Prescription Opioid Use among Patients with Chronic Noncancer Pain before and after the COVID-19 Outbreak in Taiwan: A Multicenter Prospective Observational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Instrument
2.3. Follow-Up Survey on Opioid Prescriptions
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Major Findings
4.2. Impact of COVID-19 Outbreak and Opioid Overdose
4.3. Opioid Tapering or Discontinuation and Suicide Attempts
4.4. Overall Lower Quality of Life
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kahan, M.; Mailis-Gagnon, A.; Wilson, L.; Srivastava, A. Canadian guideline for safe and effective use of opioids for chronic noncancer pain: Clinical summary for family physicians. Part 1: General population. Can. Fam. Physician 2011, 57, 1257–1266. [Google Scholar] [PubMed]
- Dowell, D.; Haegerich, T.M.; Chou, R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA 2016, 315, 1624–1645. [Google Scholar] [CrossRef] [Green Version]
- Wilson, N.; Kariisa, M.; Seth, P.; Smith, H.T.; Davis, N.L. Drug and opioid-involved overdose deaths—United States, 2017–2018. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 290–297. [Google Scholar] [CrossRef] [Green Version]
- National Institute on Drug Abuse. Overdose Death Rates, 1999–2020. Post on 20 January 2022. Available online: https://nida.nih.gov/drug-topics/trends-statistics/overdose-death-rates (accessed on 15 July 2022).
- The Draft 2022 CDC Clinical Practice Guideline for Prescribing Opioids Was Posted for a 60-Day Public Comment Period (from 10 February 2022 to 11 April 2022). Available online: https://www.cdc.gov/opioids/guideline-update/index.html (accessed on 15 July 2022).
- Tuan, W.J.; Spotts, H.; Zgierska, A.E.; Lennon, R.P. COVID-19 outcomes among adult patients treated with long-term opioid therapy for chronic non-cancer pain in the USA: A retrospective cohort study. BMJ Open 2021, 11, e056436. [Google Scholar] [CrossRef]
- Lacasse, A.; Pagé, M.G.; Dassieu, L.; Sourial, N.; Janelle-Montcalm, A.; Dorais, M.; Nguena Nguefack, H.L.; Godbout-Parent, M.; Hudspith, M.; Moor, G.; et al. Impact of the COVID-19 pandemic on the pharmacological, physical, and psychological treatments of pain: Findings from the Chronic Pain & COVID-19 Pan-Canadian Study. Pain Rep. 2021, 6, e891. [Google Scholar] [CrossRef]
- Soares, W.E., 3rd; Melnick, E.R.; Nath, B.; D’Onofrio, G.; Paek, H.; Skains, R.M.; Walter, L.A.; Casey, M.F.; Napoli, A.; Hoppe, J.A.; et al. Emergency department visits for nonfatal opioid overdose during the COVID-19 pandemic across six US health care systems. Ann. Emerg. Med. 2022, 79, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Nataraj, N.; Strahan, A.E.; Guy, G.P.; Losby, J.L.; Dowell, D. Dose tapering, increases, and discontinuity among patients on long-term high-dose opioid therapy in the United States, 2017–2019. Drug Alcohol Depend. 2022, 234, 109392. [Google Scholar] [CrossRef]
- Oliva, E.M.; Bowe, T.; Manhapra, A.; Kertesz, S.; Hah, J.M.; Henderson, P.; Robinson, A.; Paik, M.; Sandbrink, F.; Gordon, A.J.; et al. Associations between stopping prescriptions for opioids, length of opioid treatment, and overdose or suicide deaths in US veterans: Observational evaluation. BMJ 2020, 368, m283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiPrete, B.L.; Ranapurwala, S.I.; Maierhofer, C.N.; Fulcher, N.; Chelminski, P.R.; Ringwalt, C.L.; Ives, T.J.; Dasgupta, N.; Go, V.F.; Pence, B.W. Association of opioid dose reduction with opioid overdose and opioid use disorder among patients receiving high-dose, long-term opioid therapy in North Carolina. JAMA Netw. Open 2022, 5, e229191. