Biogeography of Stigmaphyllon (Malpighiaceae) and a Meta-Analysis of Vascular Plant Lineages Diversified in the Brazilian Atlantic Rainforests Point to the Late Eocene Origins of This Megadiverse Biome
Abstract
:1. Introduction
2. Results
2.1. Phylogenetic Analysis
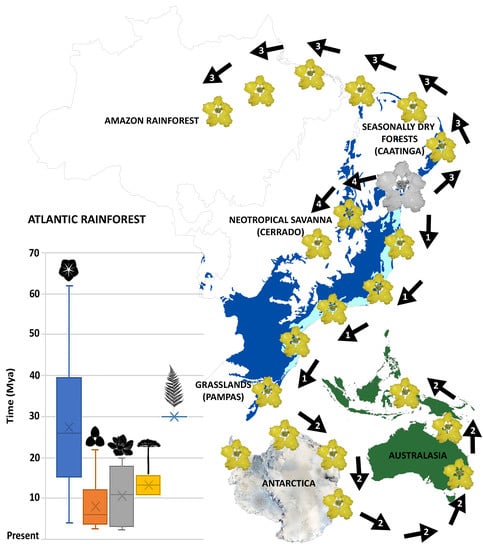
2.2. Ancestral Area Reconstruction and Divergence Times Estimation
2.3. Meta-Analysis
3. Discussion
3.1. Phylogenetics of Stigmaphyllon
3.2. Divergence Times and Biogeography of Stigmaphyllon
3.3. Time and Diversification of the Atlantic Rainforest
4. Material and Methods
4.1. Taxon Sampling and Plant Material
4.2. Molecular Protocols
4.3. Phylogenetic Analysis
4.4. Calibration
4.5. Ancestral Area Reconstruction
4.6. Meta-Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xi, Z.; Ruhfel, B.R.; Schaefer, H.; Amorim, A.M.A.; Sugumaran, M.; Wurdack, K.J.; Endress, P.K.; Matthews, M.L.; Stevens, P.F.; Mathews, S.; et al. Phylogenomics and a posteriori data partitioning resolve Cretaceous angiosperm radiation Malpighiales. Proc. Natl. Acad. Sci. USA 2012, 109, 17519–17524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, C.C.; Anderson, W.R. A complete generic phylogeny of Malpighiaceae inferred from nucleotide sequence data and morphology. Am. J. Bot. 2010, 97, 2031–2048. [Google Scholar] [CrossRef] [PubMed]
- Cameron, K.M.; Chase, M.W.; Anderson, W.R.; Hills, H.G. Molecular systematics of Malpighiaceae: Evidence from plastid rbcL and matK sequences. Am. J. Bot. 2001, 88, 1847–1862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, C.C. Madagasikaria (Malpighiaceae): A new genus from Madagascar with implications for floral evolution in Malpighiaceae. Am. J. Bot. 2002, 89, 699–706. [Google Scholar] [CrossRef] [Green Version]
- Davis, C.C.; Anderson, W.R.; Donoghue, M.J. Phylogeny of Malpighiaceae: Evidence from chloroplast ndhF and trnL-F nucleotide sequences. Am. J. Bot. 2001, 88, 1830–1846. [Google Scholar] [CrossRef] [Green Version]
- Davis, C.C.; Bell, C.D.; Fritsch, P.W.; Mathews, S. Phylogeny of Acridocarpus-Brachylophon (Malpighiaceae): Implications for tertiary tropical floras and Afroasian biogeography. Evolution 2002, 56, 2395–2405. [Google Scholar] [CrossRef]
- Davis, C.C.; Fritsch, P.W.; Bell, C.D.; Mathews, S. High-latitude tertiary migrations of an exclusively tropical clade: Evidence from Malpighiaceae. Int. J. Plant. Sci. 2004, 165, S107–S121. [Google Scholar] [CrossRef] [Green Version]
- Almeida, R.F.; Amorim, A.M.A.; Correa, A.M.S.; van den Berg, C. A new infrageneric classification for Amorimia (Malpighiaceae) based on morphological, phytochemical, and molecular evidence. Phytotaxa 2017, 313, 231–248. [Google Scholar] [CrossRef]
- Willis, C.G.; Franzone, B.F.; Xi, Z.; Davis, C.C. The establishment of Central American migratory corridors and the biogeographic origins of seasonally dry tropical forests in Mexico. Front. Genet. 2014, 5, 433. [Google Scholar] [CrossRef] [Green Version]
- Anderson, C.E. Revision of Ryssopterys and transfer to Stigmaphyllon (Malpighiaceae). Blumea 2011, 56, 73–104. [Google Scholar] [CrossRef] [Green Version]
- Anderson, C.E. Monograph of Stigmaphyllon (Malpighiaceae). Syst. Bot. Monographs 1997, 51, 1–313. [Google Scholar] [CrossRef]
- Flora do Brasil. 2020. Available online: http://floradobrasil.jbrj.gov.br/reflora/listaBrasil/ConsultaPublicaUC/ResultadoDaConsultaNovaConsulta.do#CondicaoTaxonCP (accessed on 10 November 2020).
- Scarano, F.R.; Ceotto, P. Brazilian Atlantic forest: Impact, vulnerability, and adaptation to climate change. Biodivers. Conserv. 2015, 24, 2319–2331. [Google Scholar] [CrossRef]
- Ledo, R.; Colli, G.R. The historical connections between the Amazon and the Atlantic forest revisited. J. Biogeogr. 2017, 44, 2551–2563. [Google Scholar] [CrossRef]
- Vanzolini, P.E.; Williams, E.F. The vanishing refuge: A mechanism for ecogeographic speciation. Pap. Avulsos Zool. 1981, 34, 251–255. [Google Scholar]
- Antonelli, A.; Zizka, A.; Carvalho, F.A.; Scharm, R.; Bacon, C.D.; Silvestro, D.; Condamine, F.L. Amazonia is the primary source of Neotropical biodiversity. Proc. Natl. Acad. Sci. USA 2018, 115, 6034–6039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- POWO-Plants of the World Online. 2020. Available online: http://www.plantsoftheworldonline.org/ (accessed on 10 November 2020).
