Effect of Mesos Components (MgSO4, CaCl2, KH2PO4) on In Vitro Shoot Growth of Blackberry, Blueberry, and Saskatoon
Abstract
:1. Introduction
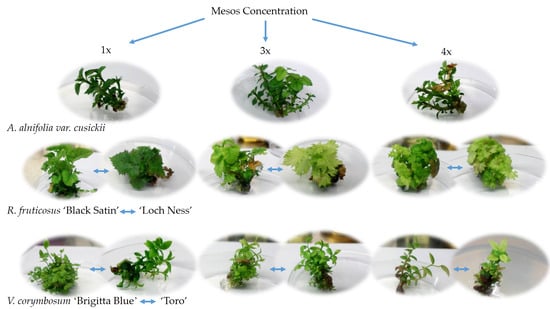
2. Results
Effect of Cultivar, Mesos Concentration, and Subcultivation on Shoot Number and Length in Studied Cultivars
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Shoot Initiation and Multiplication
4.3. Experimental Design
4.4. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Debnath, S.C. Micropropagation of small fruits. In Micropropagation of Woody Trees and Fruits; Jain, S.M., Ishii, K., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; pp. 465–506. [Google Scholar]
- Debnath, S.C. Corrigendum: Bioreactors and molecular analysis in berry crop micropropagation—A review. Can. J. Plant. Sci. 2016, 96, 382–383. [Google Scholar] [CrossRef] [Green Version]
- Mazur, W.M.; Uehara, M.; Wahala, K.; Adlercreutz, H. Phyto-oestrogen content of berries, and plasma concentrations and urinary excretion of enterolactone after a single strawberry-meal in human subjects. Br. J. Nutr. 2000, 83, 381–387. [Google Scholar] [PubMed]
- Rodriguez-Mateos, A.; Heiss, C.; Borges, G.; Crozier, A. Berry(poly) phenols and cardiovascular health. J. Agric. Food Chem. 2014, 62, 3842–3851. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.C.; Goyali, J.C. In vitro propagation and variation of Antioxidant properties in micropropagated Vaccinium berry plants—A review. Molecules 2020, 25, 788. [Google Scholar] [CrossRef] [Green Version]
- Ramage, C.M.; Williams, R.R. Mineral nutrition and plant morphogenesis. In Vitro Cell. Dev. Biol. Plant 2002, 38, 116–124. [Google Scholar] [CrossRef]
- Greenway, M.B.; Isaac, C.P.; Meagan, N.L.; John, F.H.; Gregory, C.P. A nutrient medium for diverse applications and tissue growth of plant species in vitro. In Vitro Cell. Dev. Biol. Plant 2012, 48, 403–410. [Google Scholar] [CrossRef]
- Diengngan, S.; Murthy, B.N.S. Influence of plant growth promoting substances in micropropagation of strawberry cv. Festival. Bioscan 2014, 9, 1491–1493. [Google Scholar]
- Capocasa, F.; Balducci, F.; Marcellini, M.; Bernardini, D.; Navacchi, O.; Mezzetti, B. Comparing nursery behavior, field plant yield and fruit quality of in vitro and in vivo propagated strawberry mother plants. Plant Cell Tissue Organ. Cult. 2019, 136, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, M.V.; Lopez, M.; Valdes, A.E.; Ordas, R.J. Micropropagation of three berry fruit species using nodal segments from field-grown plants. Ann. Appl. Biol. 2000, 137, 73–78. [Google Scholar] [CrossRef]
- Ružić, D.; Lazić, T. Micropropagation as means of rapid multiplication of newly developed blackberry and black currant cultivars. Agric. Conspec. 2006, 71, 149–153. [Google Scholar]
- Pruski, K.; Nowak, J.; Grainier, G. Micropropagation of four cultivars of Saskatoon berry (Amelanchier alnifolia Nutt.). Plant Cell Tissue Organ. Cult. 1990, 21, 103–109. [Google Scholar] [CrossRef]