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Matielo, H.; da Silva Oliveira, V.R.; de Oliveira, V.T.; Dale, C.S. Pain in Covid era. Front. Physiol. 2021, 12, 624154. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Shanthanna, H.; Strand, N.H.; Provenzano, D.A.; Lobo, C.A.; Eldabe, S.; Bhatia, A.; Wegener, J.; Curtis, K.; Cohen, S.P.; Narouze, S. Caring for patients with pain during the COVID-19 pandemic: Consensus recommendations from an international expert panel. Anaesthesia 2020, 75, 935–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, T.C.; Ger, L.P.; Pergolizzi, J.V.; Raffa, R.B.; Wang, J.O.; Ho, S.T. Knowledge, attitude and practice survey of prescribing opioids for chronic noncancer pain in Taiwan—Comparison of pain and non-pain physicians. Pain Med. 2019, 20, 2397–2410. [Google Scholar] [CrossRef] [Green Version]
- Taiwan Food and Drug Administration. Physician Guidelines on Clinical Use of Narcotics in Chronic Noncancer Pain. (In Chinsese, amended on 13 January 2022). Available online: https://www.fda.gov.tw/tc/lawContent.aspx?cid=183&id=3370 (accessed on 15 July 2022).
- Lin, T.C.; Hsu, C.H.; Lu, C.C.; Tsai, Y.C.; Ho, S.T. Chronic opioid therapy in patients with chronic noncancer pain in Taiwan. J. Anesth. 2010, 24, 882–887. [Google Scholar] [CrossRef]
- Lin, T.C.; Ger, L.P.; Pergolizzi, J.V., Jr.; Raffa, R.B.; Wang, J.O.; Ho, S.T. Long-term use of opioids in 210 officially registered patients with chronic noncancer pain in Taiwan: A cross-sectional study. J. Formos. Med. Assoc. 2017, 116, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.Y.; Chueh, Y.N.; Chen, C.M.; Jian, S.W.; Lai, S.K.; Liu, D.P. Taiwan’s COVID-19 response: Timely case detection and quarantine, January to June 2020. J. Formos. Med. Assoc. 2021, 120, 1400–1404. [Google Scholar] [CrossRef]
- MacDonald, I.; Hsu, J.L. Epidemiological observations on breaking COVID-19 transmission: From the experience of Taiwan. J. Epidemiol. Community Health 2021, 75, 809–812. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.T.; Lin, T.C.; Yeh, C.C.; Cheng, K.I.; Sun, W.Z.; Sung, C.S.; Wen, Y.R.; Hsieh, Y.J.; Wang, P.K.; Liu, Y.C.; et al. Gender differences in depression and sex hormones among patients receiving long-term opioid treatment for chronic noncancer pain in Taiwan—A multicenter cross-sectional study. Int. J. Environ. Res. Public Health 2021, 18, 7837. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.C.; Ho, S.T.; Ger, L.P.; Liou, H.H.; Hwang, S.L. Gender difference in long-term use of opioids among Taiwan officially registered patients with chronic noncancer pain. Medicine 2018, 97, e10805. [Google Scholar] [CrossRef] [PubMed]
- Ger, L.P.; Ho, S.T.; Sun, W.Z.; Wang, M.S.; Cleeland, C.S. Validation of the Brief Pain Inventory in a Taiwanese population. J. Pain Symptom Manag. 1999, 18, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Chung, C.W.; Yu, C.F.; Wang, J.D. Development and verification of validity and reliability of the WHOQOL-BREF Taiwan version. J. Formos. Med. Assoc. 2002, 101, 342–351. [Google Scholar] [PubMed]
- Opioid Dose Equivalence. Faculty of Pain Medicine, ANZCA—March 2019. Available online: https://www.anzca.edu.au/getattachment/6892fb13-47fc-446b-a7a2-11cdfe1c9902/PM01-(Appendix-2)-Opioid-Dose-Equivalence-Calculation-of-Oral-Morphine-Equivalent-Daily-Dose-(oMEDD).aspx (accessed on 15 July 2022).