- Korall, P.; Pryer, K.M. Global biogeography of scaly tree ferns (Cyatheaceae): Evidence for Gondwanan vicariance and limited transoceanic dispersal. J. Biogeogr. 2014, 41, 402–413. [Google Scholar] [CrossRef]
- Kranitz, M.L.; Biffin, E.; Clark, A.; Hollingsworth, M.L.; Ruhsam, M.; Gardner, M.F.; Thomas, P.; Mill, R.R.; Ennos, R.A.; Gaudeul, M.; et al. Evolutionary diversification of New Caledonian Araucaria. PLoS ONE 2014, 9, e110308. [Google Scholar] [CrossRef] [Green Version]
- Quiroga, M.P.; Mathiasen, P.; Iglesias, A.; Mill, R.R.; Premoli, A.C. Molecular, and fossil evidence disentangle the biogeographical history of Podocarpus, a key genus in plant geography. J. Biogeogr. 2016, 43, 372–383. [Google Scholar] [CrossRef]
- Richardson, J.E.; Chatrou, L.W.; Mols, J.B.; Erkens, R.H.J.; Prie, M.D. Historical biogeography of two cosmopolitan families of flowering plants: Annonaceae and Rhamnaceae. Phil. Trans. R. Soc. Lond. B 2004, 359, 1495–1508. [Google Scholar] [CrossRef] [Green Version]
- Renner, S.S.; Strijk, J.S.; Strasberg, D.; Thébaud, C. Biogeography of the Monimiaceae (Laurales): A role for East Gondwana and long-distance dispersal, but not West Gondwana. J. Biogeogr. 2010, 37, 1227–1238. [Google Scholar] [CrossRef]
- Kessous, I.M.; Neves, B.; Couto, D.R.; Paixão-Souza, B.; Pederneiras, L.C.; Moura, R.L.; Barfuss, M.H.J.; Salgueiro, F.; Costa, A.F. Historical biogeography of a Brazilian lineage of Tillandsioideae (subtribe Vrieseinae, Bromeliaceae): The Paranaean Sea hypothesized as the main vicariant event. Bot. J. Linn. Soc. 2020, 192, 625–641. [Google Scholar] [CrossRef]
- Givnish, T.J.; Barfuss, M.H.J.; Van Ee, B.; Riina, R.; Schulte, K.; Horres, R.; Gonsiska, P.A.; Jabaily, R.S.; Crayn, D.M.; Smith, J.A.C.; et al. Phylogeny, adaptative radiation, and historical biogeography in Bromeliaceae: Insights from an eight-locus plastid phylogeny. Am. J. Bot. 2011, 98, 872–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellegrini, M.O.O. Systematics of Commelinales, Focusing on Neotropical Lineages. Ph.D. Thesis, Instituto de Biociências, São Paulo, Brazil, 2019; 650p. [Google Scholar]
- Couto, R.S.; Martins, A.C.; Bolson, M.; Lopes, R.C.; Smidt, E.C.; Braga, J.M.A. Time calibrated tree of Dioscorea (Dioscoreaceae) indicates four origins of yams in the Neotropics since the Eocene. Bot. J. Linn. Soc. 2018, 188, 144–160. [Google Scholar] [CrossRef]
- Vasconcelos, T.N.C.; Alcantara, S.; Andrino, C.O.; Forest, F.; Reginato, M.; Simon, M.F.; Pirani, J.R. Fast diversification through a mosaic of evolutionary histories characterizes the endemic flora of ancient Neotropical mountains. Proc. R. Soc. B 2020, 287, 20192933. [Google Scholar] [CrossRef] [Green Version]
- Gustafsson, A.L.S.; Verola, C.F.; Antonelli, A. Reassessing the temporal evolution of orchids with new fossils and a Bayesian relaxed clock, with implications for the diversification of the rare South American genus Hoffmannseggella (Orchidaceae: Epidendroideae). BMC Evol. Biol. 2010, 10, 177. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Sanchez, E. Biogeography and divergence time estimates of woody bamboos: Insights in the evolution of Neotropical bamboos. Bol. Soc. Bot. Méx. 2011, 88, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.Z.; Zeng, C.X.; Ma, P.F.; Haevermans, T.; Zhang, Y.X.; Zhang, L.N.; Guo, Z.H.; Li, D.Z. Multi-locus plastid phylogenetic biogeography supports the Asian hypothesis of the temperate woody bamboos (Poaceae: Bambusoideae). Mol. Phylogenet. Evol. 2016, 96, 118–129. [Google Scholar] [CrossRef]
- Rapini, A.; van den Berg, C.; Liede-Schumann, S. Diversification of Asclapiadoideae (Apocynaceae) in the New World. Ann. Missouri Bot. Gard. 2007, 94, 407–422. [Google Scholar] [CrossRef]
- Mandel, J.R.; Dikow, R.B.; Siniscalchi, C.M.; Thapa, R.; Watson, L.E.; Funk, V.A. A fully resolved backbone phylogeny reveals numerous dispersals and explosive diversifications throughout the history of Asteraceae. Proc. Natl. Acad. Sci. USA 2019, 116, 14083–14088. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, L.H.M.; Lohmann, L.G. Biogeography, and evolution of Dolichandra (Bignonieae, Bignoniaceae). Bot. J. Linn. Soc. 2015, 179, 403–420. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Hernández, T.; Brown, J.W.; Schlumpberger, B.O.; Eguiarte, L.E.; Magallón, S. Beyond aridification: Multiple explanations for the elevated diversification of cacti in the New World Succulent Biome. New Phytol. 2014, 202, 1382–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meseguer, A.S.; Lobo, J.M.; Cornuault, J.; Beerling, D.; Ruhfel, B.R.; Davis, C.C.; Jousselin, E.; Sanmartín, I. Reconstructing deep-time palaeoclimate legacies in the clusioid Malpighiales unveil their role in the evolution and extinction of the boreotropical flora. Glob. Ecol. Biogeogr. 2018, 27, 616–628. [Google Scholar] [CrossRef] [Green Version]
