mHealth Apps for Self-Management of Cardiovascular Diseases: A Scoping Review
Abstract
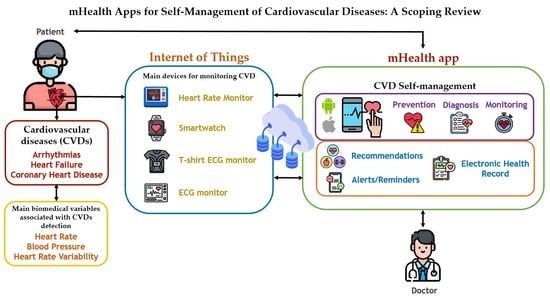
:1. Introduction
2. Materials and Methods
2.1. Research Questions
- RQ1. Which CVDs are most commonly managed by mHealth apps?
- RQ2. Which mHealth apps for CVD self-management are reported in the literature?
- RQ3. What are the main functionalities of mHealth apps for CVD self-management?
- RQ4. What are the major remarks for future work and challenges to be overcome by mHealth apps for CVD self-management?
- RQ5. Which approaches to data extraction, analysis, and management are commonly implemented in mHealth apps for CVD self-management?
- RQ6. Which wearables are commonly used to detect, monitor, and/or identify CVDs?
- RQ7. Which CVD stages are commonly managed by mHealth apps?
2.2. Inclusion and Exclusion Criteria
- ‘Cardiovascular disease’ AND (‘Self-management’ OR ‘Self-care’ OR ‘Self-monitoring’) AND (‘mHealth’ OR ‘mobile application’ OR ‘smart application’ OR ‘wearable’ OR ‘smartwatch’ OR ‘app’). The analysis of the preliminary results of this query revealed relevant search terms related to different cardiovascular disease types. Query 2 includes these search terms to expand on the relationship identified.
- (‘Heart disease’ OR ‘Cardiac issues’ OR ‘Heart failure’ OR ‘Arrhythmia’ OR ‘Coronary heart disease’ OR ‘Atrial Fibrillation’ OR ‘Hypertension’ OR ‘Cardiac arrest’ OR ‘Peripheral artery disease’) AND (‘Self-management’ OR ‘Self-care’ OR ‘Self-monitoring’) AND (‘mHealth’ OR ‘mobile application’ OR ‘smart application’ OR ‘wearable’ OR ‘smartwatch’ OR ‘app’).
2.3. Study Selection and Eligibility
- Studies on diseases other than CVDs;
- Studies conducted in domains other than health self-management;
- Studies written in languages other than English.
2.4. Data Collection and Analysis
3. Results
- Type of CVD that is managed by each mHealth app.
- Main app functionalities. Central capabilities of mHealth apps for CVD self-management, including (a) medical recommendations for patient follow-up, (b) real-time alerts before vital sign alterations, (c) medication management, (d) report of monitored parameters, (e) reminders for patient adherence to medication, physical activity, and/or dietary plans, (f) patient–physician communication via text messages, and (g) atrial fibrillation (AF) detection.
- Challenges and/or future work remarks (when applicable). Main challenges to overcome and/or suggestions for future work for mHealth apps used in CVD self-management.
- Approaches to data analysis, extraction, and management. The approaches were identified such as (a) machine learning techniques, (b) machine learning tasks, (c) big data types, and (d) device/sensor types. We identified mHealth apps relying on large datasets and big data analysis techniques. Additionally, there are apps relying on machine learning algorithms (MLAs) or techniques. Finally, we detected mHealth apps relying on sensors/wearables to obtain patient data (e.g., vital signs).
- Device and apps. Information on the wearables and web and mobile apps—either commercially available or purposefully developed in the study itself—used by each mHealth app to retrieve patient data and biomedical variables.
- CVD phase or set of phases managed by each mHealth app reviewed. The main CVD phases identified were diagnosis, prevention, monitoring, and treatment.
4. Discussion
4.1. RQ1. Which CVDs Are Most Commonly Managed by mHealth Apps?
4.2. RQ2. Which mHealth Apps for CVD Self-Management Are Reported in the Literature?
4.3. RQ3. What Are the Main Functionalities of mHealth Apps for CVD Self-Management?
- Recommendations (F1). Medical recommendations issued for patient follow-up in terms of dietary plans, physical activity, and overall health status.
- Alerts/reminders/text messages (F2). (a) Early, real-time warnings issued before potential vital signal alterations, (b) medication, physical activity, and/or dietary reminders, and (c) text messages communication between patients and physicians.
- Parameter monitoring (F3). Reports of monitored patient parameters, such as active minutes, burned calories, weight, step count, traveled distance, heart rate, blood pressure, body temperature, and physical activity.
- Medication management (F4). Control and follow-up of patient medication.
- Patient medical history (F5). Electronic health records (EHRs) including clinical data, medical history, diagnoses, medications, treatment plans, allergy test records, and laboratory and test results.
- AF detection (F6). Early detection of AF using heart rate monitoring and ECG results.
4.4. RQ4. What Are the Major Remarks for Future Work and Challenges to Be Overcome by mHealth Apps for CVD Self-Management?
4.5. RQ5. Which Approaches to Data Extraction, Analysis, and Management Are Commonly Implemented in mHealth Apps for CVD Self-Management?
4.6. RQ6. Which Wearables Are Commonly Used to Detect, Monitor, and/or Identify CVDs?
4.7. RQ7. Which CVD Stages Are Commonly Managed by mHealth Apps?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO, “Noncommunicable Diseases”, World Health Organization (WHO), 13 April 2017. Available online: https://www.who.int/en/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 7 February 2021).
