Expected Impacts of Mixing European Beech with Silver Fir on Regional Air Quality and Radiation Balance
Abstract
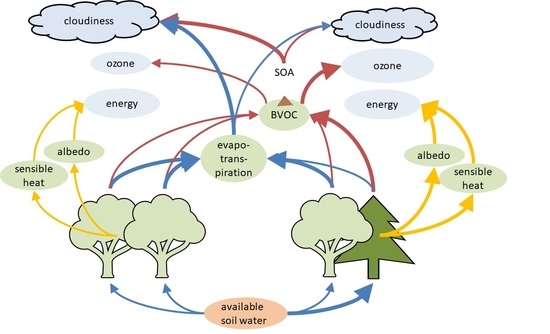
:1. Introduction
2. Materials and Methods
2.1. Investigated Area
2.2. Radiation Budget Overview
2.3. Albedo
2.4. Soil Temperature and Heat Storage Terms
2.5. Evapotranspiration and Latent Heat Flux
2.6. Secondary Radiative Effects
2.6.1. Emission of Biogenic Volatile Organic Compounds
2.6.2. Ozone and Secondary Organic Aerosol (SOA) Production
2.6.3. Cloud Condensation Nuclei and Cloud Properties
3. Results and Discussion
3.1. Land Surface Albedo
3.2. Heat Storage Dynamics
3.3. Latent Heat Exchange
3.4. Secondary Radiation Effects
3.4.1. Biogenic Volatile Organic Compounds
3.4.2. Ozone Formation
3.4.3. Secondary Organic Aerosols and Cloud Properties
3.5. Total Radiation Budget
3.6. Uncertainties and Deficiencies of the Investigations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brandl, S.; Paul, C.; Knoke, T.; Falk, W. The influence of climate and management on survival probability for Germany’s most important tree species. Forest Ecol. Manag. 2020, 458, 117652. [Google Scholar] [CrossRef]
- Conte, E.; Lombardi, F.; Battipaglia, G.; Palombo, C.; Altieri, S.; La Porta, N.; Marchetti, M.; Tognetti, R. Growth dynamics, climate sensitivity and water use efficiency in pure vs. mixed pine and beech stands in Trentino (Italy). Forest Ecol. Manag. 2018, 409, 707–718. [Google Scholar] [CrossRef]
- Pretzsch, H.; Schütze, G.; Uhl, E. Resistance of European tree species to drought stress in mixed versus pure forests: Evidence of stress release by inter-specific facilitation. Plant. Biol. 2013, 15, 483–495. [Google Scholar] [CrossRef]
- Bolte, A.; Ammer, C.; Löf, M.; Madsen, P.; Nabuurs, G.J.; Schall, P.; Spathelf, P.; Rock, J. Adaptive forest management in central Europe: Climate change impacts, strategies and integrative concept. Scand. J. For. Res. 2009, 24, 473–482. [Google Scholar] [CrossRef]
- Yousefpour, R.; Hanewinkel, M. Climate change and decision-making under uncertainty. Curr. For. Rep. 2016, 2, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Hilmers, T.; Biber, P.; Knoke, T.; Pretzsch, H. Assessing transformation scenarios from pure Norway spruce to mixed uneven-aged forests in mountain areas. Eur. J. For. Res. 2020, 139, 567–584. [Google Scholar] [CrossRef] [Green Version]
- Morin, X.; Fahse, L.; Jactel, H.; Scherer-Lorenzen, M.; García-Valdés, R.; Bugmann, H. Long-term response of forest productivity to climate change is mostly driven by change in tree species composition. Sci. Rep. 2018, 8, 5627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohn, F.J.; May, F.; Huth, A. Species composition and forest structure explain the temperature sensitivity patterns of productivity in temperate forests. Biogeosciences 2018, 15, 1795–1813. [Google Scholar] [CrossRef] [Green Version]
- Teuling, A.J.; Taylor, C.M.; Meirink, J.F.; Melsen, L.A.; Miralles, D.G.; van Heerwaarden, C.C.; Vautard, R.; Stegehuis, A.I.; Nabuurs, G.-J.; de Arellano, J.V.-G. Observational evidence for cloud cover enhancement over western European forests. Nat. Commun. 2017, 8, 14065. [Google Scholar] [CrossRef] [Green Version]
- Monson, R.K.; Holland, E.A. Biospheric trace gas fluxes and their control over tropospheric chemistry. Annu. Rev. Ecol. Syst. 2001, 32, 547–576. [Google Scholar] [CrossRef]