- Soares-Neto, R.L.; Thomas, W.W.; Barbosa, M.R.V.; Roalson, E.H. Diversification of New World Cleomaceae with emphasis on Tarenaya and the description of Iltisiella, a new genus. Taxon 2020, 69, 321–336. [Google Scholar] [CrossRef]
- Ruhfel, B.R.; Bove, C.P.; Philbrick, C.T.; Davis, C.C. Dispersal largely explains the Gondwanan distribution of the ancient tropical clusioid plant clade. Am. J. Bot. 2016, 103, 1117–1128. [Google Scholar] [CrossRef] [Green Version]
- Silva, O.L.M.; Riina, R.; Cordeiro, I. Phylogeny and biogeography of Astraea with new insights into the evolutionary history of Crotoneae (Euphorbiaceae). Mol. Phylogenet. Evol. 2020, 145, 106738. [Google Scholar] [CrossRef] [PubMed]
- Lavin, M.; Herendeen, P.S.; Wojciechowski, M.F. Evolutionary rates analysis of Leguminosae implicates a rapid diversification of lineages during the Tertiary. Syst. Biol. 2005, 54, 575–594. [Google Scholar] [CrossRef] [Green Version]
- Bruneau, A.; Mercure, M.; Lewis, G.P.; Herendeen, P.S. Phylogenetic patterns and diversification in the caesalpinioid legumes. Botany 2008, 86, 697–718. [Google Scholar] [CrossRef]
- Gagnon, E.; Ringelberg, J.J.; Bruneau, A.; Lewis, G.P.; Hughes, C.E. Global Succulent Biome phylogenetic conservatism across the pantropical Caesalpinia group (Leguminosae). New Phytol. 2019, 222, 1994–2008. [Google Scholar] [CrossRef]
- Roalson, E.H.; Roberts, W.R. Distinct processes drive diversification in different clades of Gesneriaceae. Syst. Biol. 2016, 65, 662–684. [Google Scholar] [CrossRef] [Green Version]
- Castillo, R.A.; Luebert, F.; Henning, T.; Weigend, M. Major lineages of Loasaceae subfam. Loasoideae diversified during the Andean uplift. Mol. Phylogenet. Evol. 2019, 141, 106616. [Google Scholar] [CrossRef]
- Davis, C.C.; Schaefer, H.; Xi, Z.; Baum, D.A.; Donoghue, M.J.; Harmon, L.J. Long-term morphological stasis maintained by a plant-pollinator mutualism. Proc. Natl. Acad. Sci. USA 2014, 111, 5914–5919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reginato, M.; Vasconcelos, T.N.C.; Kriebel, R.; Simões, A.O. Is dispersal mode a driver of diversification and geographical distribution in the tropical plant family Melastomataceae? Mol. Phylogenet. Evol. 2020, 148, 106815. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.F.P.; Ronsted, N.; Bruun-Lund, S.; Pereira, R.A.S.; Queiroz, L.P. Atlantic forests to the all Americas: Biogeographical history and divergence times of Neotropical Ficus (Moraceae). Mol. Phylogenet. Evol. 2018, 122, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, T.N.C.; Proença, C.E.B.; Ahmad, B.; Aguilar, D.S.; Aguilar, R.; Amorim, B.S.; Campbell, K.; Costa, I.R.; De-Carvalho, P.S.; Faria, J.E.Q.; et al. Myrteae phylogeny, calibration, biogeography and diversification patterns: Increased understanding in the most species-rich tribe of Myrtaceae. Mol. Phylogenet. Evol. 2017, 109, 113–137. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.F.; Lucas, E.; Sano, P.T.; Buerki, S.; Staggemeier, V.G.; Forest, F. Biogeographical patterns of Myrcia s.l. (Myrtaceae) and their correlation with geological and climatic history in the Neotropics. Mol. Phylogenet. Evol. 2017, 108, 34–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buerki, S.; Forest, F.; Alvarez, N.; Nylander, J.A.A.; Arrigo, N.; Sanmartín, I. An evaluation of new parsimony-based versus parametric inference methods in biogeography: A case study using the globally distributed plant family Sapindaceae. J. Biogeogr. 2011, 38, 531–550. [Google Scholar] [CrossRef]
- Särkinen, T.; Bohs, L.; Olmstead, R.G.; Knapp, S. A phylogenetic framework for evolutionary study of the nightshades (Solanaceae): A dated 1000-tip tree. BMC Evol. Biol. 2013, 13, 214. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, D.J.P.; Shimizu, G.H.; Ortiz, E.M.; Jansen, R.K.; Simpson, B.B. Historical biogeography of Vochysiaceae reveals an unexpected perspective of plant evolution in the Neotropics. Am. J. Bot 2020, 107, 1–17. [Google Scholar] [CrossRef]
- Thode, V.A.; Sanmartín, I.; Lohmann, L.G. Contrasting patterns of diversification between Amazonian and Atlantic forest clades of Neotropical lianas (Amphilophium, Bignonieae) inferred from plastid genomic data. Mol. Phylogenet. Evol. 2019, 133, 92–106. [Google Scholar] [CrossRef]
- Lohmann, L.G.; Bell, C.D.; Calió, M.F.; Winkworth, R.C. Pattern, and timing of biogeographical history in the Neotropical tribe Bignonieae (Bignoniaceae). Bot. J. Linn. Soc. 2013, 171, 154–170. [Google Scholar] [CrossRef] [Green Version]
