Unravelling the Mechanisms of Heavy Metal Tolerance: Enhancement in Hydrophilic Antioxidants and Major Antioxidant Enzymes Is Not Crucial for Long-Term Adaptation to Copper in Chlamydomonas reinhardtii
Abstract
:1. Introduction
2. Results
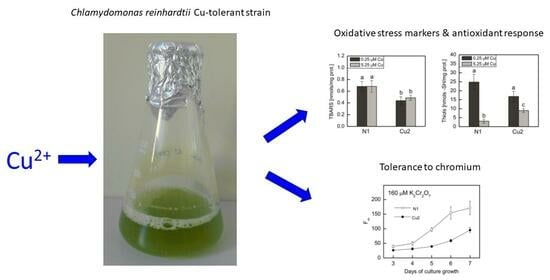
2.1. The Analysis of Oxidative Stress Markers, Photosynthetic Pigments Ratios, and Antioxidant Response in Cu-Tolerant and Non-Adapted Strain
2.2. Tolerance to Chromium in Cu-Tolerant and Non-Adapted Strain
3. Discussion
4. Materials and Methods
4.1. Algal Strains
4.2. Growth Conditions
4.3. Sample Collection, Measurement of Photosynthetic Pigments, and Cells Lysis Protocol
4.4. TBARS Measurements
4.5. The Assessment of Low-Molecular-Weight Antioxidants
4.6. The Assessment of Antioxidant Enzymes Activity
4.7. Semi-Quantitative Analysis of Cumulative Superoxide Production
4.8. The Assessment of Chromium Tolerance
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Chekroun, K.B.; Baghour, M. The role of algae in phytoremediation of heavy metals: A review. J. Mater. Environ. Sci. 2013, 4, 873–880. [Google Scholar]
- Danouche, M.; El Ghachtouli, N.; El Arroussi, H. Phycoremediation mechanisms of heavy metals using living green microalgae: Physicochemical and molecular approaches for enhancing selectivity and removal capacity. Heliyon 2021, 7, e07609. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R.K.; Agrawal, K.; Shah, M.P.; Verma, P. Bioremediation of heavy metals from wastewater: A current perspective on microalgae-based future. Lett. Appl. Microbiol. 2021, 75, 701–717. [Google Scholar] [CrossRef]
- Pluciński, B.; Waloszek, A.; Rutkowska, J.; Strzałka, K. Copper excess-induced large reversible and small irreversible adaptations in a population of Chlamydomonas reinhardtii CW15 (Chlorophyta). Acta Soc. Bot. Pol. 2018, 87, 3569. [Google Scholar] [CrossRef]
- Pluciński, B.; Waloszek, A.; Rutkowska, J.; Strzałka, K. Intrapopulation diversity of Chlamydomonas reinhardtii response to copper ions: Growth and photosynthetic performance under stress. Acta Soc. Bot. Pol. 2021, 90, 908. [Google Scholar] [CrossRef]
- Pluciński, B.; Nowicka, B.; Waloszek, A.; Rutkowska, J.; Strzałka, K. The role of antioxidant response and nonphotochemical quenching of chlorophyll fluorescence in long-term adaptation to Cu-induced stress in Chlamydomonas reinhardtii. Environ. Sci. Pollut. Res. 2023, 30, 67250–67262. [Google Scholar] [CrossRef]
- Nowicka, B. Heavy metal–induced stress in eukaryotic algae—Mechanisms of heavy metal toxicity and tolerance with particular emphasis on oxidative stress in exposed cells and the role of antioxidant response. Environ. Sci. Pollut. Res. 2022, 29, 16860–16911. [Google Scholar] [CrossRef] [PubMed]
- Burda, K.; Kruk, J.; Schmid, G.H.; Strzalka, K. Inhibition of oxygen evolution in Photosystem II by Cu(II) ions is associated with oxidation of cytochrome b559. Biochem. J. 2003, 371, 597–601. [Google Scholar] [CrossRef]
- Yruela, I. Copper in plants. Brazilian J. Plant Physiol. 2005, 17, 145–156. [Google Scholar] [CrossRef]
- DalCorso, G. Heavy metal toxicity in plants. In Plants and Heavy Metals; Furini, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–25. [Google Scholar]
- Küpper, H.; Andresen, E. Mechanisms of metal toxicity in plants. Metallomics 2016, 8, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Stoiber, T.L.; Shafer, M.M.; Armstrong, D.E. Induction of reactive oxygen species in Chlamydomonas reinhardtii in response to contrasting trace metal exposures. Environ. Toxicol. 2013, 28, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.; Sigaud-Kutner, T.C.S.; Leitão, M.A.S.; Okamoto, O.K.; Morse, D.; Colepicolo, P. Heavy metal-induced oxidative stress in algae. J. Phycol. 2003, 39, 1008–1018. [Google Scholar] [CrossRef]