- Sedlák, J.; Paprštein, F. Micropropagation of edible honeysuckle. Vědecké Práce Ovocnářské 2013, 23, 157–163. (In Czech) [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Poothong, S.; Reed, B.M. Increased CaCl2, MgSO4 and KH2PO4 improve the growth of micropropagated red raspberries. In Vitro Cell. Dev. Biol. Plant 2015, 51, 648–658. [Google Scholar] [CrossRef]
- Ivanova, M.; Van Staden, J. Nitrogen source, concentration, and NH4+:NO3− ratio influence shoot regeneration and hyperhydricity in tissue cultured Aloe polyphylla. Plant Cell Tissue Organ. Cult. 2009, 99, 167–174. [Google Scholar] [CrossRef]
- Fira, A.; Clapa, D.; Cristea, V.; Plopa, C. In vitro propagation of Lonicera kamtschatica. Agriculture 2014, 23, 90–99. [Google Scholar]
- Reed, B.M.; Wada, S.; DeNoma, J.; Niedz, R.P. Improving in vitro mineral nutrition for diverse pear germplasm. In Vitro Cell. Dev. Biol. Plant 2013, 49, 343–355. [Google Scholar] [CrossRef]
- Halloran, S.M.; Adelberg, J. A macronutrient optimization platform for micropropagation and acclimatization: Using turmeric (Curcuma longa L.) as a model plant. In Vitro Cell. Dev. Biol. Plant 2011, 47, 257–273. [Google Scholar] [CrossRef]
- Alanagh, E.N.; Garoosi, G.A.; Haddad, R.; Maleki, S.; Landín, M.; Gallego, P.P. Design of tissue culture media for efficient Prunus rootstock micropropagation using artificial intelligence models. Plant Cell Tissue Organ. Cult. 2014, 117, 349–359. [Google Scholar] [CrossRef]
- Poothong, S.; Reed, B.M. Optimizing shoot culture media for Rubus germplasm: The effects of NH4+,NO3−, and total nitrogen. In Vitro Cell. Dev. Biol. Plant 2016, 52, 265–275. [Google Scholar] [CrossRef]
- De Carvalho, A.A.; Bertolucci, S.K.V.; da Silva, G.M.; da Cunha, S.H.B.; Roza, H.L.H.; Aazza, S.; Pinto, J.E.B.P. Mesos components (CaCl2, MgSO4, KH2PO4) induced changes in growth and ascaridole content of Dysphania ambrosioides L. in vitro. Ind. Crop. Prod. 2018, 122, 28–36. [Google Scholar] [CrossRef]
- Dunstan, D.I.; Short, K.C. Improved growth of tissue cultures of the onion, Allium cepa. Physiol. Plant. 1977, 41, 70–72. [Google Scholar] [CrossRef]
- Hand, C.; Maki, S.; Reed, B.M. Modeling optimal mineral nutrition for hazelnut micropropagation. Plant Cell Tissue Organ. Cult. 2014, 119, 411–425. [Google Scholar] [CrossRef]
- Driver, J.A.; Kuniyuki, A.H. In vitro propagation of Paradox walnut rootstock. Hortscience: A Publ. Am. Soc. Hortic. Sci. 1984, 19, 507–509. [Google Scholar]
- Kovalchuk, I.Y.; Mukhitdinova, Z.; Turdiyev, T.; Madiyeva, G.; Akin, M.; Eyduran, E.; Reed, B.M. Modeling some mineral nutrient requirements for micropropagated wild apricot shoot cultures. Plant Cell Tissue Organ. Cult. 2017, 129, 325–335. [Google Scholar] [CrossRef]
- Lloyd, G.; McCown, B. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Comb. Proc. Int. Plant Propagators’ Soc. 1980, 30, 421–427. [Google Scholar]
- Hepler, P.K. Calcium: A central regulator of plant growth and development. Plant Cell 2005, 17, 2142–2155. [Google Scholar] [CrossRef]