- Sporre, M.K.; Blichner, S.M.; Karset, I.; Makkonen, R.; Berntsen, T. BVOC-aerosol-climate feedbacks investigated using NorESM. Atmos. Chem. Phys. 2019, 19, 4763–4782. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.F.; Buchholz, A.; Tillmann, R.; Kleist, E.; Wu, C.; Rubach, F.; Kiendler-Scharr, A.; Rudich, Y.; Wildt, J.; Mentel, T.F. Environmental conditions regulate the impact of plants on cloud formation. Nat. Commun. 2017, 8, 14067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arneth, A.; Makkonen, R.; Olin, S.; Paasonen, P.; Holst, T.; Kajos, M.K.; Kulmala, M.; Maximov, T.; Miller, P.A.; Schurgers, G. Future vegetation–climate interactions in Eastern Siberia: An assessment of the competing effects of CO2 and secondary organic aerosols. Atmos. Chem. Phys. 2016, 16, 5243–5262. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, J.A.; Bauhus, J. Benefits of Mixtures on Growth Performance of Silver Fir (Abies alba) and European Beech (Fagus sylvatica) Increase with Tree Size Without Reducing Drought Tolerance. Front. For. Glob. Chang. 2019, 2. [Google Scholar] [CrossRef]
- Vitasse, Y.; Bottero, A.; Cailleret, M.; Bigler, C.; Fonti, P.; Gessler, A.; Lévesque, M.; Rohner, B.; Weber, P.; Rigling, A.; et al. Contrasting resistance and resilience to extreme drought and late spring frost in five major European tree species. Glob. Chang. Biol. 2019, 25, 3781–3792. [Google Scholar] [CrossRef]
- Vitasse, Y.; Bottero, A.; Rebetez, M.; Conedera, M.; Augustin, S.; Brang, P.; Tinner, W. What is the potential of silver fir to thrive under warmer and drier climate? Eur. J. For. Res. 2019, 138, 547–560. [Google Scholar] [CrossRef]
- Rehschuh, S.; Fuchs, M.; Tejedor, J.; Schäfler-Schmid, A.; Magh, R.-K.; Burzlaff, T.; Rennenberg, H.; Dannenmann, M. Admixing fir to European beech forests improves the soil greenhouse gas balance. Forests 2019, 10, 213. [Google Scholar] [CrossRef] [Green Version]
- Magh, R.-K.; Bonn, B.; Grote, R.; Burzlaff, T.; Pfausch, S.; Rennenberg, H. Drought superimposes the positive effect of silver-fir on water relations of European beech in mature forest stands. Forests 2019, 10, 897. [Google Scholar] [CrossRef] [Green Version]
- Magh, R.-K.; Grün, M.; Knothe, V.E.; Stubenazy, T.; Tejedor, J.; Dannenmann, M.; Rennenberg, H. Silver-fir (Abies alba MILL.) neighbors improve water relations of European beech (Fagus sylvatica L.), but do not affect N nutrition. Trees Struct. Funct. 2018, 32, 337–348. [Google Scholar] [CrossRef]
- Moukhtar, S.; Bessagnet, B.; Rouil, L.; Simon, V. Monoterpene emissions from Beech (Fagus sylvatica) in a French forest and impact on secondary pollutants formation at regional scale. Atmos. Environ. 2005, 39, 3535–3547. [Google Scholar] [CrossRef]
- Moukhtar, S.; Couret, C.; Rouil, L.; Simon, V. Biogenic Volatile Organic Compounds (BVOCs) emissions from Abies alba in a French forest. Sci. Total Environ. 2006, 354, 232–345. [Google Scholar] [CrossRef] [PubMed]
- Dindorf, T.; Kuhn, U.; Ganzeveld, L.; Schebeske, G.; Ciccioli, P.; Holzke, C.; Köble, R.; Seufert, G.; Kesselmeier, J. Significant light and temperature dependent monoterpene emissions from European beech (Fagus sylvatica L.) and their potential impact on the European volatile organic compound budget. J. Geophys. Res. 2006, 111. [Google Scholar] [CrossRef] [Green Version]