- Athilingam, P.; Jenkins, B. Mobile Phone Apps to Support Heart Failure Self-Care Management: Integrative Review. JMIR Cardio 2018, 2, e10057. [Google Scholar] [CrossRef]
- Chow, C.K.; Ariyarathna, N.; Islam, S.M.S.; Thiagalingam, A.; Redfern, J. mHealth in Cardiovascular Health Care. Heart Lung Circ. 2016, 25, 802–807. [Google Scholar] [CrossRef]
- Xie, B.; Su, Z.; Zhang, W.; Cai, R. Chinese Cardiovascular Disease Mobile Apps’ Information Types, Information Quality, and Interactive Functions for Self-Management: Systematic Review. JMIR mHealth uHealth 2017, 5, e195. [Google Scholar] [CrossRef] [PubMed]
- Searcy, R.P.; Summapund, J.; Estrin, D.; Pollak, J.P.; Schoenthaler, A.; Troxel, A.B.; Dodson, J.A. Mobile Health Technologies for Older Adults with Cardiovascular Disease: Current Evidence and Future Directions. Curr. Geriatr. Rep. 2019, 8, 31–42. [Google Scholar] [CrossRef]
- Coorey, G.M.; Neubeck, L.; Mulley, J.; Redfern, J. Effectiveness, acceptability and usefulness of mobile applications for cardiovascular disease self-management: Systematic review with meta-synthesis of quantitative and qualitative data. Eur. J. Prev. Cardiol. 2018, 25, 505–521. [Google Scholar] [CrossRef]
- Dale, L.P.; Dobson, R.; Whittaker, R.; Maddison, R. The effectiveness of mobile-health behaviour change interventions for cardiovascular disease self-management: A systematic review. Eur. J. Prev. Cardiol. 2016, 23, 801–817. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, L.; Seaton, P. The Effectiveness of Self-Management Mobile Phone and Tablet Apps in Long-term Condition Management: A Systematic Review. J. Med. Internet Res. 2016, 18, e97. [Google Scholar] [CrossRef]
- Gandhi, S.; Chen, S.; Hong, L.; Sun, K.; Gong, E.; Li, C.; Yan, L.L.; Schwalm, J.-D. Effect of Mobile Health Interventions on the Secondary Prevention of Cardiovascular Disease: Systematic Review and Meta-analysis. Can. J. Cardiol. 2017, 33, 219–231. [Google Scholar] [CrossRef]
- Pearsons, A.; Hanson, C.L.; Gallagher, R.; O’Carroll, R.E.; Khonsari, S.; Hanley, J.; Strachan, F.E.; Mills, N.L.; Quinn, T.J.; McKinstry, B.; et al. Atrial fibrillation self-management: A mobile telephone app scoping review and content analysis. Eur. J. Cardiovasc. Nurs. 2021, 20, 305–314. [Google Scholar] [CrossRef]
- Villarreal, V.; Alvarez, A. Evaluation of mHealth Applications Related to Cardiovascular Diseases: A Systematic Review. Acta Inform. Med. 2020, 28, 130–137. [Google Scholar] [CrossRef]
- Neubeck, L.; Lowres, N.; Benjamin, E.; Freedman, B.; Coorey, G.; Redfern, J. The mobile revolution—using smartphone apps to prevent cardiovascular disease. Nat. Rev. Cardiol. 2015, 12, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Martínez, R.R.; Wentzel, J.; Asbjørnsen, R.A.; Noort, P.D.; van Niekerk, J.M.; Sanderman, R.; van Gemert-Pijnen, J.E. Supporting Self-Management of Cardiovascular Diseases Through Remote Monitoring Technologies: Metaethnography Review of Frameworks, Models, and Theories Used in Research and Development. J. Med. Internet Res. 2020, 22, e16157. [Google Scholar] [CrossRef]
- Hannan, A.L.; Harders, M.P.; Hing, W.; Climstein, M.; Coombes, J.S.; Furness, J. Impact of wearable physical activity monitoring devices with exercise prescription or advice in the maintenance phase of cardiac rehabilitation: Systematic review and meta-analysis. BMC Sports Sci. Med. Rehabil. 2019, 11, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marston, H.R.; Hadley, R.; Banks, D.; Duro, M.D.C.M. Mobile Self-Monitoring ECG Devices to Diagnose Arrhythmia that Coincide with Palpitations: A Scoping Review. Healthcare 2019, 7, 96. [Google Scholar] [CrossRef] [Green Version]
- Brørs, G.; Pettersen, T.R.; Hansen, T.B.; Fridlund, B.; Hølvold, L.B.; Lund, H.; Norekvål, T.M. Modes of e-Health delivery in secondary prevention programmes for patients with coronary artery disease: A systematic review. BMC Health Serv. Res. 2019, 19, 364. [Google Scholar] [CrossRef]
- Villarreal, V.; Castillo-Sanchez, G.; Hamrioui, S.; Alvarez, A.B.; Díez, I.D.L.T.; Lorenz, P. A Systematic Review of mHealth apps Evaluations for Cardiac Issues. Multidiscip. Digit. Publ. Inst. Proc. 2018, 2, 481. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, S.J.; Mills, B.; Birch, E.M.; Thompson, S.C. Smartphones in the secondary prevention of cardiovascular disease: A systematic review. BMC Cardiovasc. Disord. 2018, 18, 25. [Google Scholar] [CrossRef]
- Bochicchio, M.A.; Vaira, L.; Mortara, A.; De Maria, R. A preliminar analysis and comparison of international projects on mobile devices and mHealth Apps for heart failure. In Proceedings of the 2019 5th Experiment International Conference (exp.at’19), Madeira Island, Portugal, 12–14 June 2019; pp. 280–285. [Google Scholar]
- Allida, S.; Du, H.; Xu, X.; Prichard, R.; Chang, S.; Hickman, L.D.; Davidson, P.M.; Inglis, S.C. mHealth education interventions in heart failure. Cochrane Database Syst. Rev. 2020, 2020, CD011845. [Google Scholar]
- Schmaderer, M.S.; Struwe, L.; Loecker, C.; Lier, L.; Lundgren, S.W.; Wichman, C.; Pozehl, B.; Zimmerman, L. Mobile Health Self-management Interventions for Patients With Heart Failure. J. Cardiovasc. Nurs. 2021, 1–11. [Google Scholar] [CrossRef]
