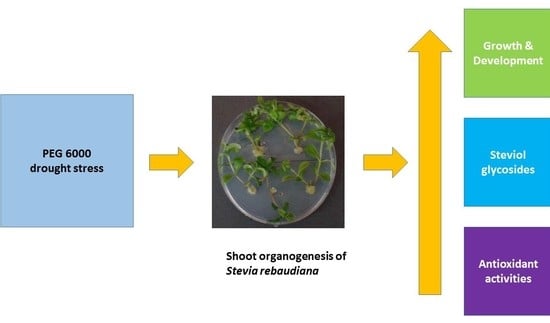
PEG 6000-Stimulated Drought Stress Improves the Attributes of In Vitro Growth, Steviol Glycosides Production, and Antioxidant Activities in Stevia rebaudiana Bertoni
Abstract
:1. Introduction
2. Results
2.1. Evaluation of Growth and Development
2.2. Estimation of Secondary Metabolites Production
3. Discussion
4. Experimental Procedure
4.1. MS Medium Preparation and Shoot Organogenesis Conditions
4.2. Extract Preparation for Steviol Glycosides Analysis
4.3. Extract Preparation for Antioxidant Activities Analysis
4.3.1. Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
4.3.2. Total Antioxidant Capacity (TAC) and Total Reducing Power (TRP)
4.3.3. DPPH-Free Radical Scavenging Activity
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Shivanna, N.; Naika, M.; Khanum, F.; Kaul, V.K. Antioxidant, anti-diabetic and renal protective properties of Stevia rebaudiana. Journal of Diabetes and its Complications 2013, 27, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Thiyagarajan, M.; Venkatachalam, P. Large scale in vitro propagation of Stevia rebaudiana (bert) for commercial application: Pharmaceutically important and antidiabetic medicinal herb. Industrial Crops and Products 2012, 37, 111–117. [Google Scholar] [CrossRef]
- Gupta, P.; Sharma, S.; Saxena, S. Effect of abiotic stress on growth parameters and steviol glycoside content in Stevia rebaudiana (Bertoni) raised in vitro. Journal of Applied Research on Medicinal and Aromatic Plants 2016, 3, 160–167. [Google Scholar] [CrossRef]
- Dey, A.; Kundu, S.; Bandyopadhyay, A.; Bhattacharjee, A. Efficient micropropagation and chlorocholine chloride induced stevioside production of Stevia rebaudiana Bertoni. C. R. Biol. 2013, 336, 17–28. [Google Scholar] [CrossRef]
- Rafiq, M.; Dahot, M.U.; Muhamamd, S.; Naqvi, H.A.; Qarshi, I.A. In vitro clonal propagation and biochemical analysis of field established stevia Rebaudiana bertoni. Pak. J. Bot. 2007, 39, 2467–2474. [Google Scholar]
- Javed, R.; Zia, M.; Yücesan, B.; Gürel, E. Abiotic stress of ZnO-PEG, ZnO-PVP, CuO-PEG and CuO-PVP nanoparticles enhance growth, sweetener compounds and antioxidant activities in shoots of Stevia rebaudiana Bertoni. IET Nanobiotechnology 2017, 11, 898–902. [Google Scholar] [CrossRef]
- Javed, R.; Yucesan, B.; Zia, M.; Gurel, E. Differential effects of plant growth regulators on physiology, steviol glycosides content, and antioxidant capacity in micropropagated tissues of Stevia rebaudiana. Biologia 2017, 72, 1156–1165. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Javed, R.; Adeel, M.; Rizwan, M.; Ao, Q.; Yang, Y. Engineered ZnO and CuO Nanoparticles Ameliorate Morphological and Biochemical Response in Tissue Culture Regenerants of Candyleaf (Stevia rebaudiana). Molecules 2020, 25. [Google Scholar] [CrossRef] [Green Version]
- Javed, R.; Mohamed, A.; Yücesan, B.; Gürel, E.; Kausar, R.; Zia, M. CuO nanoparticles significantly influence in vitro culture, steviol glycosides, and antioxidant activities of Stevia rebaudiana Bertoni. Plant Cell Tiss Organ Cult 2017, 131, 611–620. [Google Scholar] [CrossRef]
- Javed, R.; Usman, M.; Yücesan, B.; Zia, M.; Gürel, E. Effect of Zinc Oxide (ZnO) Nanoparticles on Physiology and Steviol Glycosides Production in Micropropagated Shoots of Stevia Rebaudiana Bertoni. Available online: https://pubmed.ncbi.nlm.nih.gov/27246994/ (accessed on 10 July 2020).
