Grapevine and Ozone: Uptake and Effects
Abstract
:1. Introduction
2. Materials and Methods
3. Results
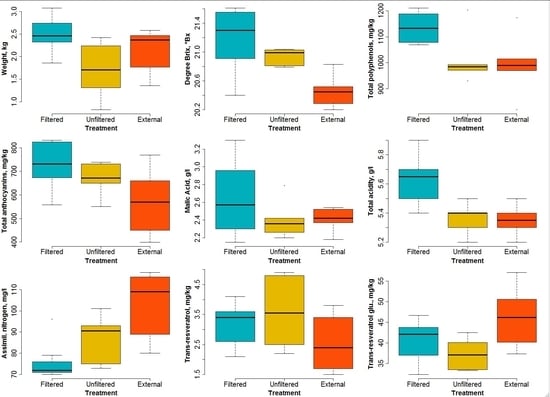
- Grape weight per bunch. There is a significant difference between the samples obtained from the filtered OTCs and from the other two treatments (p = 0.0213). However, the p value corresponding to the unfiltered versus external couple is too high (0.22) to imply any significance. Notwithstanding the difference in ozone levels between the unfiltered and external treatments, little difference in the bunch weights is detectable. This is probably due to an effect of the open top chambers in which the physical conditions were not the same as in the open air (i.e., for the external samples). However, significant differences between the weight of the bunches enclosed in the filtered and unfiltered OTCs are clearly visible (see Figure 8), with p = 0.0166. This result shows that higher ozone levels cause a reduction in yields, at least in the case of our experiment.
- Degrees Brix. This variable expresses the sugar content of the must and is widely used in the wine production industry. A rather important effect of ozone levels appears (see Figure 8), especially between samples enclosed in filtered OTCs and external samples (p = 0.0022). The difference is less clear between filtered and unfiltered samples.
- Polyphenols. Here, no clear difference appears between unfiltered and external samples, but the samples enclosed in filtered OTCs are characterized by a much higher content of polyphenols (p = 0.045). These substances are known as antioxidants and have health effects on digestion, for example. The results presented here indicate that high ozone content in air can counterbalance the health effects of polyphenols present in wine.
- Anthocyanins. These pigments present in grapes show a significant difference (p = 0.046) between plants enclosed in filtered OTCs and external ones.
- Malic acid. No significant difference was found between the three treatments due to the overlap of variability ranges for the different treatments. All p values lie above 0.2.
- Titrable acidity. Differences appear rather clearly between plants enclosed in filtered and unfiltered OTCs (p = 0.0086) and between those enclosed in filtered OTCs and under external conditions (p = 0.0055).
- Assimilable nitrogen. Here, an opposite pattern with respect to most other variables can be seen: higher ozone levels result in higher assimilable nitrogen. The variance analysis for all three treatments combined shows good significance (p = 0.0032); the highest significance appears between the external plants and those enclosed in filtered OTCs (p = 0.00067).
- Trans-resveratrol. As for malic acid, no significant difference was found between the three treatments. All p values lie above 0.1.
- Trans-resveratrol glucoside. A significant difference (p = 0.033) appears between external plants and those enclosed in unfiltered OTCs. However, for this variable, no conclusion can be drawn about the effect of ozone between filtered and unfiltered OTCs.