- Zawadzka, M.; Orlikowska, T. Factors modifying regeneration in vitro of adventitious shoots in five red raspberry cultivars. J. Fruit Ornam. Plant Res. 2006, 14, 105–115. [Google Scholar]
- Poothong, S.; Reed, B.M. Modeling the effects of mineral nutrition for improving growth and development of micropropagated red raspberries. Sci. Hortic. 2014, 165, 132–141. [Google Scholar] [CrossRef]
- Poothong, S.; Morré, J.; Maier, C.S.; Reed, B.M. Metabolic changes and improved growth in micropropagated red raspberry “Indian summer” are tied to improved mineral nutrition. In Vitro Cell. Dev. Biol. Plant 2017, 53, 579–590. [Google Scholar] [CrossRef]
- Wada, S.; Maki, S.; Niedz, R.P.; Reed, B.M. Screening genetically diverse pear species for in vitro CaCl2, MgSO4 and KH2PO4. Acta Physiol. Plant. 2015, 37, 63. [Google Scholar] [CrossRef]
- Gajdošová, A.; Ostrolucká, M.G.; Libiaková, G.; Ondrušková, E.; Šimala, D. Microclonal propagation of Vaccinium sp. and Rubus sp. and detection of genetic variability in culture in vitro. J. Fruit Ornam. Plant Res. 2006, 14, 103–119. [Google Scholar]
- Hunková, J.; Libiaková, G.; Gajdošová, A. Shoot proliferation ability of selected cultivars of Rubus spp. as influenced by genotype and cytokinin concentration. J. Cent. Eur. Agric. 2016, 17, 379–390. [Google Scholar] [CrossRef] [Green Version]
- Hunková, J.; Libiaková, G.; Fejér, J.; Gajdošová, A. Improved Amelanchier alnifolia Nutt. Ex. M. Roem. shoot proliferation by manipulating iron source. Propag. Ornam. Plants 2017, 17, 103–107. [Google Scholar]

| Effect | A. alnifolia | R. fruticosus | V. corymbosum | ||||||
|---|---|---|---|---|---|---|---|---|---|
| df | F | p | df | F | p | df | F | p | |
| cultivar | - | 1 | 44.0736 | 0.000000 | 1 | 58.484 | 0.000000 | ||
| mesos | 4 | 5.1395 | 0.000523 | 4 | 2.1595 | 0.072593 | 3 | 13.000 | 0.000000 |
| subcultivation | 1 | 0.0621 | 0.803403 | 1 | 36.4831 | 0.000000 | 1 | 7.919 | 0.005107 |
| cultivar × mesos | - | 4 | 3.3075 | 0.010924 | 3 | 9.332 | 0.000005 | ||
| cultivar × subcult. | - | 1 | 7.2468 | 0.007360 | 1 | 0.994 | 0.319241 | ||
| mesos × subcultivation | 4 | 7.7551 | 0.000006 | 4 | 2.9868 | 0.018731 | 3 | 12.761 | 0.000000 |
| cultivar × mesos×subcultivation | - | 4 | 4.5503 | 0.001296 | 3 | 10.636 | 0.000001 | ||
| Error | 275 | 464 | 447 | ||||||
| Effect | A. alnifolia | R. fruticosus | V. corymbosum | ||||||
|---|---|---|---|---|---|---|---|---|---|
| df | F | p | df | F | p | df | F | p | |
| cultivar | - | 1 | 221.390 | 0.000000 | 1 | 27.38 | 0.000000 | ||
| mesos | 4 | 24.374 | 0.000000 | 4 | 10.415 | 0.000000 | 3 | 28.36 | 0.000000 |
| subcultivation | 1 | 19.817 | 0.000009 | 1 | 20.028 | 0.000008 | 1 | 6.40 | 0.011443 |
| cultivar × mesos | - | 4 | 18.556 | 0.000000 | 3 | 10.14 | 0.000001 | ||
| cultivar × subcult. | - | 1 | 20.815 | 0.000005 | 1 | 10.27 | 0.001363 | ||
| mesos × subcult. | 4 | 17.862 | 0.000000 | 4 | 9.399 | 0.000000 | 3 | 18.98 | 0.000000 |