- Creber, R.M.M.; Maurer, M.S.; Reading, M.; Hiraldo, G.; Hickey, K.T.; Iribarren, S. Review and Analysis of Existing Mobile Phone Apps to Support Heart Failure Symptom Monitoring and Self-Care Management Using the Mobile Application Rating Scale (MARS). JMIR mHealth uHealth 2016, 4, e74. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef] [Green Version]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Zisis, G.; Carrington, M.J.; Oldenburg, B.; Whitmore, K.; Lay, M.; Huynh, Q.; Neil, C.; Ball, J.; Marwick, T.H. An m-Health intervention to improve education, self-management, and outcomes in patients admitted for acute decompensated heart failure: Barriers to effective implementation. Eur. Heart J. Digit. Health 2021, 2, 649–657. [Google Scholar] [CrossRef]
- Bohanec, M.; Tartarisco, G.; Marino, F.; Pioggia, G.; Puddu, P.E.; Schiariti, M.S.; Baert, A.; Pardaens, S.; Clays, E.; Vodopija, A.; et al. HeartMan DSS: A decision support system for self-management of congestive heart failure. Expert Syst. Appl. 2021, 186, 115688. [Google Scholar] [CrossRef]
- Heiney, S.P.; Donevant, S.B.; Adams, S.A.; Parker, P.D.; Chen, H.; Levkoff, S. A Smartphone App for Self-Management of Heart Failure in Older African Americans: Feasibility and Usability Study. JMIR Aging 2020, 3, e17142. [Google Scholar] [CrossRef]
- Koirala, B.; Himmelfarb, C.R.D.; Budhathoki, C.; Davidson, P.M. Heart failure self-care, factors influencing self-care and the relationship with health-related quality of life: A cross-sectional observational study. Heliyon 2020, 6, e03412. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Sanchez, J.; Recio-Rodriguez, J.I.; Fernandez-Delrio, A.; Sanchez-Perez, A.; Magdalena-Belio, J.F.; Gomez-Marcos, M.A.; Garcia-Ortiz, L. Using a smartphone app in changing cardiovascular risk factors: A randomized controlled trial (EVIDENT II study). Int. J. Med. Inform. 2019, 125, 13–21. [Google Scholar] [CrossRef]
- Barrett, M.; Boyne, J.; Brandts, J.; Rocca, H.-P.B.-L.; De Maesschalck, L.; De Wit, K.; Dixon, L.; Eurlings, C.; Fitzsimons, D.; Golubnitschaja, O.; et al. Artificial intelligence supported patient self-care in chronic heart failure: A paradigm shift from reactive to predictive, preventive and personalised care. EPMA J. 2019, 10, 445–464. [Google Scholar] [CrossRef] [Green Version]
- Silva, E.; Rijo, R.; Martinho, R.; Assuncao, P.; Seco, A.; Fonseca-Pinto, R. A Cardiac Rehabilitation Program Supported by mHealth Technology: The MOVIDA.eros Platform. Procedia Comput. Sci. 2018, 138, 119–124. [Google Scholar] [CrossRef]
- Foster, M. A Mobile Application for Patients with Heart Failure: Theory—and Evidence-Based Design and Testing. CIN Comput. Inform. Nurs. 2018, 36, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, B.M.; Ross, E.; Arthur, G.; Brown-Ganzert, L.; Petrin, S.; Sedlak, T.; Lear, S.A. Using Mobile-Health to Connect Women with Cardiovascular Disease and Improve Self-Management. Telemed. e-Health 2017, 23, 233–239. [Google Scholar] [CrossRef]
- De la Torre-Diez, I.; Martinez-Perez, B.; Lopez-Coronado, M.; Rodrigues, J.J.P.C.; Arambarri, J. Development and validation of a mobile health app for the self-management and education of cardiac patients. In Proceedings of the 2016 11th Iberian Conference on Information Systems and Technologies (CISTI), Gran Canaria, Spain, 15–18 June 2016; pp. 1–5. [Google Scholar]
- Rahimi, K.; Velardo, C.; Triantafyllidis, A.; Conrad, N.; Shah, S.A.; Chantler, T.; Mohseni, H.; Stoppani, E.; Moore, F.; Paton, C.; et al. A user-centred home monitoring and self-management system for patients with heart failure: A multicentre cohort study. Eur. Heart J. Qual. Care Clin. Outcomes 2015, 1, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, Y.K.; Haywood, A.; Bentley, C.L.; Parker, J.; Hawley, M.S.; Mountain, G.A.; Mawson, S. The SMART personalised self-management system for congestive heart failure: Results of a realist evaluation. BMC Med. Inform. Decis. Mak. 2014, 14, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Turchioe, M.R.; Jimenez, V.; Isaac, S.; Alshalabi, M.; Slotwiner, D.; Creber, R.M. Review of mobile applications for the detection and management of atrial fibrillation. Heart Rhythm O2 2020, 1, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Pierleoni, P.; Belli, A.; Gentili, A.; Incipini, L.; Palma, L.; Raggiunto, S.; Sbrollini, A.; Burattini, L. Real-time smart monitoring system for atrial fibrillation pathology. J. Ambient Intell. Humaniz. Comput. 2021, 12, 4461–4469. [Google Scholar] [CrossRef]
- Reverberi, C.; Rabia, G.; De Rosa, F.; Bosi, D.; Botti, A.; Benatti, G. The RITMIATM Smartphone App for Automated Detection of Atrial Fibrillation: Accuracy in Consecutive Patients Undergoing Elective Electrical Cardioversion. BioMed Res. Int. 2019, 2019, 4861951. [Google Scholar] [CrossRef] [Green Version]
- Fukuma, N.; Hasumi, E.; Fujiu, K.; Waki, K.; Toyooka, T.; Komuro, I.; Ohe, K. Feasibility of a T-Shirt-Type Wearable Electrocardiography Monitor for Detection of Covert Atrial Fibrillation in Young Healthy Adults. Sci. Rep. 2019, 9, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Bumgarner, J.M.; Lambert, C.T.; Hussein, A.A.; Cantillon, D.J.; Baranowski, B.; Wolski, K.; Lindsay, B.D.; Wazni, O.M.; Tarakji, K.G. Smartwatch Algorithm for Automated Detection of Atrial Fibrillation. J. Am. Coll. Cardiol. 2018, 71, 2381–2388. [Google Scholar] [CrossRef]