4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Popescu, S.M. The combined effects of CO2 and O3 on the physiology of the grapevine Vitis vinifera L. cv. Merlot). J. Hortic. For. Biotechnol. 2011, 15, 62–66. [Google Scholar]
- Richards, B.L.; Middleton, J.T.; Hewitt, W.B. Ozone stipple of grape leaf lesions on the upper leaf surfaces and premature leaf fall occur on grapevines in areas polluted by air-borne ozone. Calif. Agric. 1959, 13, 4–11. [Google Scholar]
- Roper, T.R.; Williams, L.E. Effects of ambient and acute partial pressures of ozone on leaf net CO2 assimilation of field-grown Vitis vinifera L. Plant Physiol. 1989, 91, 1501–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shertz, R.D.; Kender, W.D.; Musselman, R.D. Effects of ozone and sulfur dioxide on grapevines. Sci. Hortic. 1980, 13, 37–45. [Google Scholar] [CrossRef]
- Soja, G.; Eid, M.; Gangl, H.; Redl, H. Ozone sensitivity of grapevine (Vitis vinifera L.): Evidence for a memory effect in a perennial crop plant. Phyton 1997, 37, 265–270. [Google Scholar]
- Fumagalli, I.; Gimeno, B.S.; Velissariou, D. Evidence of ozone-induced adverse effects on crops in the Mediterranean region. Atmos. Environ. 2001, 35, 2583–2587. [Google Scholar] [CrossRef]
- Velissariou, D.; Gimeno, B.S.; Badiani, M.; Fumagalli, I.; Davison, A.W. Records of O3 visible injury in the ECE Mediterranean region. In Critical Levels for Ozone in Europe: Testing and Finalising the Concept; Karelampi, L., Skarby, L., Eds.; UN-ECE Workshop Report; University of Kuopio: Kuopio, Finland, 1996; pp. 343–350. [Google Scholar]
- Fuhrer, J.; Egger, A.; Lehnherr, B.; Grandjean, A.; Tschannen, W. Effects of ozone on the yield of spring wheat grown in open-top field chambers. Environ. Pollut. 1989, 60, 273–289. [Google Scholar] [CrossRef]
- Oksanen, E.; Freiwald, V.; Prozherina, N.; Rousi, M. Photosynthesis of birch is sensitive to springtime frost and ozone. Can. J. For. Res. 2005, 35, 703–712. [Google Scholar] [CrossRef]
- Soja, G.; Reichenauer, T.G.; Eid, M.; Soja, A.M.; Schaber, R.; Gangl, H. Long-term ozone exposure and ozone uptake of grapevines in open-top chambers. Atmos. Environ. 2004, 38, 2313–2321. [Google Scholar] [CrossRef]
- Lea, M.C. On the influence of ozone and some other chemical agents on germination and vegetation. Am. J. Sci. Arts 1864, 37, 373–376. [Google Scholar] [CrossRef]
- Rich, S.; Waggoner, P.E.; Tomlinson, H. Ozone uptake by bean leaves. Science 1970, 169, 79. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Waggoner, P.E.; Rich, S. Removal of ozone from the atmosphere by soil and vegetation. Nature 1974, 250, 486. [Google Scholar] [CrossRef]
- Ashmore, M.R. Assessing the future global impacts of ozone on vegetation. Plant Cell Environ. 2005, 28, 949–964. [Google Scholar] [CrossRef]
- Krupa, S.V.; Manning, W.J. Atmospheric ozone: Formation and effects on vegetation. Environ. Pollut. 1988, 50, 101–137. [Google Scholar] [CrossRef]
- Grimes, H.D.; Perkins, K.K.; Boss, W.F. Ozone degrades into hydroxyl radical under physiological conditions. Plant Physiol. 1983, 72, 1016–1020. [Google Scholar] [CrossRef] [Green Version]
- Pryor, W.A.; Church, D.F. Aldehydes, hydrogen peroxide and organic radicals, as mediators of ozone toxicity. Free Rad. Biol. Med. 1991, 11, 41–46. [Google Scholar] [CrossRef]
