Antiviral, Antioxidant, and Antihemolytic Effect of Annona muricata L. Leaves Extracts
Abstract
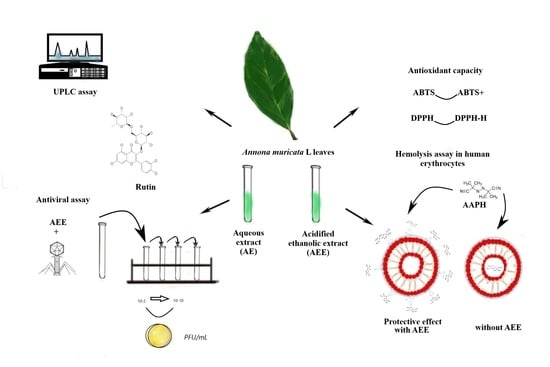
:1. Introduction
2. Results
2.1. UPLC Analysis
2.2. DPPH and ABTS Assays
2.3. Hemolysis Assays in Human Erythrocytes
2.4. Antiviral Assay
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Vegetal Material
4.3. UPLC Analysis
4.4. DPPH Assay
4.5. ABTS Assay
4.6. Hemolysis Assays in Human Erythrocytes
4.7. Bacteriophage Propagation
4.8. Antiviral Assay
4.9. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adewole, S.O.; Ojewole, J.A. Protective effects of Annona muricata Linn. (Annonaceae) leaf aqueous extract on serum lipid profiles and oxidative stress in hepatocytes of streptozotocin-treated diabetic rats. Afr. J. Tradit. Complement Altern. Med. 2008, 6, 30–41. [Google Scholar] [CrossRef] [Green Version]
- Jacobo-Herrera, N.J.; Jacobo-Herrera, F.E.; Zentella-Dehesa, A.; Andrade-Cetto, A.; Heinrich, M.; Pérez-Plasencia, C. Medicinal plants used in Mexican traditional medicine for the treatment of colorectal cancer. J. Ethnopharmacol. 2016, 179, 391–402. [Google Scholar] [CrossRef]
- Pieme, C.A.; Kumar, S.G.; Dongmo, M.S.; Moukette, B.M.; Boyoum, F.F.; Ngogang, J.Y.; Saxena, A.K. Antiproliferative activity and induction of apoptosis by Annona muricata (Annonaceae) extract on human cancer cells. BMC Complement. Altern. Med. 2014, 14, 516. [Google Scholar] [CrossRef] [Green Version]
- León-Fernández, A.E.; Obledo-Vázquez, E.N.; Vivar-Vera, M.D.A.; Sáyago-Ayerdi, S.G.; Montalvo-González, E. Evaluation of emerging methods on the polyphenol content, antioxidant capacity and qualitative presence of acetogenins in soursop pulp (Annona muricata L.). Rev. Bras. Frutic. 2015, 39, E358. [Google Scholar] [CrossRef] [Green Version]
- Biba, V.S.; Amily, A.; Sangeetha, S.; Remani, P. Anticancer, antioxidant and antimicrobial activity of Annonaceae family. World J. Pharm. Pharm. Sci. 2014, 3, 1595–1604. [Google Scholar]
- Coria-Tellez, A.V.; Montalvo-González, E.; Yahia, E.M.; Obledo-Vázquez, E.N. Annona muricata: A comprehensive review on its traditional medicinal uses, phytochemicals, pharmacological activities, mechanisms of action and toxicity. Arab. J. Chem. 2018, 11, 662–691. [Google Scholar] [CrossRef] [Green Version]
- Wahab, N.Z.A.; Ibrahim, N.; Kamarudin, M.K.A.; Lananan, F.; Juahir, H.; Ghazali, A.; Ireana Yusra, A.F. Cytotoxicity and antiviral activity of Annona muricata aqueous leaves extract against dengue virus type 2. J. Fundam. Appl. Sci. 2018, 10, 580–589. [Google Scholar] [CrossRef]
- McMinn, B.R.; Ashbolt, N.J.; Korajkic, A. Bacteriophages as indicators of faecal pollution and enteric virus removal. Lett. Appl. Microbiol. 2017, 65, 11–26. [Google Scholar] [CrossRef] [Green Version]
