit. Environ. Exp. Bot. 2006, 58, 223–233. [Google Scholar] [CrossRef]
- Gilbert, K.G.; Maule, H.G.; Rudolph, B.; Lewis, M.; Vandenburg, H.; Sales, E.; Tozzi, S.; Cooke, D.T. Quantitative Analysis of Indigo and Indigo Precursors in Leaves of Isatis Spp. and Polygonum tinctorium. Biotechnol. Prog. 2004, 20, 1289–1292. [Google Scholar] [CrossRef] [PubMed]
- Teanglum, A.; Teanglum, S.; Saithong, A. Selection of Indigo Plant Varieties and Other Plants That Yield Indigo Dye. Procedia Eng. 2012, 32, 184–190. [Google Scholar] [CrossRef]
- Chanayath, N.; Lhieochaiphant, S.; Phutrakul, S. Pigment Extraction Techniques from the Leaves of Indigofera tinctoria Linn. and Baphicacanthus cusia Brem. and Chemical Structure Analysis of Their Major Components. Chiang Mai Univ. J. 2002, 1, 149–160. [Google Scholar]
- Hartl, A.; Proaño Gaibor, A.N.; van Bommel, M.R.; Hofmann-de Keijzer, R. Searching for Blue: Experiments with Woad Fermentation Vats and an Explanation of the Colours through Dye Analysis. J. Archaeol. Sci. Rep. 2015, 2, 9–39. [Google Scholar] [CrossRef]
- Food and Feed Information Portal Database Version 1.0. Available online: https://ec.europa.eu/food/food-feed-portal/screen/food-additives/search/details/POL-FAD-IMPORT-2997 (accessed on 11 May 2023).
- PubChem Database. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2723854 (accessed on 11 May 2023).
- Hofenk de Graaff, J. The Colourful Past—Origins, Chemistry and Identification of Natural Dyestuffs; ABEGG-Stiftung and Archetype Publications: Riggisberg, Switzerland; London, UK, 2004. [Google Scholar]
- Rastegari, F.; Tohidinejad, E.; Mohammadi-Nejad, G. Effect of Drought Stress on Indigo and Seed Yield of Indigofera tinctoria Ecotypes. Indian J. Agric. Sci. 2020, 90, 1063–1067. [Google Scholar] [CrossRef]
- Rastegari, F.; Tohidinejad, E.; Mohammadi-Nejad, G. Evaluation of Growth and Yield of Indigo Ecotypes in Different Sowing Dates and Genetic Diversity Analysis Using AFLP Markers. Agron. J. 2022, 114, 1571–1580. [Google Scholar] [CrossRef]
- Angelini, L.G.; Tozzi, S.; Nassi o di Nasso, N. Differences in Leaf Yield and Indigo Precursors Production in Woad (Isatis tinctoria L.) and Chinese Woad (Isatis indigotica Fort.) Genotypes. Field Crops Res. 2007, 101, 285–295. [Google Scholar] [CrossRef]
- Spataro, G.; Negri, V. Adaptability and Variation in Isatis tinctoria L.: A New Crop for Europe. Euphytica 2008, 163, 89–102. [Google Scholar] [CrossRef]
- Hartl, A.; Vogl, C.R. The Potential Use of Organically Grown Dye Plants in the Organic Textile Industry: Experiences and Results on Cultivation and Yields of Dyer’s Chamomile (Anthemis tinctoria L.), Dyer’s Knotweed (Polygonum tinctorium Ait.), and Weld (Reseda luteola L.). J. Sustain. Agric. 2003, 23, 17–40. [Google Scholar] [CrossRef]
- Angelini, L.G.; Tozzi, S.; Nassi o di Nasso, N. Environmental Factors Affecting Productivity, Indican Content, and Indigo Yield in Polygonum tinctorium Ait., a Subtropical Crop Grown under Temperate Conditions. J. Agric. Food Chem. 2004, 52, 7541–7547. [Google Scholar] [CrossRef] [PubMed]
- Schrire, B.D.; Lavin, M.; Barker, N.P.; Forest, F. Phylogeny of the Tribe Indigofereae (Leguminosae-Papilionoideae): Geographically Structured More in Succulent-Rich and Temperate Settings than in Grass-Rich Environments. Am. J. Bot. 2009, 96, 816–852. [Google Scholar] [CrossRef] [PubMed]
- Royal Botanic Gardens Kew: Plants of the World Online: Indigofera arrecta Hochst. Ex A.Rich. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:498996-1 (accessed on 2 October 2023).