- Javed, R.; Yücesan, B.; Gurel, E. Hydrogen Peroxide-Induced Steviol Glycosides Accumulation and Enhancement of Antioxidant Activities in Leaf Tissues of Stevia rebaudiana Bertoni. Sugar Tech 2018, 20, 100–104. [Google Scholar] [CrossRef]
- Javed, R.; Yucesan, B.; Zia, M.; Gurel, E. Elicitation of Secondary Metabolites in Callus Cultures of Stevia rebaudiana Bertoni Grown Under ZnO and CuO Nanoparticles Stress. Sugar Tech 2018, 20, 194–201. [Google Scholar] [CrossRef]
- Javed, R.; Gürel, E. Salt stress by NaCl alters the physiology and biochemistry of tissue culture-grown Stevia rebaudiana Bertoni. Turk. J. Agric. For. 2019, 43, 11–20. [Google Scholar] [CrossRef]
- Mohd, K.K.; Pragati, M.; Taru, S.; Shukla, P.K.; Ramteke, P.W. Effect of adenine sulphate on in vitro mass propagation of Stevia rebaudiana Bertoni. J. Med. Plants Res. 2014, 8, 543–549. [Google Scholar] [CrossRef]
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; García-Lara, S. In vitro plant tissue culture: means for production of biological active compounds. Planta 2018, 248, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ranjbarfordoei, A.; Samson, R.; Van Damme, P.; Lemeur, R. Effects of Drought Stress Induced by Polyethylene Glycol on Pigment Content and Photosynthetic Gas Exchange of Pistacia Khinjuk and P. Mutica. Photosynthetica 2000, 38, 443–447. [Google Scholar] [CrossRef]
- Muscolo, A.; Sidari, M.; Anastasi, U.; Santonoceto, C.; Maggio, A. Effect of PEG-induced drought stress on seed germination of four lentil genotypes. Journal of Plant Interactions 2014, 9, 354–363. [Google Scholar] [CrossRef]
- Hajihashemi, S.; Geuns, J.M.C. Gene transcription and steviol glycoside accumulation in Stevia rebaudiana under polyethylene glycol-induced drought stress in greenhouse cultivation. FEBS Open Bio 2016, 6, 937–944. [Google Scholar] [CrossRef] [Green Version]
- Badran, A.E.; Alhady, M.R.A.A.; Hassan, W.A. In Vitro Evaluation of Some Traits in Stevia rebaudiana (Bertoni) under Drought Stress and Their Relationship on Stevioside Content. American Journal of Plant Sciences 2015, 6, 746–752. [Google Scholar] [CrossRef] [Green Version]
- Hajihashemi, S.; Sofo, A. The effect of polyethylene glycol-induced drought stress on photosynthesis, carbohydrates and cell membrane in Stevia rebaudiana grown in greenhouse. Acta Physiol Plant 2018, 40, 142. [Google Scholar] [CrossRef]
- Hajihashemi, S.; Ehsanpour, A.A. Antioxidant response of Stevia rebaudiana B. to polyethylene glycol and paclobutrazol treatments under in vitro culture. Appl. Biochem. Biotechnol. 2014, 172, 4038–4052. [Google Scholar] [CrossRef]
- Hajihashemi, S.; Rajabpoor, S.; Djalovic, I. Antioxidant potential in Stevia rebaudiana callus in response to polyethylene glycol, paclobutrazol and gibberellin treatments. Physiol Mol Biol Plants 2018, 24, 335–341. [Google Scholar] [CrossRef]
- Bogado Villalba, L.; Nakayama Nakashima, H. Effect of induced hydric stress with PEG 6000 on Stevia rebaudiana cv. “KH-IAN/VC-142” in vitro growth. Biotecnología Vegetal 2016, 16, 189–192. [Google Scholar]
- Gorzi, A.; Omidi, H.; Bostani, A.B. Morpho-physiological responses of Stevia (Stevia rebaudiana Bertoni) to various priming treatments under drought stress. Appl. Ecol. Env. Res. 2018, 16, 4753–4771. [Google Scholar] [CrossRef]