- Simon, M.F.; Grether, R.; Queiroz, L.P.; Skema, C.; Pennington, R.T.; Hughes, C.E. Recent assembly of the Cerrado, a neotropical plant diversity hotspot, by in situ evolution of adaptations to fire. Proc. Natl. Acad. Sci. USA 2009, 106, 20359–20364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazine, F.F.; Faria, J.E.Q.; Giaretta, A.; Vasconcelos, T.; Forest, F.; Lucas, E. Phylogeny and biogeography of the hyper-diverse genus Eugenia (Myrtaceae: Myrteae), with emphasis on E. sect. Umbellatae, the most unmanageable clade. Taxon 2018, 67, 752–769. [Google Scholar] [CrossRef]
- Davies, B.J.; Hambrey, M.J.; Smelle, J.L.; Carrivick, J.L.; Glasser, N.F. Antarctic Peninsula ice sheet evolution during the Cenozoic era. Quat. Sci. Rev. 2012, 31, 30–66. [Google Scholar] [CrossRef]
- Lewis, A.R.; Marchant, D.R.; Ashworth, A.C.; Hedenäs, L.; Hemming, S.R.; Johnson, J.V.; Leng, M.L.; Machlus, M.L.; Newton, A.E.; Raine, J.I.; et al. Mid-Miocene cooling and the extinction of tundra in continental Antarctica. Proc. Natl. Acad. Sci. USA 2008, 105, 10676–10680. [Google Scholar] [CrossRef] [Green Version]
- Chacón, J.; Assis, M.C.; Meerow, A.W.; Renner, S.S. From East Gondwana to Central America: Historical biogeography of the Alstroemeriaceae. J. Biogeogr. 2012, 39, 1806–1818. [Google Scholar] [CrossRef]
- Maciel, J.R. Estudos Taxonômicos, Filogenéticos e Biogeográficos em Aechmea (Bromeliaceae). Ph.D. Thesis, Universidade Federal de Pernambuco, Recife, Brazil, 2017; 213p. [Google Scholar]
- Morley, R.J. Origin and Evolution of Tropical Rain Forests; Wiley: Chichester, UK, 2000; p. 362. [Google Scholar]
- Zachos, J.; Pagani, M.; Sloan, L.; Thomas, E.; Billups, K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 2001, 292, 686–693. [Google Scholar] [CrossRef]
- Flower, B.P.; Kennett, J.P. The middle Miocene climate transition: East Antarctic ice sheet development, deep ocean circulation and global carbon cycling. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1994, 108, 537–555. [Google Scholar] [CrossRef]
- Prado, D.E.; Gibbs, P.E. Patterns of species distributions in the dry seasonal forests of South Am.ica. Ann. Mo. Bot. Gard. 1993, 80, 902–927. [Google Scholar] [CrossRef]
- Peres, E.A.; Pinto-da-Rocha, R.; Lohmann, L.G.; Michelangeli, F.A.; Miyaki, C.Y.; Carnaval, A.C. Patterns of species and lineage diversity in the Atlantic Rainforest of Brazil. In Neotropical Diversification: Patterns and Processes; Rull, V., Carnaval, A.C., Eds.; Springer: Cham, Switzerland, 2020; pp. 415–520. [Google Scholar]
- Karl, M.; Glasmacher, U.A.; Kollenz, S.; Franco-Magalhães, A.O.B.; Stockli, D.F.; Hackspacher, P.C. Evolution of the South Atlantic passive continental margin in southern Brazil derived from zircon and apatite (U–Th–Sm)/He and fission-track data. Tectonophysics 2013, 604, 224–244. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Almeida, R.F.; Amorim, A.M.A.; van den Berg, C. Timing the origin and past connections between Andean and Atlantic Seasonally Dry Tropical Forests in South America: Insights from the biogeographical history of Amorimia (Malpighiaceae). Taxon 2018, 67, 739–751. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Inform. 2004, 5, 113. [Google Scholar]
- Swofford, D.L. PAUP: Phylogenetic Analysis Using Parsimony and Other Methods, Version 4.0b10.; Sinauer: Sunderland, UK, 2002. [Google Scholar]
- Fitch, W.M. Towards defining the course of evolution: Minimum change for a specific tree topology. Syst. Zool. 1971, 20, 406–416. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics, and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [Green Version]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [Green Version]
- Drummond, A.J.; Suchard, M.A.; Xie, D.; Rambaut, A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol Evol. 2012, 29, 1969–1973. [Google Scholar] [CrossRef] [Green Version]
- Cai, L.; Xi, Z.; Peterson, K.; Rushworth, C.; Beaulieu, J.; Davis, C.C. Phylogeny of Elatinaceae and the tropical Gondwanan origin of the Centroplacaceae (Malpighiaceae, Elatinaceae) clade. PLoS ONE 2016, 11, e0161881. [Google Scholar] [CrossRef]
- Rambaut, A.; Suchard, M.A.; Xie, D.; Drummond, A.J. Tracer v1.6. 2014. Available online: http://beast.bio.ed.ac.uk/Tracer (accessed on 10 November 2020).
- FigTree. 2020. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 10 November 2020).
- Anderson, W.R.; Davis, C.C. Generic adjustments in Neotropical Malpighiaceae. Contr. Univ. Michigan Herb. 2007, 25, 137–166. [Google Scholar]
- Gates, B. Banisteriopsis, Diplopterys (Malpighiaceae). Flora Neotrop. 1982, 30, 1–238. [Google Scholar]
- IBGE-Instituto Brasileiro de Geografia e Estatística. Mapa de vegetação do Brasil. Rio de Janeiro, Brasil. 2012. Available online: http://www.ibge.gov.br/home/presidencia/noticias/21052004biomashtml.shtm (accessed on 10 November 2020).