| cultivar × mesos×subcultivation | - | 4 | 4.468 | 0.001363 | 3 | 1.14 | 0.329475 | ||
| Error | 1913 | 1525 | 3146 | ||||||
| Species | Cultivar | Subcultivation | Number of Shoots (±SD) | Length of Shoots in cm (±SD) |
|---|---|---|---|---|
| Mean | Mean | |||
| R. fruticosus | ‘Loch Ness’ | 1 | 2.07 ± 1.17c | 0.93 ± 0.49c |
| 2 | 2.78 ± 2.12b | 0.98 ± 0.51c | ||
| ‘Black Satin’ | 1 | 2.89 ± 2.06b | 1.51 ± 0.59a | |
| 2 | 4.49 ± 2.75a | 1.23 ± 0.48b | ||
| V. corymbosum | ‘Toro’ | 1 | 4.83 ± 2.46c | 1.15 ± 0.65a |
| 2 | 6.19 ± 2.93b | 1.04 ± 0.52b | ||
| ‘Brigitta Blue’ | 1 | 7.74 ± 5.40a | 0.94 ± 0.57c | |
| 2 | 8.53 ± 5.62a | 0.99 ± 0.51bc | ||
| A. alnifolia var. cusickii | 1 | 6.60 ± 4.91a | 1.62 ± 0.70a | |
| 2 | 6.90 ± 5.36a | 1.68 ± 0.68a |
| Species | Cultivar | Mesos Concentration | Mean Number of Shoots (±SD) | Mean Length of Shoots (±SD) |
|---|---|---|---|---|
| A. alnifolia var. cusickii | 0.5× | 7.66 ± 4.56ab | 1.61 ± 0.61b | |
| 1× | 6.07 ± 5.37bc | 1.59 ± 0.64b | ||
| 2× | 6.10 ± 3.96bc | 1.47 ± 0.64c | ||
| 3× | 8.64 ± 5.77a | 1.84 ± 0.74a | ||
| 4× | 5.02 ± 5.22c | 1.69 ± 0.75b | ||
| R. fruticosus | ‘Black Satin’ | 0.5× | 3.02 ± 1.58bc | 1.41 ± 0.58a |
| 1× | 3.77 ± 2.22ab | 1.35 ± 0.59a | ||
| 2× | 3.93 ± 2.48ab | 1.31 ± 0.52ab | ||
| 3× | 4.55 ± 3.65a | 1.21 ± 0.40bc | ||
| 4× | 3.34 ± 2.40b | 1.43 ± 0.61a | ||
| R. fruticosus | ‘Loch Ness’ | 0.5× | 2.26 ± 1.34c | 0.62 ± 0.29f |
| 1× | 2.32 ± 1.58c | 0.83 ± 0.42e | ||
| 2× | 2.30 ± 2.20c | 1.14 ± 0.54c | ||
| 3× | 2.41 ± 1.40c | 0.96 ± 0.47d | ||
| 4× | 3.04 ± 2.24bc | 1.18 ± 0.50c | ||
| V. corymbosum | ‘Brigitta Blue’ | 1× | 9.32 ± 5.90ab | 0.93 ± 0.45c |
| 2× | 10.07 ± 5.39a | 1.01 ± 0.48bc | ||
| 3× | 8.43 ± 5.81b | 0.82 ± 0.47d | ||
| 4× | 4.81 ± 3.06c | 1.20 ± 0.76a | ||
| V. corymbosum | ‘Toro’ | 1× | 5.61 ± 2.65c | 1.14 ± 0.56a |
| 2× | 5.54 ± 2.65c | 1.00 ± 0.54bc | ||
| 3× | 5.82 ± 3.11c | 1.04 ± 0.53b | ||
| 4× | 5.17 ± 2.74c | 1.18 ± 0.66a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hunková, J.; Gajdošová, A.; Szabóová, M. Effect of Mesos Components (MgSO4, CaCl2, KH2PO4) on In Vitro Shoot Growth of Blackberry, Blueberry, and Saskatoon. Plants 2020, 9, 935. https://doi.org/10.3390/plants9080935
Hunková J, Gajdošová A, Szabóová M. Effect of Mesos Components (MgSO4, CaCl2, KH2PO4) on In Vitro Shoot Growth of Blackberry, Blueberry, and Saskatoon. Plants. 2020; 9(8):935. https://doi.org/10.3390/plants9080935
Chicago/Turabian StyleHunková, Júlia, Alena Gajdošová, and Monika Szabóová. 2020. "Effect of Mesos Components (MgSO4, CaCl2, KH2PO4) on In Vitro Shoot Growth of Blackberry, Blueberry, and Saskatoon" Plants 9, no. 8: 935. https://doi.org/10.3390/plants9080935
APA StyleHunková, J., Gajdošová, A., & Szabóová, M. (2020). Effect of Mesos Components (MgSO4, CaCl2, KH2PO4) on In Vitro Shoot Growth of Blackberry, Blueberry, and Saskatoon. Plants, 9(8), 935. https://doi.org/10.3390/plants9080935