Effect of Incorporating Short-Foot Exercises in the Balance Rehabilitation of Flat Foot: A Randomized Controlled Trial
Abstract
:1. Introduction
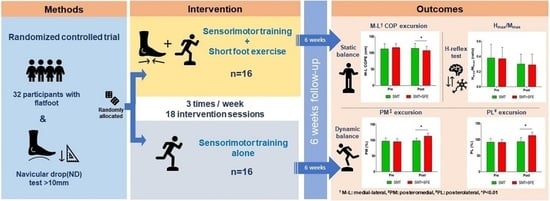
2. Materials and Methods
2.1. Study Design
2.2. Outcome Measures
2.2.1. Static Balance
2.2.2. Dynamic Balance
2.2.3. Hoffman Reflex (H-Reflex)
2.3. Interventions
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neumann, D.A. Kinesiology of the Musculoskeletal System: Foundations for Rehabilitation, 2nd ed.; Mosby: Maryland Heights, MO, USA, 2010; pp. 593–595. [Google Scholar]
- Toullec, E. Adult flatfoot. Orthop. Traumatol. Surg. Res. 2015, 101, 11–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munro, B.; Steele, J. Foot-care awareness. A survey of persons aged 65 years and older. J. Am. Podiatr. Med. Assoc. 1998, 88, 242–248. [Google Scholar] [CrossRef]
- Otsuka, R.; Yatsuya, H.; Miura, Y.; Murata, C.; Tamakoshi, K.; Oshiro, K.; Nishio, N.; Ishikawa, M.; Zhang, H.M.; Shiozawa, M.; et al. Association of flatfoot with pain, fatigue and obesity in Japanese over sixties. Nihon Koshu Eisei Zasshi 2003, 50, 988–998. [Google Scholar]
- Franco, A.H. Pes cavus and pes planus. analyses and treatment. Phys. Ther. 1987, 67, 688–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hertel, J.; Gay, M.R.; Denegar, C.R. Differences in postural control during single-leg stance among healthy individuals with different foot types. J. Athl. Train. 2002, 37, 129–132. [Google Scholar] [PubMed]
- Tsai, L.-C.; Yu, B.; Mercer, V.S.; Gross, M.T. Comparison of different structural foot types for measures of standing postural control. J. Orthop. Sports Phys. Ther. 2006, 36, 942–953. [Google Scholar] [CrossRef] [Green Version]
- LeDoux, W.R.; Shofer, J.B.; Ahroni, J.H.; Smith, D.G.; Sangeorzan, B.J.; Boyko, E. Biomechanical differences among pes cavus, neutrally aligned, and pes planus feet in subjects with diabetes. Foot Ankle Int. 2003, 24, 845–850. [Google Scholar] [CrossRef]
- Williams Iii, D.S.; McClay, I.S.; Hamill, J. Arch structure and injury patterns in runners. Clin. Biomech. 2001, 16, 341–347. [Google Scholar] [CrossRef]
- Loudon, J.K.; Jenkins, W.; Loudon, K.L. The relationship between static posture and ACL injury in female athletes. J. Orthop. Sports Phys. Ther. 1996, 24, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Kosashvili, Y.; Fridman, T.; Backstein, D.; Safir, O.; Bar Ziv, Y. The correlation between pes planus and anterior knee or in-termittent low back pain. Foot Ankle Int. 2008, 29, 910–913. [Google Scholar] [CrossRef]
- Kothari, A.; Dixon, P.C.; Stebbins, J.; Zavatsky, A.B.; Theologis, T. Are flexible flat feet associated with proximal joint problems in children? Gait Post. 2016, 45, 204–210. [Google Scholar] [CrossRef]
- Janda, V. Muscles and motor control in low back pain: Assessment and management. In Physical Therapy of the Low Back; Twomey, L.T., Ed.; Churchill Livingstone: New York, NY, USA, 1987; pp. 253–278. [Google Scholar]
- Madureira, M.M.; Takayama, L.; Gallinaro, A.L.; Caparbo, V.F.; Costa, R.A.; Pereira, R.M. Balance training program is highly effective in improving functional status and reducing the risk of falls in elderly women with osteoporosis: A randomized con-trolled trial. Osteoporos. Int. 2007, 18, 419–425. [Google Scholar] [CrossRef] [Green Version]
- Mckeon, P.O.; Ingersoll, C.D.; Kerrigan, D.C.; Saliba, E.; Bennett, B.C.; Hertel, J. Balance training improves function and postural control in those with chronic ankle instability. Med. Sci. Sports Exerc. 2008, 40, 1810–1819. [Google Scholar] [CrossRef]