- Byvoet, P.; Balis, J.U.; Shelley, S.A.; Montgomery, M.R.; Barrber, M.J. Detection of hydroxyl radicals upon interaction of ozone with aqueous media of extracellular surfactants. Arch. Biochem. Biophys. 1995, 319, 464–469. [Google Scholar] [CrossRef]
- Pell, E.J.; Schangnhaufer, C.D.; Arteca, R.N. Ozone-induced oxidative stress: Mechanisms of action and reaction. Physiol. Plant. 1997, 100, 264–273. [Google Scholar] [CrossRef]
- Anjum, N.A.; Umar, S.; Chan, M.T. (Eds.) Ascorbate-Glutathione Pathway and Stress Tolerance in Plants; Springer: Dordrecht, The Netherlands, 2010; ISBN 978-90-481-9403-2. [Google Scholar]
- Tai, A.P.K.; San Martin, M. Impacts of ozone pollution and temperature extremes on crop yields: Spatial variability, adaptation and implications for future food security. Atmos. Environ. 2017, 169, 11–21. [Google Scholar] [CrossRef]
- Van Dingenen, R.; Dentener, F.J.; Raes, F.; Krol, M.C.; Emberson, L.; Cofala, J. The global impact of ozone on agricultural crop yields under current and future air quality legislation. Atmos. Environ. 2009, 43, 604–618. [Google Scholar] [CrossRef]
- Sitch, S.; Cox, P.M.; Collins, W.J.; Huntingford, C. Indirect forcing of climate change through ozone effects on the land-carbon sink. Nature 2007, 448, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Cieslik, S.; Tuovinen, J.P.; Baumgarten, M.; Matyssek, R.; Brito, P.; Wieser, G. Gaseous exchange between forests and the atmosphere. In Climate Change, Air Pollution and Global Challenges; Matyssek, R., Clarke, N., Cudlin, P., Mikkelsen, T.N., Tuovinen, J.P., Wieser, G., Paoletti, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; part 2. [Google Scholar]
- Franz, M.; Alonso, R.; Arneth, A.; Zaehle, S. Evaluation of simulated ozone effects in forest ecosystems against biomass damage estimates from fumigation experiments. Biogeosciences 2018, 15, 6941–6957. [Google Scholar] [CrossRef] [Green Version]
- Mills, G.; Pleijel, H.; Malley, C.S.; Sinha, B.; Cooper, O.R.; Scholtz, C.S.; Neufeld, H.S.; Simpson, D.; Sharops, K.; Feng, Z.; et al. Tropospheric ozone assessment report: Present-day tropospheric ozone distribution and trends relevant to vegetation. Elementa 2018, 6, 47. [Google Scholar] [CrossRef]
- Niinemets, Ü. Responses, of forest trees to single and multiple environmental stresses from seedlings to mature plants. For. Ecol. Manag. 2010, 260, 1623–1639. [Google Scholar] [CrossRef]
- Directive 2008/50/EC of the European Parliament and of the Council. Off. J. Eur. Communities 2008, 11, 2008.
- Grünhage, L.; Jäger, H.J. From critical levels to critical loads for ozone: A discussion of a new experimental and modelling approach for establishing flux-response relationships for agricultural crops and native plant species. Environ. Pollut. 2003, 125, 99–110. [Google Scholar] [CrossRef]
- Kärenlampi, L.; Skärby, L. (Eds.) Critical Levels for Ozone in Europe: Testing and Finalizing the Concepts; UN-ECE Workshop Report; University of Kuopio: Kuopio, Finland, 1996. [Google Scholar]
- Paoletti, E.; Manning, W.J. Toward a biologically significant and usable standard for ozone that will also protect plants. Environ. Pollut. 2007, 150, 85–95. [Google Scholar] [CrossRef]
- Emberson, L.D.; Wieser, G.; Ashmore, M.R. Modelling of stomatal conductance and ozone flux of Norway spruce: Comparison with field data. Environ. Pollut. 2000, 109, 393–402. [Google Scholar] [CrossRef]
- Emberson, L.D.; Ashmore, M.R.; Cambridge, H.M.; Simpson, D.; Tuovinen, J.P. Modelling of stomatal ozone flux across Europe. Environ. Pollut. 2000, 109, 403–413. [Google Scholar] [CrossRef]