- Krivoshei, L.; Weber, S.; Burkard, T.; Maseli, A.; Brasier, N.; Kühne, M.; Conen, D.; Huebner, T.; Seeck, A.; Eckstein, J. Smart detection of atrial fibrillation. Europace 2016, 19, 753–757. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Chen, Y.; Lane, D.A.; Liu, L.; Wang, Y.; Lip, G.Y. Mobile Health Technology for Atrial Fibrillation Management Integrating Decision Support, Education, and Patient Involvement: mAF App Trial. Am. J. Med. 2017, 130, 1388–1396. [Google Scholar] [CrossRef] [Green Version]
- Evans, G.F.; Shirk, A.; Muturi, P.; Soliman, E.Z. Feasibility of Using Mobile ECG Recording Technology to Detect Atrial Fibrillation in Low-Resource Settings. Glob. Heart 2017, 12, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Halcox, J.P.; Wareham, K.; Cardew, A.; Gilmore, M.; Barry, J.P.; Phillips, C.; Gravenor, M.B. Assessment of remote heart rhythm sampling using the AliveCor heart monitor to screen for atrial fibrillation the REHEARSE-AF study. Circulation 2017, 136, 1784–1794. [Google Scholar] [CrossRef]
- Lowres, N.; Mulcahy, G.; Gallagher, R.; Ben Freedman, S.; Marshman, D.; Kirkness, A.; Orchard, J.; Neubeck, L. Self-monitoring for atrial fibrillation recurrence in the discharge period post-cardiac surgery using an iPhone electrocardiogram. Eur. J. Cardio-Thorac. Surg. 2016, 50, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Hickey, K.T.; Hauser, N.R.; Valente, L.E.; Riga, T.C.; Frulla, A.P.; Creber, R.M.; Whang, W.; Garan, H.; Jia, H.; Sciacca, R.R.; et al. A single-center randomized, controlled trial investigating the efficacy of a mHealth ECG technology intervention to improve the detection of atrial fibrillation: The iHEART study protocol. BMC Cardiovasc. Disord. 2016, 16, 152. [Google Scholar] [CrossRef] [Green Version]
- McManus, D.D.; Chong, J.W.; Soni, A.; Saczynski, J.S.; Esa, N.; Napolitano, C.; Darling, C.E.; Boyer, E.; Rosen, R.K.; Floyd, K.C.; et al. PULSE-SMART: Pulse-Based Arrhythmia Discrimination Using a Novel Smartphone Application. J. Cardiovasc. Electrophysiol. 2015, 27, 51–57. [Google Scholar] [CrossRef]
- Kakria, P.; Tripathi, N.K.; Kitipawang, P. A Real-Time Health Monitoring System for Remote Cardiac Patients Using Smartphone and Wearable Sensors. Int. J. Telemed. Appl. 2015, 2015, 373474. [Google Scholar] [CrossRef] [Green Version]
- Brouwers, R.W.; Kraal, J.J.; Traa, S.C.; Spee, R.F.; Oostveen, L.M.; Kemps, H.M. Effects of cardiac telerehabilitation in patients with coronary artery disease using a personalised patient-centred web application: Protocol for the SmartCare-CAD randomised controlled trial. BMC Cardiovasc. Disord. 2017, 17, 46. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Jiang, Y.; Nguyen, H.D.; Poo, D.C.C.; Wang, W. The effect of a smartphone-based coronary heart disease prevention (SBCHDP) programme on awareness and knowledge of CHD, stress, and cardiac-related lifestyle behaviours among the working population in Singapore: A pilot randomised controlled trial. Health Qual. Life Outcomes 2017, 15, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Athilingam, P.; Labrador, M.A.; Remo, E.F.J.; Mack, L.; San Juan, A.B.; Elliott, A.F. Features and usability assessment of a patient-centered mobile application (HeartMapp) for self-management of heart failure. Appl. Nurs. Res. 2016, 32, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Dale, L.P.; Whittaker, R.; Jiang, Y.; Stewart, R.; Rolleston, A.; Maddison, R. Text Message and Internet Support for Coronary Heart Disease Self-Management: Results From the Text4Heart Randomized Controlled Trial. J. Med. Internet Res. 2015, 17, e237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skobel, E.; Martinez-Romero, A.; Scheibe, B.; Schauerte, P.; Marx, N.; Luprano, J.; Knackstedt, C. Evaluation of a newly designed shirt-based ECG and breathing sensor for home-based training as part of cardiac rehabilitation for coronary artery disease. Eur. J. Prev. Cardiol. 2014, 21, 1332–1340. [Google Scholar] [CrossRef]
- Layton, A.M.; Whitworth, J.; Peacock, J.; Bartels, M.N.; Jellen, P.A.; Thomashow, B.M. Feasibility and Acceptability of Utilizing a Smartphone Based Application to Monitor Outpatient Discharge Instruction Compliance in Cardiac Disease Patients around Discharge from Hospitalization. Int. J. Telemed. Appl. 2014, 2014, 415868. [Google Scholar] [CrossRef] [Green Version]
- Dale, L.P.; Whittaker, R.; Jiang, Y.; Stewart, R.; Rolleston, A.; Maddison, R. Improving coronary heart disease self-management using mobile technologies (Text4Heart): A randomised controlled trial protocol. Trials 2014, 15, 71. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Zhu, Q.; Zheng, Y.; Zhu, Y.; Li, Y.; Huo, Y. Perceptions and Acceptance of mHealth in Patients With Cardiovascular Diseases: A Cross-Sectional Study. JMIR mHealth uHealth 2019, 7, e10117. [Google Scholar] [CrossRef]
- Baek, H.; Suh, J.-W.; Kang, S.-H.; Kang, S.; Lim, T.H.; Hwang, H.; Yoo, S. Enhancing User Experience Through User Study: Design of an mHealth Tool for Self-Management and Care Engagement of Cardiovascular Disease Patients. JMIR Cardio 2018, 2, e3. [Google Scholar] [CrossRef]
- Supervía, M.; López-Jimenez, F. mHealth and cardiovascular diseases self-management: There is still a long way ahead of us. Eur. J. Prev. Cardiol. 2018, 25, 974–975. [Google Scholar] [CrossRef]
- Tinsel, I.; Siegel, A.; Schmoor, C.; Poguntke, I.; Maun, A.; Niebling, W. Encouraging Self-Management in Cardiovascular Disease Prevention. Dtsch. Ärztebl. Int. 2018, 115, 469–476. [Google Scholar] [CrossRef]