- Pokorska, O.; Dewulf, J.; Amelynck, C.; Schoon, N.; Simpraga, M.; Steppe, K.; Van Langenhove, H. Isoprene and terpenoid emissions from Abies alba: Identification and emission rates under ambient conditions. Atmos. Environ. 2012, 59, 501–508. [Google Scholar] [CrossRef]
- Bonn, B.; Magh, R.-K.; Rombach, J.; Kreuzwieser, J. Biogenic isoprenoid emissions under drought stress: Different responses for isoprene and terpenes. Biogeosciences 2019, 16, 4627–4645. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Pullinen, I.; Andres, S.; Carriero, G.; Fares, S.; Goldbach, H.; Hacker, L.; Kasal, T.; Kiendler-Scharr, A.; Kleist, E.; et al. Impacts of soil moisture on de-novo monoterpene emissions from European beech, Holm oak, Scots pine, and Norway spruce. Biogeosciences 2015, 12, 177–191. [Google Scholar] [CrossRef] [Green Version]
- Bonn, B.; Bourtsoukidis, E.; Sun, T.S.; Bingemer, H.; Rondo, L.; Javed, U.; Li, J.; Axinte, R.; Li, X.; Brauers, T.; et al. The link between atmospheric radicals and newly formed particles at a spruce forest site in Germany. Atmos. Chem. Phys. 2014, 14, 10823–10843. [Google Scholar] [CrossRef] [Green Version]
- McFiggans, G.; Mentel, T.F.; Wildt, J.; Pullinen, I.; Kang, S.; Kleist, E.; Schmitt, S.; Springer, M.; Tillmann, R.; Wu, C.; et al. Secondary organic aerosol reduced by mixture of atmospheric vapours. Nature 2019, 565, 587–593. [Google Scholar] [CrossRef]
- Niinemets, Ü. What are plant-released biogenic volatiles and how they participate in landscape- to global-level processes? In Ecosystem Services from Forest Landscapes: Broadscale Considerations; Perera, A.H., Peterson, U., Pastur, G.M., Iverson, L.R., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 29–56. [Google Scholar] [CrossRef]
- Hilmers, T.; Avdagić, A.; Bartkowicz, L.; Bielak, K.; Binder, F.; Bončina, A.; Dobor, L.; Forrester, D.I.; Hobi, M.L.; Ibrahimspahić, A.; et al. The productivity of mixed mountain forests comprised of Fagus sylvatica, Picea abies, and Abies alba across Europe. Forestry 2019, 92, 512–522. [Google Scholar] [CrossRef] [Green Version]
- Mina, M.; Rio, M.; Huber, M.O.; Thürig, E.; Rohner, B. The symmetry of competitive interactions in mixed Norway spruce, silver fir and European beech forests. J. Veg. Sci. 2018, 29, 775–787. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014—Update 2015; World Soil Resources Reports 106; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015; p. 203. [Google Scholar]
- Birmili, W.; Weinhold, K.; Rasch, F.; Sonntag, A.; Sun, J.; Merkel, M.; Wiedensohler, A.; Bastian, S.; Schladitz, A.; Löschau, G.; et al. Long-term observations of tropospheric particle number size distributions and equivalent black carbon mass concentrations in the German Ultrafine Aerosol Network (GUAN). Earth Syst. Sci. Data 2016, 8, 355–382. [Google Scholar] [CrossRef] [Green Version]
- Kändler, G.; Cullmann, D. Der Wald in Baden-Württemberg—Ausgewählte Ergebnisse der dritten Bundeswaldinventur; Forstliche Versuchsanstalt Baden-Württemberg: Freiburg, Germany, 2016; p. 64. [Google Scholar]
- Gasparini, P.; Nocetti, M.; Tabacchi, G.; Tosi, V. Biomass Equations and Data for Forest Stands and Shrublands of the Eastern Alps (Trentino, Italy); PNW-GTR-688; General Technical Report: 11/01; USDA Forest Service: Washington, SC, USA, 2006; p. 14.