- Musselman, R.C.; Lefohn, A.S.; Massman, W.J.; Heath, R.L. A critical review and analysis of the use of exposure- and flux-based ozone indices for predicting vegetation effects. Atmos. Environ. 2006, 40, 1869–1888. [Google Scholar] [CrossRef]
- Alebić-Juretić, A.; Bokan-Vocelić, I.; Mifka, B.; Zatezalo, M.; Zubak, V. Impact of ozone gradient on grapevine leaves. In Proceedings of the 19th EGU General Assembly, Vienna, Austria, 23–28 April 2017; p. 5660. [Google Scholar]
- Xiao, F.; Yang, Z.Q.; Lee, K.W. Photosynthetic and physiological responses to high temperature in grapevine (Vitis vinifera L.) leaves during the seedling stage. J. Hortic. Sci. Biotechnol. 2017, 92, 2–10. [Google Scholar] [CrossRef]
- Valletta, A.; Salvatori, E.; Santamaria, A.R.; Nicoletti, M.; Toniolo, C.; Caboni, E.; Bernardini, A.; Pasqua, G.; Manes, F. Ecophysiological and phytochemical response to ozone of wine grape cultivars of Vitis vinifera L. Nat. Prod. Res. 2016, 30, 2514–2522. [Google Scholar] [CrossRef] [PubMed]
- Mattivi, F. La stima della maturazione fenolica delle uve rosse con un nuovo metodo rapido. ENOLOGO-MILANO- 2007, 43, 89. [Google Scholar]
- Gatto, P.; Vrhovsek, U.; Muth, J.; Segala, C.; Romualdi, C.; Fontana, P.; Pruefer, D.; Stefanini, M.; Moser, C.; Mattivi, F.; et al. Ripening and genotype controlled stilbene accumulation in healthy grapes. J. Agric. Food Chem. 2008, 56, 11773–11785. [Google Scholar] [CrossRef] [PubMed]








| AOT40 (ppb.h) | POD0 (mmol.m−2) | |
|---|---|---|
| External | 12,300 | 38.1 |
| Unfiltered OTC | 6100 | 32.0 |
| Filtered OTC | 2000 | 21.4 |
| External | Filtered OTC | Unfiltered OTC | |
|---|---|---|---|
| Grape weight per bunch (kg) | 2.14 ± 0.45 | 2.50 ± 0.37 | 1.72 ± 0.54 |
| Degrees Brix (°Bx) | 20.62 ± 0.35 | 21.18 ± 0.42 | 20.94 ± 0.11 |
| pH | 3.32 ± 0.0 | 3.32 ± 0.1 | 3.35 ± 0.0 |
| Titrable acidity (as tartaric acid, g/L) | 5.35 ± 0.1 | 5.56 ± 0.2 | 5.37 ± 0.1 |
| Density at 20 °C | 1.090 ± 0.0 | 1.092 ± 0.0 | 1.091 ± 0.0 |
| Tartaric acid (g/L) | 6.25 ± 0.1 | 6.19 ± 0.3 | 6.15 ± 0.1 |
| Malic acid (g/L) | 2.41 ± 0.12 | 2.65 ± 0.40 | 2.40 ± 0.10 |
| Potassium (mg/L) | 1705 ± 27.9 | 1745 ± 69.8 | 1689 ± 37.2 |
| Assimilable nitrogen (mg/L) | 103.5 ± 14.3 | 76.0 ± 8.6 | 87.1 ± 10.0 |
| Total anthocyanins (mg/kg) | 569.3 ± 131.3 | 731.0 ± 98.8 | 668.7 ± 62.8 |
| Total polyphenols (mg/kg) | 992 ± 103 | 1141 ± 53 | 972 ± 22 |
| Trans-resveratrol (mg/kg) | 2.5 ± 0.8 | 3.1 ± 0.7 | 3.6 ± 1.1 |
| Cis-resveratrol glucoside (mg/kg) | <0.1 | <0.1 | <0.1 |
| Trans-resveratrol glucoside (mg/kg) | 46.3 ± 6.7 | 40.4 ± 0.7 | 37.3 ± 3.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fumagalli, I.; Cieslik, S.; De Marco, A.; Proietti, C.; Paoletti, E. Grapevine and Ozone: Uptake and Effects. Climate 2019, 7, 140. https://doi.org/10.3390/cli7120140
Fumagalli I, Cieslik S, De Marco A, Proietti C, Paoletti E. Grapevine and Ozone: Uptake and Effects. Climate. 2019; 7(12):140. https://doi.org/10.3390/cli7120140
Chicago/Turabian StyleFumagalli, Ivano, Stanislaw Cieslik, Alessandra De Marco, Chiara Proietti, and Elena Paoletti. 2019. "Grapevine and Ozone: Uptake and Effects" Climate 7, no. 12: 140. https://doi.org/10.3390/cli7120140
APA StyleFumagalli, I., Cieslik, S., De Marco, A., Proietti, C., & Paoletti, E. (2019). Grapevine and Ozone: Uptake and Effects. Climate, 7(12), 140. https://doi.org/10.3390/cli7120140