- Shirasaki, N.; Matsushita, T.; Matsui, Y.; Murai, K. Assessment of the efficacy of membrane filtration processes to remove human enteric viruses and the suitability of bacteriophages and a plant virus as surrogates for those viruses. Water Res. 2017, 115, 29–39. [Google Scholar] [CrossRef]
- Amarillas, L.; López-Cuevas, O.; León-Félix, J.; Castro-del Campo, N.; Gerba, C.P.; Chaidez, C. Genomic analysis of broad-host-range enterobacteriophage Av-05. Genome Announc. 2015, 3, e00282-15. [Google Scholar] [CrossRef] [Green Version]
- López-Cuevas, O.; Castro-del Campo, N.; León-Félix, J.; González-Robles, A.; Chaidez, C. Characterization of bacteriophages with a lytic effect on various Salmonella serotypes and Escherichia coli O157:H7. Can. J. Microbiol. 2011, 57, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.R.; Choi, E.H.; Kim, G.T.; Park, T.S.; Shim, S.M. Bioefficacy of Graviola leaf extracts in scavenging free radicals and upregulating antioxidant genes. Food Funct. 2016, 7, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Calzada, F.; Merlin-Lucas, V.I.; Valdes, M.; Solares-Pascasio, J.I.; Garcia-Hernandez, N.; Pina-Jimenez, E.; Velazquez, C.; Barbosa, E.; Yepez-Mulia, L.; Ordoñez-Razo, R.M. Secondary metabolites and biological properties of Annona muricata. Rev. Bras. Farmacogn. 2020, 30, 305–311. [Google Scholar] [CrossRef]
- Chen, C.J.; Chang, F.R.; Teng, C.M.; Wu, Y.C. Cheritamine, A New N-fatty acyl tryptamine and other constituents from the stems of Annona cherimola. J. Chin. Chem. Soc. 2013, 46, 77–86. [Google Scholar] [CrossRef]
- Yamthe, L.R.T.; Fokou, P.V.T.; Mbouna, C.D.J.; Keumoe, R.; Ndjakou, B.L.; Djouonzo, P.T.; Mfopa, A.N.; Legac, J.; Tsabang, N.; Gut, J.; et al. Extracts from Annona Muricata L. and Annona Reticulata L. (Annonaceae) potently and selectively inhibit Plasmodium falciparum. Medicines 2015, 2, 55–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thang, T.; Hoang, L.; Tuan, N.; Dai, D.; Ogunwande, I.; Hung, N. Analysis of the leaf essential oils of Uvaria grandiflora Roxb. ex Hornem. and Uvaria microcarpa Champ. ex Benth. (Annonaceae) from Vietnam. J. Essent. Oil-Bear. Plants 2017, 20, 496–501. [Google Scholar] [CrossRef]
- Ahmad, F.B.; Nisa, N.K.; Sallehuddin, M.; Assim, Z. Chemical constituents and antiviral study of Goniothalamus velutinus. J. Fundam. Sci. 2010, 6, 72–75. [Google Scholar] [CrossRef] [Green Version]
- Kuskoski, E.M.; Asuero, A.G.; Troncoso, A.M.; Mancini-Filho, J.; Fett, R. Aplicación de diversos métodos químicos para determinar actividad antioxidante en pulpa de frutos. Cienc. Tecnol. Aliment. 2005, 4, 726–732. [Google Scholar] [CrossRef] [Green Version]
- Kingsley, A.C.; Okolie, N.P.; Eze, I.; Anionye, J.C.; Falodun, A. Phytochemical analysis, toxicity profile, and hemomodulatory properties of Annona muricata (Soursop). Egypt J. Haematol. 2017, 42, 36–44. [Google Scholar] [CrossRef]