- Luis, P.; Behnke, K.; Toepel, J.; Wilhelm, C. Parallel analysis of transcript levels and physiological key parameters allows the identification of stress phase gene markers in Chlamydomonas reinhardtii under copper excess. Plant Cell Environ. 2006, 29, 2043–2054. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Meng, Q.; Wei, Y.Y.; Yang, Z.M. Alleviation of copper-induced oxidative damage in Chlamydomonas reinhardtii by carbon monoxide. Arch. Environ. Contam. Toxicol. 2011, 61, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhu, Y.; Hu, Z.; Lei, A.; Wang, J. Towards elucidation of the toxic mechanism of copper on the model green alga Chlamydomonas reinhardtii. Ecotoxicology 2016, 25, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Hamed, S.M.; Selim, S.; Klöck, G.; AbdElgawad, H. Sensitivity of two green microalgae to copper stress: Growth, oxidative and antioxidants analyses. Ecotoxicol. Environ. Saf. 2017, 144, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Mallick, N. Copper-induced oxidative stress in the chlorophycean microalga Chlorella vulgaris: Response of the antioxidant system. J. Plant Physiol. 2004, 161, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.K.; Gaur, J.P. Heavy metal-induced proline accumulation and its role in ameliorating metal toxicity in Chlorella vulgaris. New Phytol. 1999, 143, 253–259. [Google Scholar] [CrossRef]
- Qian, H.; Li, J.; Pan, X.; Sun, L.; Lu, T.; Ran, H.; Fu, Z. Combined effect of copper and cadmium on heavy metal ion bioaccumulation and antioxidant enzymes induction in Chlorella vulgaris. Bull. Environ. Contam. Toxicol. 2011, 87, 512–516. [Google Scholar] [CrossRef]
- El-Naggar, A.H.; Sheikh, H.M. Response of the green microalga Chlorella vulgaris to the oxidative stress caused by some heavy metals. Life Sci. J. 2014, 11, 1249–1257. [Google Scholar] [CrossRef]
- Chen, Z.; Song, S.; Wen, Y.; Zou, Y.; Liu, H. Toxicity of Cu (II) to the green alga Chlorella vulgaris: A perspective of photosynthesis and oxidant stress. Environ. Sci. Pollut. Res. 2016, 23, 17910–17918. [Google Scholar] [CrossRef]
- Nagalakshmi, N.; Prasad, M.N.V. Copper-induced oxidative stress in Scenedesmus bijugatus: Protective role of free radical scavengers. Bull. Environ. Contam. Toxicol. 1998, 61, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Nagalakshmi, N.; Prasad, M.N.V. Responses of glutathione cycle enzymes and glutathione metabolism to copper stress in Scenedesmus bijugatus. Plant Sci. 2001, 160, 291–299. [Google Scholar] [CrossRef]
- Sabatini, S.E.; Juárez, Á.B.; Eppis, M.R.; Bianchi, L.; Luquet, C.M.; de Molina, M.D.C.R. Oxidative stress and antioxidant defenses in two green microalgae exposed to copper. Ecotoxicol. Environ. Saf. 2009, 72, 1200–1206. [Google Scholar] [CrossRef]
- Štork, F.; Bačkor, M.; Klejdus, B.; Hedbavny, J.; Kováčik, J. Changes of metal-induced toxicity by H2O2/NO modulators in Scenedesmus quadricauda (Chlorophyceae). Environ. Sci. Pollut. Res. 2013, 20, 5502–5511. [Google Scholar] [CrossRef]
- Nowicka, B.; Pluciński, B.; Kuczyńska, P.; Kruk, J. Prenyllipid antioxidants participate in response to acute stress induced by heavy metals in green microalga Chlamydomonas reinhardtii. Environ. Exp. Bot. 2016, 123, 98–107. [Google Scholar] [CrossRef]