- Taiwan Food and Drug Administration. Guidelines on Clinical Use of Pethidine. (In Chinese, amended on 7 November 2017). Available online: https://www.fda.gov.tw/tc/lawContent.aspx?cid=183&id=2937 (accessed on 30 November 2020).
- Lee, B.; Yang, K.-C.; Kaminski, P.; Peng, S.; Odabas, M.; Gupta, S.; Green, H.D., Jr.; Ahn, Y.-Y.; Perry, B.L. Substitution of nonpharmacologic therapy with opioid prescribing for pain during the COVID-19 pandemic. JAMA Netw. Open 2021, 4, e2138453. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.E., Jr.; Sloan, P.A. The opioid crisis in the United States: Chronic pain physicians are the answer, not the cause. Anesth. Analg. 2017, 125, 1432–1434. [Google Scholar] [CrossRef]
- Taiwan Ministry of Health and Welfare. 2020 Cause of Death Statistics. Last Updated 1 October 2021. Available online: https://www.mohw.gov.tw/cp-5256-63399-2.html (accessed on 15 July 2022).
- Darzi, M.T.; Pourhadi, S.; Hosseinzadeh, S.; Ahmadi, M.H.; Dadian, M. Comparison of quality of life in low back pain patients and healthy subjects by using WHOQOL-BREF. J. Back Musculoskelet. Rehabil. 2014, 27, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lim, Y.H.; Hong, S.J.; Jeong, J.H.; Choi, H.R.; Park, S.K.; Kim, J.E.; Park, E.H.; Kim, J.H. Multicenter survey of symptoms, work life, economic status, and quality of life of complex regional pain syndrome patients. Korean J. Pain 2021, 34, 288–303. [Google Scholar] [CrossRef]

| Decreased MMEs (Greater than 2.5%) | Equal MMEs (±2.5%) | Increased MMEs (Greater than 2.5%) | p Value | |
|---|---|---|---|---|
| n (%) | 31 (30.1%) | 27 (26.2%) | 45 (43.7%) | |
| Male, n = 63 | 17 (54.8%) | 19 (70.4%) | 27 (60.0%) | 0.470 a |
| Female, n = 40 | 14 (45.2%) | 8 (29.6%) | 18 (40.0%) | |
| Age, year | 54.7 ± 11.9 (33 to 92) | 53.0 ± 11.0 (39 to 89) | 53.0 ± 14.3 (30 to 94) | 0.824 b |
| Height, cm | 164.4 ± 9.7 (146 to 185) | 164.2 ± 5.9 (152 to 176) | 162.2 ± 15.3 (100 to 185) | 0.992 c |
| Weight, kg | 64.7 ± 14.5 (40 to 97) | 62.0 ± 13.6 (41 to 99) | 61.1 ± 17.1 (30 to 100) | 0.593 b |
| BMI, kg/m2 | 23.9 ± 4.7 (15.4 to 34.4) | 23.0 ± 4.9 (14.5 to 34.3) | 23.1 ± 5.0 (15.6 to 33.1) | 0.726 b |
| Follow up duration, days | 531.3 ± 56.5 (365 to 619) | 535.8 ± 55.8 (454 to 637) | 528.8 ± 60.8 (357 to 634) | 0.881 b |
| Pain duration, months | 148.7 ± 99.7 (14 to 485), 120 | 117.7 ± 87.8 (12 to 300), 89 | 190.8 ± 111.4 (24 to 480), 192 | 0.013 b,* |