- Flora of China; Li, A.; Bao, B.; Grabovskaya-Borodina, A.E.; Hong, S.; McNeill, J.; Mosyakin, S.L.; Ohba, H.; Park, C. eFloras, Flora of China (Vol. 5, Polygonaceae); Missouri Botanical Garden: St. Louis, MO, USA; Harvard University Herbaria: Cambridge, MA, USA, 2022; Available online: http://www.efloras.org (accessed on 28 October 2022).
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger Climate Classification Updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Duke, J.A. Handbook of Legumes of World Economic Importance; Plenum Press: New York, NY, USA; London, UK, 1981. [Google Scholar]
- Thüringer Landesanstalt für Landwirtschaft. Anbautelegramm Färberknöterich (Polygonum tinctorium Ait.); Thüringer Ministerium für Landwirtschaft, Naturschutz und Umwelt: Jena, Germany, 2009. [Google Scholar]
- Theisen, H. Herstellung eines Batak-Tuches (ulos sibolang sitolun tuho). In Archiv für Völkerkunde; Selbstverlag: Vienna, Austria, 1982; pp. 59–94. [Google Scholar]
- Dhanraj, D.; Kaliaperumal, S. Comparative Evaluation of Various Extraction Methods on Quantity and Quality of Indigo Dye from Indigofera tinctoria L. J. Trop. Agric. 2021, 59, 162–170. [Google Scholar]
- Stoker, K.G.; Cooke, D.T.; Hill, D.J. An Improved Method for the Large-Scale Processing of Woad (Isatis tinctoria) for Possible Commercial Productions of Woad Indigo. J. Agric. Eng. Res. 1998, 71, 315–320. [Google Scholar] [CrossRef]
- Rahayuningsih, E.; Fatimah, W.S.; Sapto Pamungkas, M.; Marfitania, T. Effect of Physicochemical Process Variables on Natural Indigo Dye Production from Strobilanthes cusia Leaves by Response Surface Methodology. Indones. J. Chem. 2022, 22, 342–351. [Google Scholar] [CrossRef]
- Sandoval-Salas, F.; Gschaedler-Mathis, A.; Vilarem, G.; Méndez-Carreto, C. Effect of Harvest Time on Dye Production in Indigofera suffruticosa MILL. Agrociencia 2006, 40, 585–591. [Google Scholar]
- Sales, E.; Kanhonou, R.; Baixauli, C.; Giner, A.; Cooke, D.; Gilbert, K.; Arrillaga, I.; Segura, J.; Ros, R. Sowing Date, Transplanting, Plant Density and Nitrogen Fertilization Affect Indigo Production from Isatis Species in a Mediterranean Region of Spain. Ind. Crops Prod. 2006, 23, 29–39. [Google Scholar] [CrossRef]
- Orsini, R.; Aquilanti, L.; Osimani, A.; Serrani, L.; Baldini, G.; Seddaiu, G.; De Sanctis, G.; Santilocchi, R. Isatis tinctoria L.: Biomass Production and Indigo Dye Yield as Influenced by Mineral or Organic Nitrogen Fertilization. Agrochimica 2012, LVI, 292–307. [Google Scholar]
- Garcia, M.; (Plantes et Couleurs, Lauris, France). Personal communication, 1 October 2015.