- Lindroth, A.; Mölder, M.; Lagergren, F. Heat storage in forest biomass improves energy balance closure. Biogeosciences 2010, 7, 301–313. [Google Scholar] [CrossRef] [Green Version]
- Prata, A.J. A new long-wave formula for estimating downward clear-sky radiation at the surface. Q. J. R. Meteorol. Soc. 1996, 122, 1127–1151. [Google Scholar] [CrossRef]
- Wilber, A.C.; Kratz, D.P.; Gupta, S.K. Surface Emissivity Maps for Use in Retrievals of Satellite Longwave Radiation; NASA/TP-1999-209362; National Aeronautics and Space Administration: Langley Research Center Hampton: Hampton, VA, USA, 1999; p. 35.
- Glass, S.; Zelinka, S. Moisture relations and physical properties of wood. In Wood Handbook—Wood as An Engineering Material, Ross, R.J., Ed.; United States Department of Agriculture—Forest Service—Forest Products Laboratory: Washington, DC, USA, 2010. [Google Scholar]
- Thom, A.S. Momentum, mass and heat exchange of plant communities. In Vegetation and the Atmosphere; Monteith, J.L., Ed.; Academic Press: London, UK; New York, NY, USA, 1975; Volume 1, pp. 57–109. [Google Scholar]
- Monteith, J.L. Evaporation and surface temperature. Q. J. R. Meteorol. Soc. 1981, 107, 1–27. [Google Scholar] [CrossRef]
- Penman, H.L. Natural evaporation from open water, bare soil and grass. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1948, 193, 120–145. [Google Scholar] [CrossRef] [Green Version]
- Penman, H.L.; Long, I.F. Weather in wheat: An essay in mirometeorology. Q. J. R. Meteorol. Soc. 1960, 86, 1650. [Google Scholar] [CrossRef]
- Gracia, C.A.; Tello, E.; Sabaté, S.; Bellot, J. GOTILWA: An integrated model of water dynamics and forest growth. In Ecology of Mediterranean Evergreen Oak Forests, 137th ed.; Rodà, F., Retana, J., Gracia, C.A., Bellot, J., Eds.; Springer-Verlag: Berlin, Germany, 1999; pp. 163–179. [Google Scholar]
- Haas, E.; Klatt, S.; Fröhlich, A.; Werner, C.; Kiese, R.; Grote, R.; Butterbach-Bahl, K. LandscapeDNDC: A process model for simulation of biosphere-atmosphere-hydrosphere exchange processes at site and regional scale. Landsc. Ecol. 2013, 28, 615–636. [Google Scholar] [CrossRef]
- Pereira, A.R.; Pruitt, W.O. Adaptation of the Thornthwaite scheme for estimating daily reference evapotranspiration. Agric. Water Manag. 2004, 66, 251–257. [Google Scholar] [CrossRef]
- Thornthwaite, C.W. An approach toward a rational classification of climate. Geogr. Rev. 1948, 38, 55. [Google Scholar] [CrossRef]
- Willmott, C.J.; Rowe, C.M.; Mintz, Y. Climatology of the terrestrial seasonal water cycle. Int. J. Climatol. 1985, 5, 589–606. [Google Scholar] [CrossRef]
- Burgess, S.S.O.; Adams, M.A.; Turner, N.C.; Beverly, C.R.; Ong, C.K.; Khan, A.A.H.; Bleby, T.M. An improved heat pulse method to measure low and reverse rates of sap flow in woody plants. Tree Physiol. 2001, 21, 589–598. [Google Scholar] [CrossRef]
- Kreuzwieser, J.; Scheerer, U.; Rennenberg, H. Metabolic origin of acetaldehyde emitted by poplar (Populus tremula × P. alba) trees. J. Exp. Bot. 1999, 50, 757–765. [Google Scholar] [CrossRef] [Green Version]
- Yáñez-Serrano, A.M.; Fasbender, L.; Kreuzwieser, J.; Dubbert, D.; Haberstroh, S.; Lobo-do-Vale, R.; Caldeira, M.C.; Werner, C. Volatile diterpene emission by two Mediterranean Cistaceae shrubs. Sci. Rep. 2018, 8, 6855. [Google Scholar] [CrossRef] [PubMed]