- WWF-World Wildlife Fund. Tropical and Subtropical Dry Broadleaf Forests. 2020. Available online: http://www.worldwildlife.org/biomes/tropical-and-subtropical-dry-broadleaf-forests (accessed on 10 November 2020).
- Yu, Y.; Harris, A.J.; Blair, C.; He, X.J. RASP (Reconstruct Ancestral State in Phylogenies): A tool for historical biogeography. Mol. Phylogenet. Evol. 2015, 87, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Ree, R.H.; Smith, S.A. Maximum likelihood inference of geographic range evolution by dispersal, local extinction, and cladogenesis. Syst. Biol. 2008, 57, 4–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ree, R.H.; Sanmartín, I. Conceptual and statistical problems with the DEC+J model of founder-event speciation and its comparison with DEC via model selection. J. Biogeogr. 2018, 45, 741–749. [Google Scholar] [CrossRef]




| Nodes | Max | Age Mean | Min | Ancestral Area Reconstruction | Dispersal/Vicariance Event |
|---|---|---|---|---|---|
| 1 | 40.0 | 39.95 | 35.0 | Atlantic Forest/Caatinga | Dispersal |
| 2 | 41.29 | 36.39 | 29.92 | Atlantic Forest/Caatinga | Dispersal |
| 3 | 34.09 | 26.47 | 19.35 | Atlantic Forest | Dispersal |
| 4 | 22.14 | 14.06 | 6.76 | Caatinga | Dispersal |
| 5 | 30.14 | 22.41 | 15.43 | Atlantic Forest | Dispersal |
| 6 | 19.87 | 10.18 | 2.18 | Atlantic Forest/Asian Rainforests | Vicariance |
| 7 | 26.14 | 19.19 | 12.71 | Atlantic Forest | Dispersal |
| 8 | 12.35 | 6.25 | 1.06 | Atlantic Forest/Caatinga/Amazon Forest | Vicariance |
| 9 | 24.05 | 17.45 | 11.64 | Atlantic Forest | - |
| 10 | 16.22 | 9.4 | 3.18 | Atlantic Forest | Dispersal |
| 11 | 1.81 | 0.72 | 0.03 | Atlantic Forest/Caatinga | Dispersal |
| 12 | 21.40 | 15.21 | 10.06 | Atlantic Forest | Dispersal/Vicariance |
| 13 | 18.30 | 12.89 | 8.25 | Atlantic Forest | - |
| 14 | 12.16 | 7.45 | 3.30 | Atlantic Forest | - |
| 15 | 8.70 | 4.57 | 1.42 | Atlantic Forest | - |
| 16 | 8.29 | 4.39 | 0.95 | Atlantic Forest | - |
| 17 | 17.19 | 12.01 | 7.32 | Atlantic Forest | Dispersal/Vicariance |
| 18 | 1.84 | 0.54 | 0.0 | Amazon Forest | - |
| 19 | 16.0 | 11.09 | 6.88 | Atlantic Forest | Dispersal |
| 20 | 13.05 | 8.52 | 4.43 | Atlantic Forest | - |
| 21 | 10.87 | 6.73 | 3.04 | Atlantic Forest | Dispersal |
| 22 | 8.49 | 4.81 | 1.28 | Atlantic Forest | - |
| 23 | 13.49 | 8.98 | 4.88 | Atlantic Forest/Caatinga | Dispersal |
| 24 | 0.36 | 0.30 | 0.10 | Caatinga | - |
| 25 | 10.35 | 6.06 | 2.27 | Atlantic Forest/Caatinga | Dispersal |
| 26 | 0.86 | 0.27 | 0.0 | Atlantic Forest/Caatinga | Dispersal |
| Family | Genus/Lineage | BAF spp./Genus | Phytophysiognomy | Max | Mean | Min | Reference |
|---|---|---|---|---|---|---|---|
| Ferns | |||||||
| Cyatheaceae | Cyathea Sm. | 23/290 | Rainforest | ? | 30.0 | ? | [18] |
| Gymnosperms | |||||||
| Araucariaceae | Araucaria Juss. | 1/20 | Rainforest | ? | 10.0 | ? | [19] |
| Podocarpaceae | Podocarpus L’Hér. ex Pers. | 2/115 | Rainforest | ? | 15.0 | ? | [20] |
| Magnoliids | |||||||
| Annonaceae | Hornschuchia Nees | 10/10 | Rainforest | ? | 20.0 | ? | [21] |
| Lauraceae | Phyllostemonodaphne Koesterm. | 1/1 | Rainforest | ? | ? | ? | - |
| Lauraceae | Urbanodendron Mez | 3/3 | Rainforest | ? | ? | ? | - |
| Monimiaceae | Macropeplus Perkins | 4/4 | Rainforest | ? | 11.26 | ? | [22] |
| Monimiaceae | Grazielanthus Peixoto and Per.-Moura | 1/1 | Rainforest | ? | 3.85 | ? | [22] |
| Monimiaceae | Hennecartia J.Poiss. | 1/1 | Rainforest | ? | 15.58 | ? | [22] |
| Monimiaceae | Macrotorus Perkins | 1/1 | Rainforest | ? | 11.26 | ? | [22] |
| Monimiaceae | Mollinedia Ruiz and Pav. | 32/55 | Rainforest | ? | 2.20 | ? | [22] |
| Monocots | |||||||
| Amaryllidaceae | Griffinia Ker Gawl. | 17/22 | Grassland | ? | ? | ? | - |
| Araceae | Asterostigma Fisch. and C.A. Mey. | 8/10 | Rainforest | ? | ? | ? | - |
| Araceae | Dracontioides Engl. | 2/2 | Rainforest | ? | ? | ? | - |