- Martorella, G.; Graven, L.; Schluck, G.; Bérubé, M.; Gélinas, C. Nurses’ Perception of a Tailored Web-Based Intervention for the Self-Management of Pain After Cardiac Surgery. SAGE Open Nurs. 2018, 4, 237796081880627. [Google Scholar] [CrossRef] [Green Version]
- Johnston, N.; Bodegard, J.; Jerström, S.; Åkesson, J.; Brorsson, H.; Alfredsson, J.; Albertsson, P.A.; Karlsson, J.-E.; Varenhorst, C. Effects of interactive patient smartphone support app on drug adherence and lifestyle changes in myocardial infarction patients: A randomized study. Am. Heart J. 2016, 178, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Gökalp, M.O.; Kayabay, K.; Akyol, M.A.; Koçyiğit, A.; Eren, P.E. Big data in mHealth. In Current and Emerging mHealth Technologies: Adoption, Implementation, and Use; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 241–256. [Google Scholar]
- Khan, Z.F.; Alotaibi, S.R. Applications of Artificial Intelligence and Big Data Analytics in m-Health: A Healthcare System Perspective. J. Healthc. Eng. 2020, 2020, 8894694. [Google Scholar] [CrossRef] [PubMed]
- Baladrón, C.; de Diego, J.J.G.; Amat-Santos, I.J. Big data and new information technology: What cardiologists need to know. Rev. Esp. Cardiol. 2021, 74, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Code, R. Wearable technology in healthcare. Nat. Biotechnol. 2019, 37, 376. [Google Scholar]
- Singhal, A.; Cowie, M.R. The Role of Wearables in Heart Failure. Curr. Heart Fail. Rep. 2020, 17, 125–132. [Google Scholar] [CrossRef]
- Dagher, L.; Shi, H.; Zhao, Y.; Marrouche, N.F. Wearables in cardiology: Here to stay. Heart Rhythm 2020, 17, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Kario, K. Management of Hypertension in the Digital Era: Small Wearable Monitoring Devices for Remote Blood Pressure Monitoring. Hypertension 2020, 76, 640–650. [Google Scholar] [CrossRef]
- Dunn, J.; Runge, R.; Snyder, M. Wearables and the medical revolution. Pers. Med. 2018, 15, 429–448. [Google Scholar] [CrossRef] [Green Version]
- Ambhore, S. Early Detection of Cardiovascular Diseases Using Deep Convolutional Neural Network & Fourier Wavelet Transform. Available online: https://www.sciencedirect.com/science/article/pii/S2214785320392324 (accessed on 20 January 2021).
- Mohan, S.; Thirumalai, C.; Srivastava, G. Effective Heart Disease Prediction Using Hybrid Machine Learning Techniques. IEEE Access 2019, 7, 81542–81554. [Google Scholar] [CrossRef]
- Khan, M.A. An IoT Framework for Heart Disease Prediction Based on MDCNN Classifier. IEEE Access 2020, 8, 34717–34727. [Google Scholar] [CrossRef]
- Raj, S. An Efficient IoT-Based Platform for Remote Real-Time Cardiac Activity Monitoring. IEEE Trans. Consum. Electron. 2020, 66, 106–114. [Google Scholar] [CrossRef]
- Khan, M.A.; Algarni, F. A Healthcare Monitoring System for the Diagnosis of Heart Disease in the IoMT Cloud Environment Using MSSO-ANFIS. IEEE Access 2020, 8, 122259–122269. [Google Scholar] [CrossRef]




| Area | Keywords | Related Concepts |
|---|---|---|
| Cardiovascular disease | Self-management Self-care Self-monitoring Heart disease Cardiac issues Heart failure Arrhythmia Coronary heart disease Atrial Fibrillation (AF) Hypertension Cardiac arrest Peripheral artery disease | mHealth mobile application smart application wearable smartwatch app |
| Study Reference | CVD | Main App Functionalities | Challenges and/or Future Work Remarks | Approaches | Device or Web/Mobile Application | CVD Phase |
|---|---|---|---|---|---|---|
| Zisis et al. [27] | Heart failure | Medical recommendations, reminders, weight control | Computer skills of the patient, hearing problems, impaired vision, and cognitive impairment | Supervised machine learning (classification) | Smartphone or Tablet, Heart Failure app | Monitoring, treatment |
| Bohanec et al. [28] | Heart failure | Nutrition management, managing medication intake, psychological support, daily Exercise management, monitoring biomedical variables, medical recommendations | Increased adaptation to the patients’ lifestyle, add methods for recognizing patients’ activities, and integrating the optimization module in a smart-home environment | Supervised machine learning (random forest algorithm), classic differential evolution algorithm, and IoT device (heart rate, blood pressure) | Wristband, Blood pressure monitor, HeartMan Web app | Monitoring, treatment |
| Heiney et al. [29] | Heart failure | Text messages for communication between patients and physicians, weight and symptoms control, medical recommendations, medication management | Disparate population with low literacy, low health literacy, and limited smartphone use | IoT device (heart rate) | Smartphone, Healthy Heart app | Monitoring, treatment |
| Koirala et al. [30] | Heart failure | Medical recommendations | Implement the app in a real environment | Big data type (unstructured data), Supervised machine learning | Smartphone | Prevention, diagnosis |
| Gonzalez-Sanchez et al. [31] | Heart failure | Medical recommendations | Overcome patient resistance behavior toward using technology Add more functionality to the mobile app | Unsupervised machine learning | Smartphone, Evident II app | Prevention |