- Tripathi, B.N.; Mehta, S.K.; Amar, A.; Gaur, J.P. Oxidative stress in Scenedesmus sp. during short- and long-term exposure to Cu2+ and Zn2+. Chemosphere 2006, 62, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.D.; Soares, E.V. Short- and long-term exposure to heavy metals induced oxidative stress response in Pseudokirchneriella subcapitata. Clean-Soil Air Water 2016, 44, 1578–1583. [Google Scholar] [CrossRef]
- Filová, A.; Fargašová, A.; Molnárová, M. Cu, Ni, and Zn effects on basic physiological and stress parameters of Raphidocelis subcapitata algae. Environ. Sci. Pollut. Res. 2021, 28, 58426–58441. [Google Scholar] [CrossRef]
- Nikookar, K.; Moradshahi, A.; Hosseini, L. Physiological responses of Dunaliella salina and Dunaliella tertiolecta to copper toxicity. Biomol. Eng. 2005, 22, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, B.; Zyzik, M.; Kapsiak, M.; Ogrodzińska, W.; Kruk, J. Oxidative stress limits growth of Chlamydomonas reinhardtii (Chlorophyta, Chlamydomonadales) exposed to copper ions at the early stage of culture growth. Phycologia 2021, 60, 303–313. [Google Scholar] [CrossRef]
- Lu, L.; Wu, Y.; Ding, H.; Zhang, W. The combined and second exposure effect of copper (II) and chlortetracycline on fresh water algae, Chlorella pyrenoidosa and Microcystis aeruginosa. Environ. Toxicol. Pharmacol. 2015, 40, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-T.; Chang, S.-J.; Chou, T.-L. Intracellular proline accumulation in some algae exposed to copper and cadmium. Bot. Bull. Acad. Sin. 1995, 36, 89–93. [Google Scholar]
- León-Vaz, A.; León, R.; Giráldez, I.; Vega, J.M.; Vigara, J. Impact of heavy metals in the microalga Chlorella sorokiniana and assessment of its potential use in cadmium bioremediation. Aquat. Toxicol. 2021, 239, 105941. [Google Scholar] [CrossRef] [PubMed]
- Sauser, K.R.; Liu, J.K.; Wong, T.Y. Identification of a copper-sensitive ascorbate peroxidase in the unicellular green alga Selenastrum capricornutum. BioMetals 1997, 10, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Dewez, D.; Geoffroy, L.; Vernet, G.; Popovic, R. Determination of photosynthetic and enzymatic biomarkers sensitivity used to evaluate toxic effects of copper and fludioxonil in alga Scenedesmus obliquus. Aquat. Toxicol. 2005, 74, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, U.; Khan, S.M.; Khalid, N.; Ahmad, Z.; Jehangir, S.; Lho, L.H.; Han, H.; Raposo, A. Detoxifying the heavy metals: A multipronged study of tolerance strategies against heavy metals toxicity in plants. Front. Plant Sci. 2023, 14, 1154571. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.R.; Isikhuemhen, O.S.; Anike, F.N. Fungal–metal interactions: A review of toxicity and homeostasis. J. Fungi 2021, 7, 225. [Google Scholar] [CrossRef]
- Leopold, I.; Günther, D.; Schmidt, J.; Neumann, D. Phytochelatins and heavy metal tolerance. Phytochemistry 1999, 50, 1323–1328. [Google Scholar] [CrossRef]
- Riyazuddin, R.; Nisha, N.; Ejaz, B.; Khan, M.I.R.; Kumar, M.; Ramteke, P.W.; Gupta, R. A comprehensive review on the heavy metal toxicity and sequestration in plants. Biomolecules 2021, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, B.; Pluciński, B.; Kuczyńska, P.; Kruk, J. Physiological characterization of Chlamydomonas reinhardtii acclimated to chronic stress induced by Ag, Cd, Cr, Cu and Hg ions. Ecotoxicol. Environ. Saf. 2016, 130, 133–145. [Google Scholar] [CrossRef]
- Nowicka, B.; Fesenko, T.; Walczak, J.; Kruk, J. The inhibitor-evoked shortage of tocopherol and plastoquinol is compensated by other antioxidant mechanisms in Chlamydomonas reinhardtii exposed to toxic concentrations of cadmium and chromium ions. Ecotoxicol. Environ. Saf. 2020, 191, 110241. [Google Scholar] [CrossRef]