| Asparagaceae | Herreria Ruiz and Pav. | 6/8 | Rainforest | ? | ? | ? | - |
| Bromeliaceae | Alcantarea (E.Morren ex Mez) Harms | 30/40 | Rainforest | 5.5 | 3.3 | 1.5 | [23] |
| Bromeliaceae | Araeococcus Brongn. | 6/9 | Rainforest | ? | 3.5 | ? | [24] |
| Bromeliaceae | Billbergia Thunb. | 35/63 | Rainforest | ? | 4.5 | ? | [24] |
| Bromeliaceae | Canistropsis (Mez) Leme | 11/12 | Rainforest | ? | 3.5 | ? | [24] |
| Bromeliaceae | Stigmatodon Leme, G.K.Br. and Barfuss | 18/18 | Rainforest | 6.4 | 5.5 | 2.8 | [23] |
| Bromeliaceae | Vriesea Lindl. | 167/255 | Rainforest | 6.8 | 5.0 | 3.3 | [23] |
| Commelinaceae | Siderasis Raf. | 6/6 | Rainforest | 16.69 | 8.57 | 2.26 | [25] |
| Commelinaceae | Dichorisandra J.C.Mikan | 40/52 | Rainforest | 6.38 | 2.78 | 0.32 | [25] |
| Dioscoreaceae | Dioscorea L. | 81/628 | Rainforest | 30.0 | 22.0 | 15.0 | [26] |
| Iridaceae | Neomarica Sprague | 27/27 | Grassland | ? | 6.5 | ? | [27] |
| Marantaceae | Ctenanthe Eichler | 11/15 | Rainforest | ? | ? | ? | - |
| Marantaceae | Maranta L. | 20/37 | Rainforest | ? | ? | ? | - |
| Marantaceae | Saranthe Eichler | 8/10 | Rainforest | ? | ? | ? | - |
| Marantaceae | Thalia L. | 4/6 | Rainforest | ? | ? | ? | - |
| Orchidaceae | Bifrenaria Lindl. | 17/21 | Rainforest | ? | 13.0 | ? | [28] |
| Orchidaceae | Capanemia Barb.Rodr. | 6/9 | Rainforest | ? | ? | ? | - |
| Orchidaceae | Centroglossa Barb.Rodr. | 6/6 | Rainforest | ? | ? | ? | - |
| Orchidaceae | Cirrhaea Lindl. | 7/7 | Rainforest | ? | ? | ? | - |
| Orchidaceae | Hoehneella Ruschi | 2/2 | Rainforest | ? | ? | ? | - |
| Orchidaceae | Isabelia Barb. Rodr. | 3/3 | Rainforest | ? | ? | ? | - |
| Orchidaceae | Lankesterella Ames | 7/11 | Rainforest | ? | ? | ? | - |
| Orchidaceae | Loefgrenianthus Hoehne | 1/1 | Rainforest | ? | ? | ? | - |
| Orchidaceae | Miltonia Lindl. | 19/19 | Rainforest | ? | ? | ? | - |
| Orchidaceae | Phymatidium Lindl. | 9/9 | Rainforest | ? | ? | ? | - |
| Orchidaceae | Pseudolaelia Porto and Brade | 10/15 | Rainforest | ? | ? | ? | - |
| Poaceae | Chusquea Kunth | 45/185 | Rainforest | ? | 9.0 | ? | [29] |
| Poaceae | Merostachys Spreng. | 44/53 | Rainforest | ? | ? | ? | - |
| Poaceae | Olyra L. | 9/15 | Rainforest | ? | 14.0 | ? | [30] |
| Poaceae | Raddia Bertol. | 9/12 | Rainforest | ? | 22.0 | ? | [30] |
| Eudicots | |||||||
| Acanthaceae | Herpetacanthus Nees | 14/21 | Rainforest | ? | ? | ? | - |
| Apocynaceae | Bahiella J.F.Morales | 2/2 | Rainforest | ? | ? | ? | - |
| Apocynaceae | Peplonia Decne. | 8/13 | Rainforest | ? | 13.0 | ? | [31] |
| Asteraceae | Barrosoa R.M.King and H.Rob. | 7/11 | Rainforest | ? | ? | ? | - |
| Asteraceae | Disynaphia Hook. and Arn. ex DC. | 10/14 | Rainforest | ? | ? | ? | - |
| Asteraceae | Grazielia R.M.King and H.Rob. | 7/12 | Rainforest | ? | ? | ? | - |
| Asteraceae | Pamphalea DC. | 8/9 | Rainforest | ? | ? | ? | - |
| Asteraceae | Stifftia J.C.Mikan | 4/6 | Rainforest | ? | 34.0 | ? | [32] |
| Asteraceae | Piptocarpha R.Br. | 24/50 | Rainforest | ? | ? | ? | - |
| Bignoniaceae | Dolichandra Cham. | 8/9 | Rainforest | 38.3 | 31.3 | 25.2 | [33] |
| Bignoniaceae | Paratecoma Kuhlm. | 1/1 | Rainforest | ? | ? | ? | - |
| Bignoniaceae | Zeyheria Mart. | 2/2 | Rainforest | ? | ? | ? | - |
| Cactaceae | Rhipsalis Gaertn. | 37/43 | Rainforest | 11.82 | 7.67 | 4.26 | [34] |
| Callophyllaceae | Kielmeyera Mart. and Zucc. | 24/50 | Rainforest | 23.0 | 15.54 | 10.0 | [35] |
| Cleomaceae | Tarenaya Raf. | 14/14 | Rainforest | 18.39 | 16.60 | 14.7 | [36] |
| Clusiaceae | Tovomitopsis Planch. and Triana | 2/2 | Rainforest | 34.0 | 20.46 | 13.0 | [37] |
| Erythroxylaceae | Erythroxylum P.Browne | 71/259 | Rainforest | ? | ? | ? | - |
| Euphorbiaceae | Brasiliocroton P.E.Berry and Cordeiro | 2/2 | Rainforest | 58.42 | 48.13 | 39.6 | [38] |
| Euphorbiaceae | Astraea Klotzsch | 10/14 | Rainforest | 34.38 | 19.05 | 4.46 | [38] |