- Pradhan, N.; Singh, P.; Dwivedi, P.; Pandey, D.K. Evaluation of sodium nitroprusside and putrescine on polyethylene glycol induced drought stress in Stevia rebaudiana Bertoni under in vitro condition. Industrial Crops and Products 2020, 154, 112754. [Google Scholar] [CrossRef]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.-J. The Role of the Plant Antioxidant System in Drought Tolerance. Antioxidants (Basel) 2019, 8. [Google Scholar] [CrossRef] [Green Version]
- Alhaithloul, H.A.; Soliman, M.H.; Ameta, K.L.; El-Esawi, M.A.; Elkelish, A. Changes in Ecophysiology, Osmolytes, and Secondary Metabolites of the Medicinal Plants of Mentha piperita and Catharanthus roseus Subjected to Drought and Heat Stress. Biomolecules 2020, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Said, E.M.; Rania A, M.; Rana Al, A.; Gehan, S. Drought Stress Tolerance and Enhancement of Banana Plantlets In Vitro. Austin J. Biotechnol. Bioeng. 2015, 2, 1040. [Google Scholar]
- Liu, Y.; Meng, Q.; Duan, X.; Zhang, Z.; Li, D. Effects of PEG-induced drought stress on regulation of indole alkaloid biosynthesis in Catharanthus roseus. J. Plant Interact. 2017. [Google Scholar] [CrossRef] [Green Version]
- Murashige, T.; Skoog, F.K. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol Plant 1962, 15. [Google Scholar] [CrossRef]
- Khan, S.; Ur-Rehman, T.; Mirza, B.; Ul-Haq, I.; Zia, M. Antioxidant, Antimicrobial, Cytotoxic and Protein Kinase Inhibition Activities of Fifteen Traditional Medicinal Plants From Pakistan. Pharm Chem J 2017, 51, 391–398. [Google Scholar] [CrossRef]


| Conc. of PEG (%) | Nodal Explants Shooting (%) | Mean Shoot Length (cm) | Mean No. of Nodes Per Explant | Mean No. of Leaves Per Regenerated Shoot | FW of Shoots Per Explant (g) |
|---|---|---|---|---|---|
| 0 | 82.5 | 4.1 ± 0.01 e | 4.4 ± 0.01 e | 13.1 ± 0.01 c | 0.16 ± 0.01 d |
| 0.5 | 84.7 | 4.3 ± 0.01 d | 4.6 ± 0.01 d | 13.6 ± 0.02 c | 0.24 ± 0.01 c |
| 1.0 | 86.4 | 4.5 ± 0.01 c | 4.8 ± 0.01 c | 14.8 ± 0.01 b | 0.38 ± 0.01 b |
| 2.0 | 88.9 | 4.7 ± 0.02 b | 5.0 ± 0.01 b | 16.5 ± 0.02 a | 0.45 ± 0.01 a |
| 4.0 | 92.4 | 5.1 ± 0.01 a | 5.3 ± 0.01 a | 17.9 ± 0.01 a | 0.52 ± 0.01 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, M.A.; Javed, R.; Adeel, M.; Rizwan, M.; Yang, Y. PEG 6000-Stimulated Drought Stress Improves the Attributes of In Vitro Growth, Steviol Glycosides Production, and Antioxidant Activities in Stevia rebaudiana Bertoni. Plants 2020, 9, 1552. https://doi.org/10.3390/plants9111552
Ahmad MA, Javed R, Adeel M, Rizwan M, Yang Y. PEG 6000-Stimulated Drought Stress Improves the Attributes of In Vitro Growth, Steviol Glycosides Production, and Antioxidant Activities in Stevia rebaudiana Bertoni. Plants. 2020; 9(11):1552. https://doi.org/10.3390/plants9111552
Chicago/Turabian StyleAhmad, Muhammad Arslan, Rabia Javed, Muhammad Adeel, Muhammad Rizwan, and Yuesuo Yang. 2020. "PEG 6000-Stimulated Drought Stress Improves the Attributes of In Vitro Growth, Steviol Glycosides Production, and Antioxidant Activities in Stevia rebaudiana Bertoni" Plants 9, no. 11: 1552. https://doi.org/10.3390/plants9111552
APA StyleAhmad, M. A., Javed, R., Adeel, M., Rizwan, M., & Yang, Y. (2020). PEG 6000-Stimulated Drought Stress Improves the Attributes of In Vitro Growth, Steviol Glycosides Production, and Antioxidant Activities in Stevia rebaudiana Bertoni. Plants, 9(11), 1552. https://doi.org/10.3390/plants9111552