| Barret et al. [32] | Heart failure | Medical recommendations | Measure patient variables Greater focus on CVD asymptomatic patients | Unsupervised machine learning | Smartphone, Abby Web app | Prevention, treatment |
| Silva et al. [33] | Heart failure | Medical recommendations | Ensure interoperability of mHealth apps for remote monitoring, Heart rate measurement automation | Unsupervised machine learning | Smartphone, MOVIDA.eros app | Monitoring, treatment |
| Foster [34] | Heart failure | Medical recommendations, alerts | Implement the app in a real environment | Unsupervised machine learning | Smartphone, HF mobile app | Monitoring, treatment |
| Sakakibara et al. [35] | Heart failure | Medical recommendations, alerts, medication management | Implement the app in a real environment | Big data type (unstructured data) | Smartphone, mobile app | Prevention, treatment |
| De la Torre-Diez et al. [36] | Heart failure | Medical recommendations, alerts | Integrate the app system with EMR systems, Improve the usability of the mobile app, Add serious games to the app | Unsupervised machine learning | Smartphone, Heartkeeper app | Treatment |
| K. Rahimi et al. [37] | Heart failure | Medical recommendations, alerts, medication management | Integrate the app system with EMR systems, Increase wearable precision | Unsupervised machine learning, IoT device (heart rate, sensor Sp02) | Smartphone, SUPPORT-HF app, Oximeter | Monitoring, treatment |
| Bartlett et al. [38] | Heart failure | Step count calculation, weight control, blood pressure control | Overcome technological problems | IoT device (heart rate, blood pressure) | SMART Personalized Self-Management System (PSMS), HTC HD2 phone, MiFi device, mobile app | Monitoring, treatment |
| Turchioe et al. [39] | Arrhythmia | Medical recommendations | Overcome patient resistance to technology | Unsupervised machine learning | Smartphone | Prevention, monitoring |
| Pierleoni et al. [40] | Arrhythmia | Medical recommendations, alerts | Implement application in a real environment | Big data type (unstructured data), Unsupervised machine learning | Smartphone | Monitoring, treatment |
| Reverberi et al. [41] | Arrhythmia | AF detection | Implement algorithm for AF detection | IoT device (heart rate, ECG), Supervised machine learning (classification) | HR monitor of the chest-strap type, RITMIA app | Prevention |
| Fukuma et al. [42] | Arrhythmia | AF detection | Increase patient monitoring time | IoT device (heart rate, ECG) | T-Shirt-type wearable, ECG monitor, Hitoe Transmitter 01, smartphone | Prevention, treatment |
| Bumgarner et al. [43] | Arrhythmia | AF detection | Increase sample size, Increase the performance of the KB smartwatch algorithm, Review the real-time display of the ECG recording | IoT device (heart rate, blood pressure), Unsupervised machine learning | Kardia Band, Apple Watch, KB app | Prevention, monitoring |
| Krivoshei et al. [44] | Arrhythmia | AF detection, monitoring of heart rate, pulse wave analysis | Test the algorithm on a smartwatch | Unsupervised machine learning | Smartphone, iPhone 4S | Prevention |
| Guo et al. [45] | Arrhythmia | Medical recommendations, medication management, alerts, medical record | Overcome patient resistance to using technology | Supervised machine learning | Smartphone, mAF app | Treatment |
| Evans et al. [46] | Arrhythmia | AF detection | Extend study to other hospitals serving low-resource areas, Ensure interoperability with further systems | IoT device (heart rate, blood pressure), Supervised machine learning (classification) | AliveCor Kardia mobile ECG device, iPhone and iPad | Diagnosis, monitoring |
| Halcox et al. [47] | Arrhythmia | AF detection | The relatively high false-positive rate in the minor proportion of those reported as AF by the device | IoT device (heart rate, blood pressure), Supervised machine learning (classification) | AliveCor Kardia device, iPad | Diagnosis, monitoring |
| Lowres et al. [48] | Arrhythmia | iPhone handheld electrocardiogram (iECG) | Using iECG self-monitoring among other patient groups | Supervised machine learning | iPhone and AliveCor Heart monitor (iECG) | Monitoring |
| Hickey et al. [49] | Arrhythmia | AF detection | Implement the application in a real environment | IoT device (heart rate, blood pressure), Supervised machine learning (classification) | AliveCor Kardia mobile ECG device, iPhone | Diagnosis, monitoring |
| McManus et al. [50] | Arrhythmia | AF detection | Improve pulse recording and app performance | IoT device (heart rate), Supervised machine learning (classification) | PULSE-SMART app, iPhone 4S | Diagnosis, monitoring |
| Kakria et al. [51] | Arrhythmia | Alerts, monitoring of heart rate, blood pressure, and temperature | Solve the problem of delayed alarms in remote areas | IoT device (heart rate, blood pressure, stress level) | Smartphone, Zephyr BT system, G plus sensor, the Omron Wireless Upper Arm blood pressure monitor | Diagnosis, monitoring |
| Brouwers et al. [52] | Coronary heart disease | Medical recommendations, alerts | Sedentary patients | IoT device (heart rate) | Patient-centered web app, accelerometer, heart rate monitor | Monitoring, treatment |
| Zhang et al. [53] | Coronary heart disease | Medical recommendations | Ensure interoperability of applications for remote monitoring | Big data type (unstructured data), Unsupervised machine learning | Smartphone, Care4Heart app | Prevention |