| Opioid therapy duration, months | 108.8 ± 82.4 (4 to 300), 87 | 107.3 ± 88.4(6 to 300), 78 | 125.4 ± 100.1 (6 to 420), 120 | 0.640 b |
| <12 months, n = 6 (5.8%) | 2 (6.5%) | 1 (3.7%) | 3 (6.7%) | 0.893 d |
| 12 to <36 months, n = 14 (13.6%) | 3 (9.7%) | 5 (18.5%) | 6 (13.3%) | |
| ≥36 months, n = 83 (80.6%) | 26 (83.9%) | 21 (77.8%) | 36 (80.0%) | |
| Pain severity in the past week | ||||
| Worst, 0–10 | 8.2 ± 2.0 (3 to 10) | 8.4 ± 1.4 (6 to 10) | 9.0 ± 1.5 (3 to 10) | 0.074 b |
| Least, 0–10 | 4.9 ± 2.7 (0 to 10) | 4.3 ± 1.9 (1 to 10) | 5.0 ± 2.8 (0 to 10) | 0.505 b |
| Average, 0–10 | 6.4 ± 2.4 (2 to 10) | 5.8 ± 2.1 (3 to 10) | 6.8 ± 2.3 (2 to 10) | 0.186 b |
| Pain reduction after medication, % | 48.1 ± 21.7 (10 to 100) | 49.3 ± 14.7 (10 to 80) | 45.8 ± 21.7 (0 to 90) | 0.700 c |
| Daily function pain interference in the past week, in average, 0–10 | 4.3 ± 2.9 (0 to 9.6) | 4.2 ± 2.4 (0 to 9.6) | 5.4 ± 2.4 (0 to 10) | 0.114 b |
| Marital status | ||||
| Married couple, n = 47 (45.6%) | 19 (61.3%) | 15 (55.6%) | 13 (28.9%) | 0.010 a,# |
| Single/divorced/widowed, n = 56 (54.4%) | 12 (38.7%) | 12 (44.4%) | 32 (71.1%) | |
| Work status before pandemic | ||||
| Employed, n = 20 (19.4%) | 7 (22.6%) | 5 (18.5%) | 8 (17.8%) | 0.727 d |
| Housekeeping, n = 16 (15.5%) | 5 (16.1) | 4 (14.8%) | 7 (15.6%) | |
| Retired, n = 23 (22.3%) | 9 (29.0%) | 7 (25.9%) | 7 (15.6%) | |
| Unemployed, n = 44 (42.7%) | 10 (32.2%) | 11 (40.7%) | 23 (51.1%) | |
| Jobless due to pain, n = 81 (78.6%) | 25 (80.6%) | 21 (77.8%) | 35 (77.8%) | 0.948 a |
| Decreased MMEs (Greater than 2.5%) | Equal MMEs (±2.5%) | Increased MMEs (Greater than 2.5%) | p-Value | |
|---|---|---|---|---|
| n (%) | 31 (30.1%) | 27 (26.2%) | 45 (43.7%) | |
| MMEs, mg/day | ||||
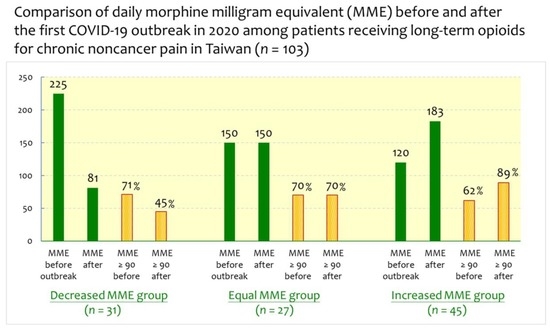
| Before pandemic, n = 103 | 231.1 ± 173.4 | 202.6 ± 153.4 | 203.4 ± 232.2 | 0.804 a |
| 206.5 ± 191.1 (8.0 to 1350.0), 150.0 | (22.9 to 705.0), 225.0 | (34.0 to 608.4), 150.0 | (8.0 to 1350.0), 120.0 | |
| During pandemic, n = 103 | 127. 1 ± 136.9 | 202.9 ± 153.9 | 284.9 ± 291.2 | 0.001 b |
| 215.9 ± 229.5 (0.0 to 1470.0), 140.0 | (0.0 to 660.0), 81.3 | (34.0 to 612.6), 150.0 | (28.8 to 1470.0), 182.8 | |
| Difference | −104.1 ± 120.2 | 0.3 ± 1.1 | 81.5 ± 109.1 | <0.001 b |
| (−600.0 to −1.7), −60.0 | (−1.3 to 4.2), 0.0 | (8.8 to 595.8), 55.7 | ||