- Guenther, A.; Hewitt, C.N.; Erickson, D.; Fall, R.; Geron, C.; Graedel, T.; Harley, P.; Klinger, L.; Lerdau, M.; McKay, W.A.; et al. A global model of natural volatile organic compound emissions. J. Geophys. Res. 1995, 100, 8873–8892. [Google Scholar] [CrossRef]
- Guenther, A.; Karl, T.; Harley, P.; Wiedinmyer, C.; Palmer, P.I.; Geron, C. Estimates of global terrestrial isoprene emissions using MEGAN (Model of Emissions of Gases and Aerosols from Nature). Atmos. Chem. Phys. 2006, 6, 3181–3210. [Google Scholar] [CrossRef] [Green Version]
- Butler, T.M.; Lawrence, M.G.; Taraborrelli, D.; Lelieveld, J. Multi-day ozone production potential of volatile organic compounds calculated with a tagging approach. Atmos. Environ. 2011, 45, 4082–4090. [Google Scholar] [CrossRef]
- Bonn, B.; Kreuzwieser, J.; Sander, F.; Yousefpour, R.; Baggio, T.; Adewale, O. The uncertain role of biogenic VOC for boundary-layer ozone concentration: Example Investigation of Emissions from Two Forest Types with a Box Model. Climate 2017, 5, 78. [Google Scholar] [CrossRef]
- Jenkin, M.E.; Young, J.C.; Rickard, A.R. The MCM v3.3.1 degradation scheme for isoprene. Atmos. Chem. Phys. 2015, 15, 11433–11459. [Google Scholar] [CrossRef] [Green Version]
- Spracklen, D.V.; Bonn, B.; Carslaw, K.S. Boreal forests, aerosols and the impacts on clouds and climate. Phil. Trans. R. Soc. A Math. Phys. Eng. Sci. 2008, 366, 4613–4626. [Google Scholar] [CrossRef]
- Mueller, R.; Trentmann, J.; Träger-Chatterjee, C.; Posselt, R.; Stöckli, R. The Role of the Effective Cloud Albedo for Climate Monitoring and Analysis. Remote Sens. 2011, 3, 2305–2320. [Google Scholar] [CrossRef] [Green Version]
- Bonn, B. Biogene Terpenemissionen und Sekundäre Organische Aerosolpartikelbildung: Ein Weg von Nadelwäldern Klimarückkopplungsprozesse zu Steuern. Prof. Thesis, J.W. Goethe Universität Frankfurt, Frankfurt, Germany, 2013. [Google Scholar]
- Weligepolage, K.; Gieske, A.S.M.; Su, Z. Effect of spatial resolution on estimating surface albedo: A case study in Speulderbos forest in The Netherlands. Int. J. Appl. Earth Obs. Geoinf. 2013, 23, 18–28. [Google Scholar] [CrossRef]
- Otto, J.; Berveiller, D.; Bréon, F.M.; Delpierre, N.; Geppert, G.; Granier, A.; Jans, W.; Knohl, A.; Kuusk, A.; Longdoz, B.; et al. Forest summer albedo is sensitive to species and thinning: How should we account for this in Earth system models? Biogeosciences 2014, 11, 2411–2427. [Google Scholar] [CrossRef] [Green Version]
- Holst, T.; Hauser, S.; Kirchgäßner, A.; Matzarakis, A.; Mayer, H.; Schindler, D. Measuring and modelling plant area index in beech stands. Int. J. Biometeorol. 2004, 48, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Kutsch, W.L.; Liu, C.; Hörmann, G.; Herbst, M. Spatial heterogeneity of ecosystem carbon fluxes in a broadleaved forest in Northern Germany. Glob. Chang. Biol. 2005, 11, 70–88. [Google Scholar] [CrossRef]
- Davi, H.; Gillmann, M.; Ibanez, T.; Cailleret, M.; Bontemps, A.; Fady, B.; Lefebre, F. Diversity of leaf unfolding dynamics among tree species: New insights from a study along an altitudinal gradient. Agric. For. Meteorol. 2011, 151, 1504–1513. [Google Scholar] [CrossRef]