- Paterno, M.V.; Myer, G.D.; Ford, K.R.; Hewett, T.E. Neuromuscular training improves single-limb stability in young female athletes. J. Orthop. Sports Phys. Ther. 2004, 34, 305–316. [Google Scholar] [CrossRef] [Green Version]
- Taube, W.; Kullmann, N.; Leukel, C.; Kurz, O.; Amtage, F.; Gollhofer, A. Differential reflex adaptations following sensorimotor and strength training in young elite athletes. Int. J. Sports Med. 2007, 28, 999–1005. [Google Scholar] [CrossRef]
- Freeman, M.A.R.; Wyke, B. Articular contributions to limb muscle reflexes. The effects of partial neurectomy of the knee-joint on postural reflexes. Br. J. Surg. 2005, 53, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Maki, B.E.; Perry, S.D.; Norrie, R.G.; McIlroy, W.E. Effect of facilitation of sensation from plantar foot-surface boundaries on postural stabilization in young and older adults. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 1999, 54, 281–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, I.; Okubo, J. The role of the plantar mechanoreceptor in equilibrium control. Ann. N. Y. Acad. Sci. 1981, 374, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Brachman, A.; Kamieniarz, A.; Michalska, J.; Pawłowski, M.; Słomka, K.J.; Juras, G. Balance training programs in athletes—A systematic review. J. Hum. Kinet. 2017, 58, 45–64. [Google Scholar] [CrossRef] [Green Version]
- Moon, D.C.; Kim, K.; Lee, S.K. Immediate effect of short-foot exercise on dynamic balance of subjects with excessively pronated feet. J. Phys. Ther. Sci. 2014, 26, 117–119. [Google Scholar] [CrossRef] [Green Version]
- Liebenson, C. Sensory motor training. J. Bodyw. Mov. Ther. 2001, 5, 21–27. [Google Scholar] [CrossRef]
- Jung, D.-Y.; Kim, M.-H.; Koh, E.-K.; Kwon, O.-Y.; Cynn, H.-S.; Lee, W.-H. A comparison in the muscle activity of the abductor hallucis and the medial longitudinal arch angle during toe curl and short foot exercises. Phys. Ther. Sport 2011, 12, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.-Y.; Koh, E.-K.; Kwon, O.-Y. Effect of foot orthoses and short-foot exercise on the cross-sectional area of the abductor hallucis muscle in subjects with pes planus: A randomized controlled trial1. J. Back Musculoskelet. Rehabil. 2011, 24, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Prentice, W.E. Rehabilitation Techniques for Sports Medicine and Athletic Training, 5th ed.; McGraw Hill Higher Education: New York, NY, USA, 2011; pp. 590–597. [Google Scholar]
- Lynn, S.K.; Padilla, R.A.; Tsang, K.K. Differences in static-and dynamic balance task performance after 4 weeks of intrinsic-foot-muscle training: The short foot exercise versus the towel-curl exercise. J. Sport Rehabil. 2012, 21, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, E.P.; Cook, P.G. Effect of plantar intrinsic muscle training on medial longitudinal arch morphology and dynamic function. Man. Ther. 2013, 18, 425–430. [Google Scholar] [CrossRef] [PubMed]
- McKeon, P.O.; Hertel, J.; Bramble, D.; Davis, I. The foot core system: A new paradigm for understanding intrinsic foot muscle function. Br. J. Sports Med. 2014, 49, 290. [Google Scholar] [CrossRef] [Green Version]
- Mignogna, C.A.; Welsch, L.A.; Hoch, M.C. The effects of short-foot exercises on postural control: A critically appraised topic. Int. J. Athl. Ther. Train. 2016, 21, 8–12. [Google Scholar] [CrossRef]
- Page, P. Sensorimotor training: A “global” approach for balance training. J. Body Mov. Ther. 2006, 10, 77–84. [Google Scholar] [CrossRef]
- Shinohara, J.; Gribble, P. Five-toed socks decrease static postural control among healthy individuals as measured with time-to-boundary analysis. In Proceedings of the American Society of Biomechanics Annual Meeting, State College, PA, USA, 28 August 2009. [Google Scholar]