| Ratio of difference, % | −44.0 ± 28.9 | 0.1 ± 0.3 | 84.2 ± 135.6 | <0.001 b |
| (−100.0 to −5.0), −46.7 | (−0.5 to 1.3), 0.0 | (3.0 to 800.0), 40.0 | ||
| Opioids < 12 months, n = 6 (5.8%) | 107.5 ± 31.8 | 60.0 | 43.5 ± 43.8 | 0.377 b |
| Opioids 12 to < 36 months, n = 14 (13.6%) | 137.8 ± 156.5 | 190.6 ± 133.0 | 204.3 ± 202.0 | 0.501 b |
| Opioids ≥ 36 months, n = 83 (80.6%) | 251.4 ± 177.1 | 212.3 ± 160.9 | 216.6 ± 243.9 | 0.406 b |
| MMEs, mg/day/kg body weight | ||||
| Before pandemic | 3.6 ± 2.7 | 3.4 ± 2.4 | 3.6 ± 4.3 | 0.942 a |
| During pandemic | 2.1 ± 2.3 | 3.4 ± 2.5 | 4.9 ± 4.8 | <0.001 b |
| MMEs ≥ 200 mg/day | ||||
| Before pandemic, n = 43 (41.7%) | 17 (54.8%) | 11 (40.7%) | 15 (33.3%) | 0.173 c |
| During pandemic, n = 38 (36.9%) | 6 (19.4%) | 11 (40.7%) | 21 (46.7%) | 0.047 c |
| MMEs ≥ 100 mg/day | ||||
| Before pandemic, n = 64 (62.1%) | 22 (71.0%) | 18 (66.7%) | 24 (53.3%) | 0.253 c |
| During pandemic, n = 70 (68.0%) | 14 (45.2%) | 18 (66.7%) | 38 (84.4%) | 0.001 c |
| MMEs ≥ 90 mg/day | ||||
| Before pandemic, n = 69 (67.0%) | 22 (71.0%) | 19 (70.4%) | 28 (62.2%) | 0.662 c |
| During pandemic, n = 73 (70.9%) | 14 (45.2%) | 19 (70.4%) | 40 (88.9%) | <0.001 c |
| Strong opioids during pandemic, n = 103 | ||||
| Oral morphine, n = 57 (55.3%) | 15 (48.4%) | 14 (51.9%) | 28 (62.2%) | 0.449 c |
| MMEs of oral morphine, mg/day | 155.5 ± 100.8 | 211.5 ± 171.1 | 252.6 ± 229.9 | 0.286 a |
| Fentanyl patch, n = 19 (18.4%) | 1 (3.2%) | 8 (29.6%) | 10 (22.2%) | 0.013 d |
| MMEs of fentanyl patch, mg/day | 28.8 | 202.2 ± 101.6 | 278.9 ± 222.9 | - |
| Oxycodone, n = 30 (29.1%) | 8 (25.8%) | 6 (22.2%) | 16 (35.6%) | 0.430 c |
| MMEs of oxycodone, mg/day | 136.8 ± 213.7 | 63.7 ± 44.5 | 130.0 ± 143.8 | 0.623 a |
| Concomitant adjuvants during pandemic | ||||
| Gabapentinoids, n = 39 (37.9%) | 10 (32.3%) | 10 (37.0%) | 19 (42.2%) | 0.675 c |
| NSAID, n = 8 (7.8%) | 3 (9.7%) | 2 (7.4%) | 3 (6.7%) | 0.898 d |
| Antidepressants, n = 20 (19.4%) | 6 (19.4%) | 4 (14.8%) | 10 (22.2%) | 0.744 c |
| SSRIs, n = 11 (10.7%) | 1 (3.2%) | 3 (11.1%) | 7 (15.6%) | 0.231 d |
| Tricyclic antidepressant, n = 10 (9.7%) | 5 (16.1%) | 1 (3.7%) | 4 (8.9%) | 0.331 d |
| Benzodiazepine before, n = 33 (32.0%) | 9 (29.0%) | 9 (33.3%) | 15 (33.3%) | 0.912 c |
| Decreased MMEs (Greater than 2.5%) | Equal MMEs (±2.5%) | Increased MMEs (Greater than 2.5%) | p-Value | |