| Euphorbiaceae | Croton L. | 98/1157 | Rainforest | 40.92 | 39.03 | 37.0 | [38] |
| Fabaceae | Dahlstedtia Malme | 9/16 | Rainforest | ? | ? | ? | - |
| Fabaceae | Holocalyx Micheli | 1/1 | Rainforest | 52.1 | 1.3 | 28.8 | [39] |
| Fabaceae | Moldenhawera Schrad. | 8/11 | Rainforest | ? | 48.0 | ? | [40] |
| Fabaceae | Parapiptadenia Brenan | 5/6 | Rainforest | ? | 11.0 | ? | [41] |
| Fabaceae | Paubrasilia Gagnon, H.C.Lima and G.P.Lewis | 1/1 | Rainforest | ? | 48.0 | ? | [41] |
| Fabaceae | Schizolobium Vogel | 1/1 | Rainforest | ? | 40.5 | ? | [41] |
| Gentianaceae | Calolisianthus (Griseb.) Gilg | 4/4 | Rainforest | ? | ? | ? | - |
| Gentianaceae | Chelonanthus (Griseb.) Gilg | 4/5 | Rainforest | ? | ? | ? | - |
| Gentianaceae | Deianira Cham. and Schltdl. | 4/7 | Rainforest | ? | ? | ? | - |
| Gentianaceae | Prepusa Mart. | 5/6 | Rainforest | ? | ? | ? | - |
| Gentianaceae | Senaea Taub. | 1/2 | Rainforest | ? | ? | ? | - |
| Gentianaceae | Tetrapollinia Maguire and B.M.Boom | 1/1 | Rainforest | ? | ? | ? | - |
| Gesneriaceae | Codonanthe (Mart.) Hanst. | 8/9 | Rainforest | ? | 7.0 | ? | [42] |
| Gesneriaceae | Nematanthus Schrad. | 32/32 | Rainforest | ? | 7.0 | ? | [42] |
| Gesneriaceae | Paliavana Vand. | 4/6 | Rainforest | ? | 7.0 | ? | [42] |
| Gesneriaceae | Sinningia Nees | 70/75 | Rainforest | ? | 14.0 | ? | [42] |
| Gesneriaceae | Vanhouttea Lem. | 9/10 | Rainforest | ? | 7.0 | ? | [42] |
| Loasaceae | Blumenbachia Schrad. | 7/12 | Rainforest | 4.22 | 27.5 | 20.7 | [43] |
| Malpighiaceae | Barnebya (Griseb.) W.R.Anderson and B.Gates | 1/2 | Rainforest | ? | 62.0 | ? | [44] |
| Melastomataceae | Behuria Cham. | 17/17 | Rainforest | ? | 8.50 | ? | [45] |
| Melastomataceae | Bertolonia Raddi | 27/27 | Rainforest | ? | 9.26 | ? | [45] |
| Melastomataceae | Huberia DC. | 13/17 | Rainforest | ? | 8.50 | ? | [45] |
| Melastomataceae | Physeterostemon R.Goldenb. and Amorim | 5/5 | Rainforest | ? | 2.50 | ? | [45] |
| Melastomataceae | Pleiochiton Naudin ex A. Gray | 12/12 | Rainforest | ? | 4.43 | ? | [45] |
| Melastomataceae | Pleroma D.Don | 46/85 | Rainforest | ? | 12.26 | ? | [45] |
| Moraceae | Clarisia Ruiz and Pav. | 2/2 | Rainforest | ? | ? | ? | - |
| Moraceae | Ficus L. | 38/874 | Rainforest | ? | 25.0 | ? | [46] |
| Myrtaceae | Accara Landrum | 1/1 | Rainforest | ? | 32.0 | ? | [47] |
| Myrtaceae | Blepharocalyx O.Berg. | 3/4 | Rainforest | ? | 40.0 | ? | [47] |
| Myrtaceae | Calyptranthes Sw. | 35/35 | Rainforest | ? | 24.0 | ? | [47] |
| Myrtaceae | Curitiba Salywon and Landrum | 1/1 | Rainforest | ? | 40.0 | ? | [47] |
| Myrtaceae | Eugenia L. | 254/1149 | Rainforest | ? | 44.0 | ? | [47] |
| Myrtaceae | Myrceugenia O.Berg. | 33/45 | Rainforest | ? | 25.0 | ? | [47] |
| Myrtaceae | Myrcia DC. | 200/609 | Rainforest | ? | 28.0 | ? | [48] |
| Myrtaceae | Myrciaria O.Berg. | 18/27 | Rainforest | ? | 26.0 | ? | [48] |
| Myrtaceae | Myrrhinium Schott | 1/1 | Rainforest | ? | 29.0 | ? | [47] |
| Myrtaceae | Neomitranthes D.Legrand | 15/15 | Rainforest | ? | 12.0 | ? | [47] |
| Myrtaceae | Plinia L. | 29/78 | Rainforest | ? | 37.0 | ? | [47] |
| Myrtaceae | Psidium L. | 39/91 | Rainforest | ? | 20.0 | ? | [47] |
| Rubiaceae | Bathysa C. Presl | 6/10 | Rainforest | ? | ? | ? | - |
| Rubiaceae | Bradea Standl. ex Brade | 6/6 | Rainforest | ? | ? | ? | - |
| Rubiaceae | Coccocypselum P.Browne | 14/22 | Rainforest | ? | ? | ? | - |
| Rutaceae | Conchocarpus J.C.Mikan | 40/48 | Rainforest | ? | ? | ? | - |
| Rutaceae | Metrodorea A.St.-Hil | 3/6 | Rainforest | ? | ? | ? | - |
| Rutaceae | Neoraputia Emmerich ex Kallunki | 5/6 | Rainforest | ? | ? | ? | - |
| Sapindaceae | Cardiospermum L. | 6/10 | Rainforest | ? | 23.0 | ? | [49] |
| Sapindaceae | Thinouia Triana and Planch. | 7/11 | Rainforest | ? | ? | ? | - |
| Solanaceae | Petunia Juss. | 10/16 | Grassland | 11.5 | 8.49 | 5.5 | [50] |