- Rai, U.N.; Singh, N.K.; Upadhyay, A.K.; Verma, S. Chromate tolerance and accumulation in Chlorella vulgaris L.: Role of antioxidant enzymes and biochemical changes in detoxification of metals. Bioresour. Technol. 2013, 136, 604–609. [Google Scholar] [CrossRef]
- Nies, D.H. Microbial heavy-metal resistance. Appl. Microbiol. Biotechnol. 1999, 51, 730–750. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Elbaz, A.; Wei, Y.Y.; Meng, Q.; Zheng, Q.; Yang, Z.M. Mercury-induced oxidative stress and impact on antioxidant enzymes in Chlamydomonas reinhardtii. Ecotoxicology 2010, 19, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Kampfenkel, K.; Van Montagu, M.; Inzé, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Förster, B.; Osmond, C.B.; Pogson, B.J. Improved survival of very high light and oxidative stress is conferred by spontaneous gain-of-function mutations in Chlamydomonas. Biochim. Biophys. Acta-Bioenerg. 2005, 1709, 45–57. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]




| Strain | Chl a/Chl b | Car/(Chl a + Chl b) | ||
|---|---|---|---|---|
| Basal Cu | Increased Cu | Basal Cu | Increased Cu | |
| N1 | 1.96 ± 0.04 a | 1.99 ± 0.08 a | 0.22 ± 0.01 a | 0.22 ± 0.01 a |
| Cu2 | 2.14 ± 0.05 b | 2.09 ± 0.06 b | 0.23 ± 0.01 a | 0.23 ± 0.01 a |
| Asc | Thiols | Pro | CAT | APX | SOD | TBARS | Carn | Cuconc | Cuadapt | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.959 | −0.761 | 0.348 | −0.221 | 0.448 | 0.272 | 0.026 | −0.846 | −0.201 | Asc |
| 1 | −0.778 | 0.227 | −0.236 | 0.364 | 0.091 | 0.008 | −0.890 | −0.112 | Thiols | |
| 1 | −0.338 | 0.309 | −0.400 | −0.120 | 0.071 | 0.854 | 0.238 | Pro | ||
| 1 | −0.351 | 0.955 | 0.863 | −0.546 | −0.322 | −0.952 | CAT | |||
| 1 | −0.233 | −0.351 | 0.572 | 0.173 | 0.437 | APX | ||||
| 1 | 0.778 | −0.443 | −0.441 | −0.920 | SOD | |||||
| 1 | −0.577 | −0.067 | −0.824 | TBARS | ||||||
| 1 | −0.055 | 0.592 | Carn | |||||||
| 1 | 0.169 | Cuconc | ||||||||
| 1 | Cuadapt |
| F | p | |
|---|---|---|
| Strain | 82.04 | <2 × 10−16 |
| Cr concentration | 2118.536 | <2 × 10−16 |
| Day | 2786.637 | <2 × 10−16 |
| Strain × Cr concentration | 130.374 | <2 × 10−16 |
| Strain × day | 7.889 | 0.00518 |
| Cr concentration × day | 80.66 | <2 × 10−16 |
| Strain × Cr concentration × day | 95.519 | <2 × 10−16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziuba, J.; Nowicka, B. Unravelling the Mechanisms of Heavy Metal Tolerance: Enhancement in Hydrophilic Antioxidants and Major Antioxidant Enzymes Is Not Crucial for Long-Term Adaptation to Copper in Chlamydomonas reinhardtii. Plants 2024, 13, 999. https://doi.org/10.3390/plants13070999
Dziuba J, Nowicka B. Unravelling the Mechanisms of Heavy Metal Tolerance: Enhancement in Hydrophilic Antioxidants and Major Antioxidant Enzymes Is Not Crucial for Long-Term Adaptation to Copper in Chlamydomonas reinhardtii. Plants. 2024; 13(7):999. https://doi.org/10.3390/plants13070999
Chicago/Turabian StyleDziuba, Julia, and Beatrycze Nowicka. 2024. "Unravelling the Mechanisms of Heavy Metal Tolerance: Enhancement in Hydrophilic Antioxidants and Major Antioxidant Enzymes Is Not Crucial for Long-Term Adaptation to Copper in Chlamydomonas reinhardtii" Plants 13, no. 7: 999. https://doi.org/10.3390/plants13070999
APA StyleDziuba, J., & Nowicka, B. (2024). Unravelling the Mechanisms of Heavy Metal Tolerance: Enhancement in Hydrophilic Antioxidants and Major Antioxidant Enzymes Is Not Crucial for Long-Term Adaptation to Copper in Chlamydomonas reinhardtii. Plants, 13(7), 999. https://doi.org/10.3390/plants13070999