- Tsai, F.J.; Lin, C.W.; Lai, C.C.; Lan, Y.C.; Lai, C.H.; Hung, C.H.; Hsueh, K.C.; Lin, T.H.; Chang, H.C.; Wan, L. Kaempferol inhibits enterovirus 71 replication and internal ribosome entry site (IRES) activity through FUBP and HNRP proteins. Food Chem. 2011, 128, 312–322. [Google Scholar] [CrossRef]
- Cyboran, S.; Oszmianski, J.; Kleszczynska, H. Interaction between plant polyphenols and the erythrocyte membrane. Cell. Mol. Biol. Lett. 2012, 17, 77–88. [Google Scholar] [CrossRef]
- Soni, S.; Malik, J.K.; Sarankar, S.K.; Soni, H. Rutin as a potent inhibitor of dihydrofolate reductase: A computational design and docking. EAS J. Pharm. Pharmacol. 2019, 1, 130–134. [Google Scholar]
- Yu, J.; Shao, S.; Liu, B.; Wang, Z.; Jiang, Y.-Z.; Li, Y.; Chen, F.; Liu, B. Emergency antiviral drug discovery during a pandemic—A case study on the application of natural compounds to treat COVID-19. ChemRxiv 2020. [Google Scholar] [CrossRef]
- Elmi, A.; Sayem, S.A.J.; Ahmed, M.; Mohamed, F. Natural compounds from Djiboutian medicinal plants as inhibitors of COVID-19 by in silico investigations. ChemRxiv 2020. [CrossRef]
- Ling, X.Y.; Tao, J.L.; Sun, X.; Yuan, B. Exploring material basis and mechanism of Lianhua Qingwen. Prescription against coronavirus based on network pharmacology. Chin. Tradit. Herbal Drugs 2020, 51, 1723–1730. [Google Scholar]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Mohammad, H.F.; et al. Pharmacological effects of gallic acid in health and diseases: A mechanistic Review. Iran J. Basic Med. Sci. 2019, 22, 225–237. [Google Scholar]
- Hinkov, A.; Angelova, P.; Marchev, A.; Hodzhev, J.; Tsvetkov, V.; Dragolova, D.; Todorov, D.; Shishkova, K.; Kapchina-Toteva, V.; Blundell, R.; et al. Nepeta nuda ssp. nuda L. water extract: Inhibition of replication of some strains of Human Alpha herpes virus (genus Simplex virus) in vitro, mode of action and NMR-based metabolomics. J. Herb. Med. 2020, 21, 100334. [Google Scholar] [CrossRef]
- Lane, T.; Anantpadma, M.; Freundlich, J.S.; Davey, R.A.; Madrid, P.B.; Ekins, S. The natural product eugenol is an inhibitor of the Ebola virus in vitro. Pharm. Res. 2019, 36, 104. [Google Scholar] [CrossRef]
- Astirin, O.P.; Artanti, A.N.; Fitria, M.S.; Perwitasari, E.A.; Prayitno, A. Annona muricata linn leaf induce apoptosis in cancer cause virus. J. Cancer Ther. 2013, 4, 1244–1250. [Google Scholar] [CrossRef] [Green Version]