| Athilingam [54] | Coronary heart disease | Medical recommendations, alerts, medication management | Overcome patient resistance to using technology Replace current sensor with handheld sensor | IoT device (heart rate), Supervised machine learning | Smartphone, HeartMapp, BioHarness Bluetooth sensor | Monitoring, treatment |
| Dale et al. [55] | Coronary heart disease | Text messages for communication of patients and physicians | Implement the app in a real environment | Big data type (structured data) | Smartphone | Treatment |
| Skobel et al. [56] | Coronary heart disease | Exercise module, activity level monitoring | Automatic arrhythmia detection | IoT device (heart rate, ECG, respiration, activity), Supervised machine learning | HeartCycle’s guided exercise (GEX) system, tablet or laptop, portable PDA for ECG display, shirt with sensors | Diagnosis, monitoring |
| AM et al. [57] | Coronary heart disease | Educational material, medication reminders, and activity level monitoring | Train medical personnel and patients | IoT device (heart rate) | Smartphone | Monitoring, treatment |
| Dale et al. [58] | Coronary heart disease | Text messages for communication of patients and physicians, medical recommendations, weight control | Implement app in a real environment | IoT device (heart rate) | Smartphone, web app Text4Heart | Treatment |
| Jiang et al. [59] | Several (coronary heart disease and hypertension) | Alerts, medication management | Achieve acceptance of mHealth solutions among older patient populations, Improve app design | Supervised machine learning (Regression) | Smartphone, mobile app | Treatment |
| Baek et al. [60] | Several (atrial fibrillation, hypertension, chest pain, vasovagal syncope, variant angina, and dyspnea on exertion) | Medical recommendations, alerts, diary, weight control | Improve app usability, Integrate app system with EMR (Electronic Medical Record) systems | IoT device (heart rate) | Smartphone | Treatment, monitoring |
| Supervía & López-Jimenez [61] | Several (heart failure, coronary heart disease, tachycardias, arrhythmia, and hypertension) | Medical recommendations | Guarantee patient data protection and confidentiality | Unsupervised machine learning | Smartphone | Treatment |
| Tinsel et al. [62] | Several (heart failure, Coronary heart disease, tachycardias, arrhythmia, and hypertension) | Medical recommendations, alerts | Overcome patient resistance to using technology | IoT device (heart rate) | Mobile app | Prevention, treatment |
| Martorella et al. [63] | Several (heart failure, coronary heart disease, tachycardias, arrhythmia and hypertension) | Medical recommendations, medication management | Screen questionnaire to tailor content according to chronic postsurgical pain (CPSP) risk factors | Not specified | Web app | Monitoring, treatment |
| Johnston et al. [64] | Several (myocardial infarction, angina pectoris, heart failure, atrial fibrillation, embolic stroke, peripheral artery disease, hypertension) | Medication management, text messaging, reminders, e-diary, exercise module, BMI module, and blood pressure module | Improve patient self-reported drug adherence | IoT device (heart rate) | Smartphone, web-based app | Treatment |
| CVD | Study | Mobile App Name | Android | iOS |
|---|---|---|---|---|
| Heart failure | Zisis et al. [27] | Heart Failure app | ✓ | |
| Bohanec et al. [28] | HeartMan | ✓ | ||
| Heiney et al. [29] | Healthy Heart | ✓ | ||
| Gonzalez-Sanchez et al. [31] | Evident II | ✓ | ||
| Barret et al. [32] | Abby | ✓ | ||
| Silva et al. [33] | MOVIDA.eros | ✓ | ✓ | |
| Foster [34] | HF mobile app | ✓ | ✓ | |
| Sakakibara et al. [35] Bartlett et al. [38] | Not specified | ✓ | ||
| De la Torre-Diez et al. [36] | HeartKeeper | ✓ | ||
| K. Rahimi et al. [37] | SUPPORT-HF | ✓ | ||
| Arrhythmia | Reverberi et al. [41] | RITMIA | ✓ | |
| Bumgarner et al. [43] Evans et al. [46] Halcox et al. [47] Lowres et al. [48] Hickey et al. [49] | Kardia app | ✓ | ||
| Krivoshei et al. [44] | Unstated | ✓ | ||
| Guo et al. [45] | mAF app | ✓ | ✓ | |
| McManus et al. [50] | PULSE-SMART | ✓ | ||
| Kakria et al. [51] | Not specified | ✓ | ||
| Coronary heart disease | Zhang et al. [53] | Care4Heart | ✓ | ✓ |
| Athilingam [54] | HeartMapp | ✓ | ||
| AM et al. [57] | Not specified | ✓ | ||
| Dale et al. [58] | Text4Heart | ✓ | ||
| Other CVDs | Jiang et al. [59] | Not specified | ✓ | |
| Supervía & López-Jimenez [61] Tinsel et al. [62] | Not specified | ✓ | ✓ |
| CVD | Study | F1 | F2 | F3 | F4 | F5 | F6 |
|---|---|---|---|---|---|---|---|
| Heart failure | Zisis et al. [27] | ✓ | ✓ | ✓ | ✓ | ||
| Bohanec et al. [28] | ✓ | ✓ | ✓ | ||||
| Heiney et al. [29] | ✓ | ✓ | ✓ | ✓ | |||
| Koirala et al. [30] | ✓ | ||||||
| Gonzalez-Sanchez et al. [31] | ✓ | ||||||
| Barret et al. [32] | ✓ | ||||||
| Silva et al. [33] | ✓ | ||||||
| Foster [34] | ✓ | ✓ | |||||
| Sakakibara et al. [35] | ✓ | ✓ | ✓ | ||||
| De la Torre-Diez et al. [36] | ✓ | ✓ | |||||
| K. Rahimi et al. [37] | ✓ | ✓ | ✓ | ||||
| Bartlett et al. [38] | ✓ | ||||||
| Arrhythmia | Turchioe et al. [39] | ✓ | |||||
| Pierleoni et al. [40] | ✓ | ✓ | |||||
| Reverberi et al. [41] | ✓ | ||||||
| Fukuma et al. [42] | ✓ | ||||||
| Bumgarner et al. [43] | ✓ | ||||||
| Krivoshei et al. [44] | ✓ | ✓ | |||||
| Guo et al. [45] | ✓ | ✓ | ✓ | ✓ | |||