|---|---|---|---|---|
| n (%) | 31 (30.1%) | 27 (26.2%) | 45 (43.7%) | |
| WHO Quality of Life score | ||||
| Global Quality of Life, 1–5 | 2.42 ± 0.96 | 2.78 ± 0.75 | 2.18 ± 0.86 | 0.020 a |
| Global Health, 1–5 | 2.06 ± 0.93 | 2.30 ± 0.91 | 1.89 ± 0.71 | 0.139 a |
| Domain 1: Physical, 4–20 | 9.37 ± 3.19 | 10.01 ± 2.64 | 9.02 ± 3.22 | 0.412 a |
| Domain 2: Psychological, 4–20 | 10.22 ± 3.49 | 10.62 ± 2.21 | 9.87 ± 3.53 | 0.607 b |
| Domain 3: Social, 4–20 | 11.65 ± 3.21 | 12.00 ± 2.51 | 9.74 ± 3.26 | 0.004 a |
| Domain 4: Environmental, 4–20 | 11.50 ± 2.83 | 11.93 ± 2.76 | 11.23 ± 3.03 | 0.619 a |
| Total score of 4 domains, 16–80 | 42.75 ± 11.49 | 44.56 ± 8.67 | 39.86 ± 11.68 | 0.193 a |
| Constipation, n = 62 (60.2%) | 20 (64.5%) | 19 (70.4%) | 23 (51.1%) | 0.228 c |
| Laxative before pandemic, n = 28 (27.2%) | 7 (22.6%) | 8 (29.6%) | 13 (28.9%) | 0.787 c |
| Laxative during pandemic, n = 35 (34.0%) | 8 (25.8%) | 9 (33.3%) | 18 (40.0%) | 0.437 c |
| Depression diagnosis, n = 50 (48.5%) | 13 (41.9%) | 18 (66.7%) | 19 (42.2%) | 0.090 c |
| Suicidal ideation | ||||
| Always or frequently, n = 14 (13.6%) | 7 (22.6%) | 1 (3.7%) | 6 (13.3%) | 0.303 d |
| Sometimes, n = 26 (25.2%) | 6 (19.4%) | 7 (25.9%) | 13 (28.9%) | |
| Seldom or never, n = 63 (61.2%) | 18 (58.1%) | 19 (70.4%) | 26 (57.8%) | |
| Beck Depression Inventory score, 0–63 | 20.9 ± 15.8 | 18.2 ± 12.3 | 24.3 ± 15.8 | 0.244 a |
| 0–18 (minimal or mild), n = 50 | 15 (48.4%) | 16 (59.6%) | 19 (42.2%) | 0.375 c |
| 19–63 (moderate or severe), n = 53 | 16 (51.6%) | 11 (40.7%) | 26 (57.8%) | |
| Decreased MMEs (Greater than 2.5%) | Equal MMEs (±2.5%) | Increased MMEs (Greater than 2.5%) | p-Value | |
|---|---|---|---|---|
| n (%) | 31 (30.1%) | 27 (26.2%) | 45 (43.7%) | |
| Afraid of opioid addiction | ||||
| Strongly agree or agree, n = 33 (32.0%) | 7 (22.6%) | 8 (29.6%) | 18 (40.0%) | 0.161 a |
| Uncertain, n = 12 (11.7%) | 2 (7.4%) | 6 (22.3%) | 4 (8.9%) | |
| Strongly disagree or disagree, n = 58 (56.3%) | 22 (71.0%) | 13 (48.1%) | 23 (51.1%) | |
| Willing to stop opioids | ||||
| Strongly agree or agree, n = 26 (25.2%) | 6 (19.4%) | 8 (29.6%) | 12 (26.7%) | 0.886 a |
| Uncertain, n = 16 (15.5%) | 6 (19.3%) | 4 (14.8%) | 6 (13.3%) | |
| Strongly disagree or disagree, n = 61 (59.2%) | 19 (61.3%) | 15 (55.6%) | 27 (60.0%) | |