- Zirlewagen, D.; von Wilpert, K. Modeling water and ion fluxes in a highly structured, mixed-species stand. For. Ecol. Manag. 2001, 143, 27–37. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics—From Air Pollution to Climate Change, 3rd ed.; Wiley: New York, NY, USA, 2016. [Google Scholar]
- Holst, T.; Mayer, H. Radiation components of beech stands in Southwest Germany. Meteorol. Zeitschrift 2005, 14, 107–115. [Google Scholar] [CrossRef]
- Schmid, H.P.; Grimmond, C.S.; Cropley, F.; Offerle, B.; Su, H.B. Measurements of CO2 and energy fluxes over a mixed hardwood forest in the mid-western United States. Agric. For. Meteorol. 2000, 103, 357–374. [Google Scholar] [CrossRef]
- Tiwari, S.; Grote, R.; Churkina, G.; Butler, T. Ozone damage, detoxification and the role of isoprenoids—New impetus for integrated models. Funct. Plant Biol. 2016, 43, 324–326. [Google Scholar] [CrossRef] [Green Version]
- Vogt, R.; Bernhofer, C.; Gay, L.W.; Jaeger, L.; Parlow, E. The available energy over a Scots Pine plantation: What’s up for partitioning? Theor. Appl. Climatol. 1996, 53, 23–31. [Google Scholar] [CrossRef]
- Oliphant, A.J.; Grimmond, C.S.B.; Zutter, H.N.; Schmid, H.P.; Su, H.-B.; Scott, S.L.; Offerle, B.; Randolph, J.C.; Ehman, J. Heat storage and energy balance fluxes for a temperate deciduous forest. Agric. For. Meteorol. 2004, 126, 185–201. [Google Scholar] [CrossRef]
- Leuzinger, S.; Körner, C. Tree species diversity affects canopy leaf temperatures in a mature temperate forest. Agric. For. Meteorol. 2007, 146, 29–37. [Google Scholar] [CrossRef]
- Bernard, M.; Boulanger, V.; Dupouey, J.-L.; Laurent, L.; Montpied, P.; Morin, X.; Picard, J.-F.; Saïd, S. Deer browsing promotes Norway spruce at the expense of silver fir in the forest regeneration phase. For. Ecol. Manag. 2017, 400, 269–277. [Google Scholar] [CrossRef]
- Kupferschmid, A.D. Selective browsing behaviour of ungulates influences the growth of Abies alba differently depending on forest type. For. Ecol. Manag. 2018, 429, 317–326. [Google Scholar] [CrossRef]
- Töchterle, P.; Yang, F.; Rehschuh, S.; Rehschuh, R.; Ruehr, N.K.; Rennenberg, H.; Dannenmann, M. Hydraulic Water Redistribution by Silver Fir (Abies alba Mill.) Occurring under Severe Soil Drought. Forests 2020, 11, 162. [Google Scholar] [CrossRef] [Green Version]
- Dani, K.G.S.; Jamie, I.M.; Prentice, I.C.; Atwell, B.J. Species-specific photorespiratory rate, drought tolerance and isoprene emission rate in plants. Plant Signal. Behav. 2015, 10, e990830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cáceres, M.D.; Martínez-Vilalta, J.; Coll, L.; Llorens, P.; Casals, P.; Poyatos, R.; Pausas, J.G.; Brotons, L. Coupling a water balance model with forest inventory data to predict drought stress: The role of forest structural changes vs. climate changes. Agric. For. Meteorol. 2015, 213, 77–90. [Google Scholar] [CrossRef] [Green Version]
- Vereecken, H.; Weihermüller, L.; Assouline, S.; Šimůnek, J.; Verhoef, A.; Herbst, M.; Archer, N.; Mohanty, B.; Montzka, C.; Vanderborght, J.; et al. Infiltration from the Pedon to Global Grid Scales: An Overview and Outlook for Land Surface Modeling. Vadose Zone J. 2019, 18. [Google Scholar] [CrossRef]