| Evans et al. [46] | ✓ | ||||||
| Halcox et al. [47] | ✓ | ||||||
| Lowres et al. [48] | ✓ | ||||||
| Hickey et al. [49] | ✓ | ||||||
| McManus et al. [50] | ✓ | ||||||
| Kakria et al. [51] | ✓ | ✓ | |||||
| Coronary heart disease | Brouwers et al. [52] | ✓ | ✓ | ||||
| Zhang et al. [53] | ✓ | ||||||
| Athilingam [54] | ✓ | ✓ | ✓ | ||||
| Dale et al. [55] | ✓ | ||||||
| Skobel et al. [56] | ✓ | ||||||
| AM et al. [57] | ✓ | ✓ | |||||
| Dale et al. [58] | ✓ | ✓ | ✓ | ||||
| Several | Jiang et al. [59] | ✓ | ✓ | ||||
| Baek et al. [60] | ✓ | ✓ | ✓ | ✓ | |||
| Supervía & López-Jimenez [61] | ✓ | ||||||
| Tinsel et al. [62] | ✓ | ✓ | |||||
| Martorella et al. [63] | ✓ | ✓ | |||||
| Johnston et al. [64] | ✓ | ✓ | ✓ | ✓ |
| CVD | Study | Machine Learning Techniques and Tasks | Big Data Types | IoT Devices/Sensors |
|---|---|---|---|---|
| Heart failure | Zisis et al. [27] | ✓ | ✓ | |
| Bohanec et al. [28] | ✓ | ✓ | ||
| Heiney et al. [29] | ✓ | |||
| Koirala et al. [30] | ✓ | ✓ | ||
| Gonzalez-Sanchez et al. [31] | ✓ | |||
| Barret et al. [32] | ✓ | |||
| Silva et al. [33] | ✓ | |||
| Foster [34] | ✓ | |||
| Sakakibara et al. [35] | ✓ | |||
| De la Torre-Diez et al. [36] | ✓ | |||
| K. Rahimi et al. [37] | ✓ | ✓ | ||
| Bartlett et al. [38] | ✓ | |||
| Arrhythmia | Turchioe et al. [39] | ✓ | ||
| Pierleoni et al. [40] | ✓ | ✓ | ||
| Reverberi et al. [41] | ✓ | |||
| Fukuma et al. [42] | ✓ | |||
| Bumgarner et al. [43] | ✓ | ✓ | ||
| Krivoshei et al. [44] | ✓ | |||
| Guo et al. [45] | ✓ | |||
| Evans et al. [46] | ✓ | ✓ | ||
| Halcox et al. [47] | ✓ | ✓ | ||
| Lowres et al. [48] | ✓ | |||
| Hickey et al. [49] | ✓ | ✓ | ||
| McManus et al. [50] | ✓ | ✓ | ||
| Kakria et al. [51] | ✓ | |||
| Coronary heart disease | Brouwers et al. [52] | ✓ | ||
| Zhang et al. [53] | ✓ | ✓ | ||
| Athilingam [54] | ✓ | |||
| Skobel et al. [56] | ✓ | ✓ | ||
| AM et al. [57] | ✓ | |||
| Dale et al. [58] | ✓ | |||
| Several | Jiang et al. [59] | ✓ | ||
| Baek et al. [60] | ✓ | |||
| Supervía & López-Jimenez [61] | ✓ | |||
| Tinsel et al. [62] | ✓ |
| CVD | Study | W1 | W2 | W3 | W4 | W5 |
|---|---|---|---|---|---|---|
| Heart failure | Bohanec et al. [28] | ✓ | ✓ | |||
| Bartlett et al. [38] | ✓ | |||||
| Arrhythmia | Reverberi et al. [41] | ✓ | ||||
| Fukuma et al. [42] | ✓ | |||||
| Bumgarner et al. [43] | ✓ | ✓ | ||||
| Evans et al. [46] | ✓ | ✓ | ||||
| Halcox et al. [47] | ✓ | ✓ | ||||
| Lowres et al. [48] | ✓ | ✓ | ||||
| Hickey et al. [49] | ✓ | ✓ | ||||
| Kakria et al. [51] | ✓ | ✓ | ||||
| Coronary heart disease | Brouwers et al. [52] | ✓ | ||||
| Athilingam [54] | ✓ | ✓ | ||||
| Skobel et al. [56] | ✓ | ✓ |
| CVD | Study | Prevention | Diagnosis | Monitoring | Treatment |
|---|---|---|---|---|---|
| Heart failure | Zisis et al. [27] | ✓ | ✓ | ||
| Bohanec et al. [28] | ✓ | ✓ | |||
| Heiney et al. [29] | ✓ | ✓ | |||
| Koirala et al. [30] | ✓ | ✓ | |||
| Gonzalez-Sanchez et al. [31] | ✓ | ||||
| Barret et al. [32] | ✓ | ✓ | |||
| Silva et al. [33] | ✓ | ✓ | |||
| Foster [34] | ✓ | ✓ | |||
| Sakakibara et al. [35] | ✓ | ✓ | |||
| De la Torre-Diez et al. [36] | ✓ | ||||
| K. Rahimi et al. [37] | ✓ | ✓ | |||
| Bartlett et al. [38] | ✓ | ✓ | |||
| Arrhythmia | Turchioe et al. [39] | ✓ | ✓ | ||
| Pierleoni et al. [40] | ✓ | ✓ | |||
| Reverberi et al. [41] | ✓ | ||||
| Fukuma et al. [42] | ✓ | ✓ | |||
| Bumgarner et al. [43] | ✓ | ✓ | |||
| Krivoshei et al. [44] | ✓ | ||||
| Guo et al. [45] | ✓ | ||||
| Evans et al. [46] | ✓ | ✓ | |||
| Halcox et al. [47] | ✓ | ✓ | |||
| Lowres et al. [48] | ✓ | ||||
| Hickey et al. [49] | ✓ | ✓ | |||
| McManus et al. [50] | ✓ | ✓ | |||
| Kakria et al. [51] | ✓ | ✓ | |||
| Coronary heart disease | Brouwers et al. [52] | ✓ | ✓ | ||
| Zhang et al. [53] | ✓ | ||||
| Athilingam [54] | ✓ | ✓ | |||
| Dale et al. [55] | ✓ | ||||
| Skobel et al. [56] | ✓ | ✓ | |||
| AM et al. [57] | ✓ | ✓ | |||
| Dale et al. [58] | ✓ | ||||
| Several | Jiang et al. [59] | ✓ | |||
| Baek et al. [60] | ✓ | ✓ | |||
| Supervía & López-Jimenez [61] | ✓ | ||||
| Tinsel et al. [62] | ✓ | ✓ | |||
| Martorella et al. [63] | ✓ | ✓ | |||
| Johnston et al. [64] | ✓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Ramos, N.A.; Alor-Hernández, G.; Colombo-Mendoza, L.O.; Sánchez-Cervantes, J.L.; Rodríguez-Mazahua, L.; Guarneros-Nolasco, L.R. mHealth Apps for Self-Management of Cardiovascular Diseases: A Scoping Review. Healthcare 2022, 10, 322. https://doi.org/10.3390/healthcare10020322
Cruz-Ramos NA, Alor-Hernández G, Colombo-Mendoza LO, Sánchez-Cervantes JL, Rodríguez-Mazahua L, Guarneros-Nolasco LR. mHealth Apps for Self-Management of Cardiovascular Diseases: A Scoping Review. Healthcare. 2022; 10(2):322. https://doi.org/10.3390/healthcare10020322
Chicago/Turabian StyleCruz-Ramos, Nancy Aracely, Giner Alor-Hernández, Luis Omar Colombo-Mendoza, José Luis Sánchez-Cervantes, Lisbeth Rodríguez-Mazahua, and Luis Rolando Guarneros-Nolasco. 2022. "mHealth Apps for Self-Management of Cardiovascular Diseases: A Scoping Review" Healthcare 10, no. 2: 322. https://doi.org/10.3390/healthcare10020322
APA StyleCruz-Ramos, N. A., Alor-Hernández, G., Colombo-Mendoza, L. O., Sánchez-Cervantes, J. L., Rodríguez-Mazahua, L., & Guarneros-Nolasco, L. R. (2022). mHealth Apps for Self-Management of Cardiovascular Diseases: A Scoping Review. Healthcare, 10(2), 322. https://doi.org/10.3390/healthcare10020322