- Chan, W.-J.J.; McLachlan, A.J.; Hanrahan, J.R.; Harnett, J.E. The safety and tolerability of Annona muricata leaf extract: A systematic review. J. Pharm. Pharmacol. 2020, 72, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Ruiz, K.L.; Ruiz-Cruz, S.; Cira-Chávez, L.A.; Gassos-Ortega, L.E.; Ornelas-Paz, J.J.; Del-Toro-Sánchez, C.L.; Márquez-Ríos, E.; López-Mata, M.A.; Rodríguez-Félix, F. Evaluation of antioxidant capacity, protective effect on human erythrocytes and phenolic compound identification in two varieties of plum fruit (Spondias spp.) by UPLC-MS. Molecules 2018, 23, 3200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moein, S.; Moein, M.R. Relationship between antioxidant properties and phenolics in Zhumeria majdae. J. Med. Plants Res. 2010, 7, 517–521. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 9, 1231–1237. [Google Scholar] [CrossRef]
- Lu, J.; Jin, Y.; Liu, G.; Zhu, N.; Gui, M.; Yu, A.; Li, X. Flavonoids from the leaves of Actinidia Kolomikta. Chem. Nat. Compd. 2010, 2, 205–208. [Google Scholar] [CrossRef]
- Goodridge, L.; Gallaccio, A.; Griffiths, M.W. Morphological, host range, and genetic characterization of two coliphages. Appl. Environ. Microbiol. 2003, 9, 5364–5371. [Google Scholar] [CrossRef] [Green Version]


| Phenolic Compounds | mg/g | ||
|---|---|---|---|
| AE | AEE | ||
| 1 | Gallic acid (3,4,5-trihydroxybenzoic acid) | ND | 3.13 ± 0.14 |
| 2 | Rutin (quercetin 3-O-rutinoside) | 1.20 ± 0.06 | 6.52 ± 0.59 |
| 3 | Naringenin (5,7-dihydroxy-(2-4-hydroxyphenyl) chroman-4-one) | ND | 5.22 ± 0.75 |
| 4 | Vanillin (4-hydroxy-3-methoxybenzaldehyde) | ND | 3.60 ± 0.17 |
| 5 | Eugenol (4-allyl-2-methoxyphenol) | ND | 1.40 ± 0.04 |
| Extracts | DPPH (mmol TE/g) | ABTS (mmol TE/g) |
|---|---|---|
| AE | 2.97 ± 0.40 a | 17.93 ± 0.31 b |
| AEE | 23.61 ± 0.42 b | 24.91 ± 0.16 b |
| Extracts | Antihemolytic Effect (%) |
|---|---|
| AE | 34.16 ± 0.13 a |
| AEE | 51.21 ± 0.36 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balderrama-Carmona, A.P.; Silva-Beltrán, N.P.; Gálvez-Ruiz, J.-C.; Ruíz-Cruz, S.; Chaidez-Quiroz, C.; Morán-Palacio, E.F. Antiviral, Antioxidant, and Antihemolytic Effect of Annona muricata L. Leaves Extracts. Plants 2020, 9, 1650. https://doi.org/10.3390/plants9121650
Balderrama-Carmona AP, Silva-Beltrán NP, Gálvez-Ruiz J-C, Ruíz-Cruz S, Chaidez-Quiroz C, Morán-Palacio EF. Antiviral, Antioxidant, and Antihemolytic Effect of Annona muricata L. Leaves Extracts. Plants. 2020; 9(12):1650. https://doi.org/10.3390/plants9121650
Chicago/Turabian StyleBalderrama-Carmona, Ana Paola, Norma Patricia Silva-Beltrán, Juan-Carlos Gálvez-Ruiz, Saúl Ruíz-Cruz, Cristóbal Chaidez-Quiroz, and Edgar Felipe Morán-Palacio. 2020. "Antiviral, Antioxidant, and Antihemolytic Effect of Annona muricata L. Leaves Extracts" Plants 9, no. 12: 1650. https://doi.org/10.3390/plants9121650
APA StyleBalderrama-Carmona, A. P., Silva-Beltrán, N. P., Gálvez-Ruiz, J. -C., Ruíz-Cruz, S., Chaidez-Quiroz, C., & Morán-Palacio, E. F. (2020). Antiviral, Antioxidant, and Antihemolytic Effect of Annona muricata L. Leaves Extracts. Plants, 9(12), 1650. https://doi.org/10.3390/plants9121650