- Hartl, A. “Wiener Blau”—Naturindigo aus Wien. Endbericht zum Forschungsprojekt; Unpublished Final Report of the Research Project “Viennese Blue—Natural Indigo from Vienna” to the Funding Organisation Vienna Anniversary Foundation for Higher Education: Vienna, Austria, 2017. [Google Scholar]
- Hartl, A. Natural Indigo from Strobilanthes cusia (Nees) Kuntze—Extraction Experiments and Indigo Quality Analysis. Final Report (Updated Version); Unpublished. Short-Term Research Support to the Project “Developing and Promoting Market-Based Agroforestry Options and Integrated Landscape Management for Smallholder Forestry in Indonesia” (FST/2016/141), funded by ACIAR (Australian Centre for International Agricultural Research): Vienna, Austria, 2018. [Google Scholar]
- Bechtold, T.; Burtscher, E.; Bobleter, O. Konzentrationsbestimmung von Indigo in Ansatz- und Prozessbädern. Text. Prax. Int. 1992, 47, 44–49. [Google Scholar]
- Schanda, J. (Ed.) Colorimetry: Understanding the CIE System; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 21 March 2023).
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 21 March 2023).
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix (Version 0.92). Available online: https://github.com/taiyun/corrplot (accessed on 22 April 2021).
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.7. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 22 April 2021).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]







| Species | Parameter | Extraction Methods | Statistical Significance | ||
|---|---|---|---|---|---|
| MME | sLPE | ||||
| Persicaria tinctoria (“Senbon”, “Maruba” and “Kojyoko”) | Indigotin in indigo (%) | 43.84 | 4.50 | *** | |
| Indigo extraction yield (g/kg fresh weight) | 1.34 | 15.33 | *** | ||
| Indigotin extraction yield (g/kg fresh weight) | 0.61 | 0.69 | * | ||
| CIELAB colour coordinates: | L* | 9.76 | 32.71 | *** | |
| a* | 2.75 | −4.14 | *** | ||
| b* | −10.25 | −11.97 | *** | ||
| Indigofera arrecta (Burkina Faso and unknown provenance) | Indigotin in indigo (%) | 53.48 | 6.23 | *** | |
| Indigo extraction yield (g/kg fresh weight) | 1.38 | 18.54 | *** | ||
| Indigotin extraction yield (g/kg fresh weight) | 0.75 | 1.17 | n.s. | ||
| CIELAB colour coordinates: | L* | 11.69 | 32.78 | *** | |
| a* | 3.40 | −6.27 | *** | ||
| b* | −9.81 | 1.23 | * | ||
| Indigofera arrecta (unknown provenance) | MME | mod.MME | |||
| Indigotin in indigo (%) | 58.13 | 58.03 | n.s. | ||
| Indigo extraction yield (g/kg fresh weight) | 1.44 | 2.17 | *** | ||
| Indigotin extraction yield (g/kg fresh weight) | 0.84 | 1.25 | *** | ||
| CIELAB colour coordinates: | L* | 12.12 | 10.15 | *** | |
| a* | 3.23 | 2.44 | *** | ||
| b* | −8.72 | −8.52 | n.s. | ||
| sLPE | mod.sLPE | ||||
| Indigotin in indigo (%) | 8.26 | 6.37 | *** | ||
| Indigo extraction yield (g/kg fresh weight) | 19.28 | 22.59 | *** | ||
| Indigotin extraction yield (g/kg fresh weight) | 1.59 | 1.44 | n.s. | ||
| CIELAB colour coordinates: | L* | 25.13 | 26.24 | ** | |
| a* | −5.94 | −3.80 | *** | ||
| b* | −3.87 | −9.42 | ** | ||
 |
| Estimate | Std. Error | t Value | Pr(>|t|) | ||
|---|---|---|---|---|---|
| (Intercept) | 50.8313 | 13.0766 | 3.887 | 0.00026 | *** |
| L* | −2.1510 | 0.7518 | −2.861 | 0.00580 | ** |