| Taking more pain medication than prescribed | ||||
| Always or frequently, n= 5 (4.9%) | 2 (6.5%) | 2 (7.4%) | 1 (2.2%) | 0.413 a |
| Sometimes, n = 12 (11.7%) | 5 (16.1%) | 1 (3.7%) | 6 (13.4%) | |
| Seldom or never, n = 86 (83.5%) | 24 (77.4%) | 24 (88.9%) | 38 (84.4%) | |
| Borrowing pain medication from someone else | ||||
| Always or frequently, n = 0 (0.0%) | 0 | 0 | 0 | 1.000 a |
| Sometimes, n = 2 (1.9%) | 1 (3.2%) | 0 | 1 (2.2%) | |
| Seldom or never, n = 101 (98.1%) | 30 (96.8%) | 27 (100.0%) | 44 (97.8%) | |
| Visiting the emergency room for more pain medication | ||||
| Always or frequently, n = 5 (4.9%) | 2 (6.5%) | 1 (3.7%) | 2 (4.4%) | 0.008 a,* |
| Sometimes, n = 20 (19.4%) | 7 (22.5%) | 0 | 13 (28.9%) | |
| Seldom or never, n = 78 (75.7%) | 22 (71.0%) | 26 (96.3%) | 30 (66.7%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-L.; Ho, S.-T.; Sun, W.-Z.; Tsai, Y.-C.; Cheng, K.-I.; Liu, Y.-C.; Hsieh, Y.-J.; Wen, Y.-R.; Wang, P.-K.; Sung, C.-S.; et al. Prescription Opioid Use among Patients with Chronic Noncancer Pain before and after the COVID-19 Outbreak in Taiwan: A Multicenter Prospective Observational Study. Healthcare 2022, 10, 2460. https://doi.org/10.3390/healthcare10122460
Chen J-L, Ho S-T, Sun W-Z, Tsai Y-C, Cheng K-I, Liu Y-C, Hsieh Y-J, Wen Y-R, Wang P-K, Sung C-S, et al. Prescription Opioid Use among Patients with Chronic Noncancer Pain before and after the COVID-19 Outbreak in Taiwan: A Multicenter Prospective Observational Study. Healthcare. 2022; 10(12):2460. https://doi.org/10.3390/healthcare10122460
Chicago/Turabian StyleChen, Jia-Lin, Shung-Tai Ho, Wei-Zen Sun, Yu-Chuan Tsai, Kuang-I Cheng, Yen-Chin Liu, Yi-Jer Hsieh, Yeong-Ray Wen, Po-Kai Wang, Chun-Sung Sung, and et al. 2022. "Prescription Opioid Use among Patients with Chronic Noncancer Pain before and after the COVID-19 Outbreak in Taiwan: A Multicenter Prospective Observational Study" Healthcare 10, no. 12: 2460. https://doi.org/10.3390/healthcare10122460
APA StyleChen, J. -L., Ho, S. -T., Sun, W. -Z., Tsai, Y. -C., Cheng, K. -I., Liu, Y. -C., Hsieh, Y. -J., Wen, Y. -R., Wang, P. -K., Sung, C. -S., Yeh, C. -C., & Lin, T. -C. (2022). Prescription Opioid Use among Patients with Chronic Noncancer Pain before and after the COVID-19 Outbreak in Taiwan: A Multicenter Prospective Observational Study. Healthcare, 10(12), 2460. https://doi.org/10.3390/healthcare10122460