| Tree Species | Area Fraction [%] | Foliage Dry Weight [g/m2] | Albedo | ERISO [µgC gDW−1 h−1]/β ISO [K−1] | ERMT [µgC gDW−1 h−1]/β MT [K−1] | ERSQT [µgC gDW−1 h−1]/β SQT [K−1] |
|---|---|---|---|---|---|---|
| Picea abies | 42.8 | 177 ± 31 | 0.09 | 0.05 | 0.886/0.11 | 0.35/0.11 |
| Abies alba | 18.5 | 174 ± 54 | 0.076 | 0.04 | 22.24/0.14 | 0.13/0.04 |
| Fagus sylvatica | 15.3 | 180 ± 14 | 0.13 | 0 | 49.1/0.31 | 0.1/0.11 |
| Pseudotsuga menziesii | 5.1 | 141 ± 45 | 0.09 | 0.008 | 0.064/0.26 | 0/- |
| Pinus sylvestris | 5.0 | 77 ± 39 | 0.101 | 0.1 | 1.4/0.09 | 0.28/0.188 |
| Quercus robur/petraea | 3.1 | 179 ± 83 | 0.18 | 62.8 | 13.1/0.121 | 0.6/0.11 |
| Betula spp. | 1.9 | 105 ± 31 | 0.167 | 0 | 0.7/0.09 | 0.31/0.18 |
| Alnus spp. | 1.2 | 109 ± 92 | 0.16 | 0.018 | 0.13/0.09 | 0.0044/0.145 |
| Carpinus betulus | 1.2 | 132 ± 110 | 0.17 | 0.1 | 0.0093/0.09 | 0.011/0.145 |
| Tilia spp. | 1.2 | 110 ± 92 | 0.17 | 0.077 | 0.51/0.09 | 0.0067/0.145 |
| Acer spp. | 1.2 | 132 ± 49 | 0.17 | 0.1 | 1.8/0.09 | 0/ - |
| Populus spp. | 1.2 | 85 ± 31 | 0.18 | 67.3 | 3.45/0.09 | 0/ - |
| Pure Stand (BB) | Mixed Stand (BF) | |||
|---|---|---|---|---|
| Beech | Beech | Fir | Total | |
| Da | 24.3 | 22.9 | ||
| Dg | 25.4 | 18.6 | 32.1 | |
| Ha | 21.2 | 18.1 | ||
| Hg | 22.9 | 21.4 | 21.4 | |
| N ha−1 | 567 | 400 | 200 | 600 |
| BA ha−1 | 28.6 | 10.8 | 19.9 | 30.7 |
| VOL ha−1 | 261 | 77 | 170 | 247 |
| Wtot ha−1 | 159 | 48 | 91 | 139 |
| Radiation Effect | Scale * | BB Site (L) or Present Mixture (R) [W m−2] | Intermixture of 50% Fir into All Current Beech Stands (BF, Scenario) [W m−2] | Difference between Present Mixture and Scenario [W m−2] |
|---|---|---|---|---|
| Fglobal_d·(1 − α) | L | 105.6 | 112.2 | 6.6 |
| FET | L | −48.0 | −66.1 | −18.1 |
| Fatmos | R | 299.8 | 299.8 | 0.000 |
| FO3 ** | R | 0.284 | 0.308 | 0.023 |
| FSOA ** | R | −0.089 | −0.087 | 0.002 |
| FCCN ** | R | 0.769 | 0.749 | −0.021 |
| ΣFi = −FH *** | L | 357.4 | 345.8 | −11.6 |
| Tmean (°C) **** | L | 10.4 | 7.0 | −3.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonn, B.; Kreuzwieser, J.; Magh, R.-K.; Rennenberg, H.; Schindler, D.; Sperlich, D.; Trautmann, R.; Yousefpour, R.; Grote, R. Expected Impacts of Mixing European Beech with Silver Fir on Regional Air Quality and Radiation Balance. Climate 2020, 8, 105. https://doi.org/10.3390/cli8100105
Bonn B, Kreuzwieser J, Magh R-K, Rennenberg H, Schindler D, Sperlich D, Trautmann R, Yousefpour R, Grote R. Expected Impacts of Mixing European Beech with Silver Fir on Regional Air Quality and Radiation Balance. Climate. 2020; 8(10):105. https://doi.org/10.3390/cli8100105
Chicago/Turabian StyleBonn, Boris, Jürgen Kreuzwieser, Ruth-Kristina Magh, Heinz Rennenberg, Dirk Schindler, Dominik Sperlich, Raphael Trautmann, Rasoul Yousefpour, and Rüdiger Grote. 2020. "Expected Impacts of Mixing European Beech with Silver Fir on Regional Air Quality and Radiation Balance" Climate 8, no. 10: 105. https://doi.org/10.3390/cli8100105
APA StyleBonn, B., Kreuzwieser, J., Magh, R. -K., Rennenberg, H., Schindler, D., Sperlich, D., Trautmann, R., Yousefpour, R., & Grote, R. (2020). Expected Impacts of Mixing European Beech with Silver Fir on Regional Air Quality and Radiation Balance. Climate, 8(10), 105. https://doi.org/10.3390/cli8100105