| a* | 9.7976 | 2.3863 | 4.106 | 0.00012 | *** |
| b* | 0.4692 | 1.1977 | 0.392 | 0.69661 | |
| L*:a* | −0.4253 | 0.1164 | −3.654 | 0.00054 | *** |
| L*:b* | −0.0398 | 0.0691 | −0.577 | 0.56576 | |
| a*:b* | −0.2023 | 0.2308 | −0.877 | 0.38418 | |
| L*:a*:b* | 0.0044 | 0.0114 | 0.391 | 0.69728 |
| Species, Provenances (of Seeds 1, Harvested Plant Material or Indigo Samples) and Code for Extraction Methods (Number of Samples in Brackets) | ||||||
|---|---|---|---|---|---|---|
| Indigofera tinctoria | Indigofera suffruticosa | Indigofera arrecta | Persicaria tinctoria | Strobilanthes cusia | Wrightia laevis | |
| Standardised extraction in Austria (own cultivation in Vienna) | Burkina Faso: MME (3) 2 Maldives: MME (3) | Venezuela: MME (3) 2 Ecuador: MME (3) Brazil: MME (3) | Mali: MME (3) Burkina Faso: MME (3) + sLPE (3) unknown provenance: MME (3) + mod.MME (3) 2 sLPE (3) + mod.sLPE (3) 2 | “Senbon”: MME (3) + sLPE (3) “Kojyoko”: MME (3) + sLPE (3) “Maruba”: MME (3) + sLPE (3) | - | - |
| Standardised extraction in China (local practice cultivation in three villages in Hainan) | Yu Long: MME (3) | Yu Dao: MME (3) | - | - | Xin Cun: MME (3) | Xin Cun: Tree 1: MME (3) 3 Tree 2: MME (3) 4 |
| Total samples | 9 | 12 | 21 | 18 | 3 | 6 |
| Local practice extraction in China (three villages in Hainan) | Yu Dao: extraction with lime, LPE (1) Yu Long: extraction with lime, LPE (1) + ADD (4) | - | - | - | Xin Cun: extraction with lime, LPE (1) + ADD (2) | Xin Cun: extraction with lime, LPE (1) |
| Local practice extraction in India (six villages in Uttarakhand) | Simalta: 1st extraction without lime + 2nd extraction with lime, ADD (2) Digoli: 1st extraction without lime + 2nd extraction with lime, ADD (2) Thanga: 1st extraction without lime + 2nd extraction with lime, ADD (2) Chankana: 1st extraction without lime + 2nd extraction with lime, ADD (2) | - | - | - | Simalta: extraction without lime, ADD (1) Tripuradevi: extraction without lime, ADD (1) Dharamghar: 1st extraction without lime + 2nd extraction with lime, ADD (2) | - |
| Local practice extraction in Indonesia (two locations in Java and Bali) | - | - | - | - | Temangung/Java: extraction with lime, ADD (1) Ubud/Bali: extraction without lime, ADD (2) | - |
| Total samples | 14 | 0 | 0 | 0 | 10 | 1 |
| TOTAL SAMPLES (standardised + local practice) | 23 | 15 | 21 | 18 | 13 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hartl, A.; Polleichtner, A.; Novak, J. “Purplish Blue” or “Greenish Grey”? Indigo Qualities and Extraction Yields from Six Species. Plants 2024, 13, 918. https://doi.org/10.3390/plants13070918
Hartl A, Polleichtner A, Novak J. “Purplish Blue” or “Greenish Grey”? Indigo Qualities and Extraction Yields from Six Species. Plants. 2024; 13(7):918. https://doi.org/10.3390/plants13070918
Chicago/Turabian StyleHartl, Anna, Andrea Polleichtner, and Johannes Novak. 2024. "“Purplish Blue” or “Greenish Grey”? Indigo Qualities and Extraction Yields from Six Species" Plants 13, no. 7: 918. https://doi.org/10.3390/plants13070918
APA StyleHartl, A., Polleichtner, A., & Novak, J. (2024). “Purplish Blue” or “Greenish Grey”? Indigo Qualities and Extraction Yields from Six Species. Plants, 13(7), 918. https://doi.org/10.3390/plants13070918