| Vochysiaceae | Callisthene Mart. | 8/8 | Rainforest | 30.0 | 22.0 | 14.0 | [51] |
| Species | Voucher (Herbarium Acronym) | ETS | ITS | PHYC |
|---|---|---|---|---|
| Bronwenia cinerascens (Benth.) W.R.Anderson and C.C.Davis | Nee 48, 658 (SP) | KR054586.1 | HQ246821.1 * | HQ246987.1 * |
| Diplopterys pubipetala (A.Juss.) W.R.Anderson and C.C.Davis | Francener 1126 (SP) | KR092986 | HQ246821.1 * | HQ247045.1 * |
| Stigmaphyllon alternifolium A.Juss. | Almeida 501 (SP) | KR054612.1 | - | - |
| Stigmaphyllon angustilobum A.Juss. | Almeida 503 (SP) | KR054591.1 | MT559811 | - |
| Stigmaphyllon arenicola C.E.Anderson | Sebastiani 5 (SP) | KR054589.1 | - | - |
| Stigmaphyllon auriculatum (Cav.) A.Juss. | Almeida 584 (HUEFS) | KR054592.1 | MT559812 | - |
| Stigmaphyllon blanchetii C.E.Anderson | Almeida 525 (SP) | KR054615.1 | MT559813 | - |
| Stigmaphyllon bonariense A.Juss. | Queiroz 13, 530 (HUEFS) | KR054607.1 | - | - |
| Stigmaphyllon caatingicola 1 R.F.Almeida and Amorim | Almeida 577 (HUEFS) | KR054595.1 | MT559814 | - |
| Stigmaphyllon caatingicola 2 R.F.Almeida and Amorim | Almeida 629 (HUEFS) | MT490608 | MT559815 | - |
| Stigmaphyllon cavernulosum C.E.Anderson | Cardoso 2083 (HUEFS) | KR054609.1 | - | - |
| Stigmaphyllon ciliatum (Lam.) A.Juss. | Almeida 541 (SP) | KR054590.1 | - | HQ247151.1 * |
| Stigmaphyllon finlayanum A.Juss. | Pace 457 (SPF) | KR054599.1 | - | HQ247152.1 * |
| Stigmaphyllon gayanum A.Juss. | Almeida 500 (SP) | KR054610.1 | - | - |
| Stigmaphyllon harleyi C.E.Anderson | Santos 378 (HUEFS) | KR054594.1 | MT581513 | - |
| Stigmaphyllon jatrophifolium A.Juss. | Filho s.n. (SP369102) | KR054614.1 | - | - |
| Stigmaphyllon lalandianum A.Juss. | Kollmann 4279 (CEPEC) | KR054596.1 | - | - |
| Stigmaphyllon lindenianum A.Juss. | Aguillar 718 (SP) | KR054603.1 | - | HQ247153.1 * |
| Stigmaphyllon macropodum A.Juss. | Almeida 538 (SP) | KR054602.1 | MT559816 | - |
| Stigmaphyllon paralias A.Juss. | Almeida 509 (SP) | KR054593.1 | KY421909.1 | AF500566.1 * |
| Stigmaphyllon puberulum A.Juss. | Perdiz 732 (HUEFS) | KR054600.1 | - | - |
| Stigmaphyllon salzmannii A.Juss. | Almeida 526 (SP) | KR054616.1 | MT559817 | - |
| Stigmaphyllon saxicola C.E.Anderson | Badini 24,261 (HUEFS) | KR054605.1 | MT559818 | - |
| Stigmaphyllon sinuatum (DC.) A.Juss. | Amorim 3159 (CEPEC) | KR054608.1 | - | - |
| Stigmaphyllon timoriense (DC) C.E.Anderson | Anderson 796 (US) | - | - | AF500545.1 * |
| Stigmaphyllon urenifolium A.Juss. | Guedes 13,932 (HUEFS) | KR054604.1 | - | - |
| Stigmaphyllon vitifolium A.Juss. | DalCol 233 (HUEFS) | KR054598.1 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Almeida, R.F.; van den Berg, C. Biogeography of Stigmaphyllon (Malpighiaceae) and a Meta-Analysis of Vascular Plant Lineages Diversified in the Brazilian Atlantic Rainforests Point to the Late Eocene Origins of This Megadiverse Biome. Plants 2020, 9, 1569. https://doi.org/10.3390/plants9111569
de Almeida RF, van den Berg C. Biogeography of Stigmaphyllon (Malpighiaceae) and a Meta-Analysis of Vascular Plant Lineages Diversified in the Brazilian Atlantic Rainforests Point to the Late Eocene Origins of This Megadiverse Biome. Plants. 2020; 9(11):1569. https://doi.org/10.3390/plants9111569
Chicago/Turabian Stylede Almeida, Rafael Felipe, and Cássio van den Berg. 2020. "Biogeography of Stigmaphyllon (Malpighiaceae) and a Meta-Analysis of Vascular Plant Lineages Diversified in the Brazilian Atlantic Rainforests Point to the Late Eocene Origins of This Megadiverse Biome" Plants 9, no. 11: 1569. https://doi.org/10.3390/plants9111569
APA Stylede Almeida, R. F., & van den Berg, C. (2020). Biogeography of Stigmaphyllon (Malpighiaceae) and a Meta-Analysis of Vascular Plant Lineages Diversified in the Brazilian Atlantic Rainforests Point to the Late Eocene Origins of This Megadiverse Biome. Plants, 9(11), 1569. https://doi.org/10.3390/plants9111569