- Janda, V.; Vavrova, M.; Herbenova, A.; Veverkova, M. Sensory motor stimulation. In Rehabilitation of the Spine; Liebenson, C., Ed.; Lippincott, Williams, & Wilkins: Baltimore, MD, USA, 2007; pp. 513–530. [Google Scholar]
- Michell, T.B.; Ross, S.E.; Blackburn, J.T.; Hirth, C.J.; Guskiewicz, K.M. Functional balance training, with or without exercise sandals, for subjects with stable or unstable ankles. J. Athl. Train. 2007, 41, 393–398. [Google Scholar]
- Sauer, L.D.; Saliba, S.A.; Ingersoll, C.D.; Kerrigan, D.C.; Pietrosimone, B.P.; Hertel, J. Effects of rehabilitation incorporating short foot exercises on self-reported function, static and dynamic balance in chronic ankle instability patients. J. Athl. Train. 2010, 45, 67. [Google Scholar]
- Rothermel, S.A.; Hale, S.A.; Hertel, J.; Denegar, C.R. Effect of active foot positioning on the outcome of a balance training program. Phys. Ther. Sport 2004, 5, 98–103. [Google Scholar] [CrossRef]
- McPoil, T.G.; Cornwall, M.W.; Medoff, L.; Vicenzino, B.; Forsberg, K.; Hilz, D. Arch height change during sit-to-stand: An alternative for the navicular drop test. J. Foot Ankle Res. 2008, 1, 3. [Google Scholar] [CrossRef] [Green Version]
- Blackburn, T.; Guskiewicz, K.M.; Petschauer, M.A.; Prentice, W.E. Balance and joint stability: The relative contributions of proprioception and muscular strength. J. Sport Rehabil. 2000, 9, 315–328. [Google Scholar] [CrossRef] [Green Version]
- Plisky, P.J.; Gorman, P.P.; Butler, R.J.; Kiesel, K.B.; Underwood, F.B.; Elkins, B. The reliability of an instrumented device for measuring components of the star excursion balance test. N. Am. J. Sports Phys. 2009, 4, 92–99. [Google Scholar]
- Hertel, J.; Miller, S.J.; Denegar, C.R. Intratester and intertester reliability during the star excursion balance tests. J. Sport Rehabil. 2000, 9, 104–116. [Google Scholar] [CrossRef] [Green Version]
- Gribble, P.A.; Hertel, J. Considerations for normalizing measures of the star excursion balance test. Meas. Phys. Educ. Exerc. Sci. 2003, 7, 89–100. [Google Scholar] [CrossRef]
- Sefton, J.M.; Hicks-Little, C.A.; Koceja, D.M.; Cordova, M.L. Modulation of soleus H-reflex by presynaptic spinal mechanisms during varying surface and ankle brace conditions. Neurophysiol. Clin. Neurophysiol. 2007, 37, 15–21. [Google Scholar] [CrossRef]
- Trimble, M.H.; Koceja, D.M. Effect of a reduced base of support in standing and balance training on the soleus H-reflex. Int. J. Neurosci. 2001, 106, 1–20. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988; pp. 20–27. [Google Scholar]
- Kavounoudias, A.; Roll, R.; Roll, J.P. Foot sole and ankle muscle inputs contribute jointly to human erect posture regulation. J. Physiol. 2001, 532, 869–878. [Google Scholar] [CrossRef]
- Slobounov, S.; Newell, K.M. Postural dynamics as a function of skill level and task constraints. Gait Posture 1994, 2, 85–93. [Google Scholar] [CrossRef]
- Gribble, P.A.; Hertel, J.; Plisky, P. Using the star excursion balance test to assess dynamic postural-control deficits and out-comes in lower extremity injury: A literature and systematic review. J. Athl. Train. 2012, 47, 339–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zech, A.; Hübscher, M.; Vogt, L.; Banzer, W.; Hänsel, F.; Pfeifer, K. Balance training for neuromuscular control and performance enhancement: A systematic review. J. Athl. Train. 2010, 45, 392–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong-Yu, I.S.; Mak, M.K. Task-and context-specific balance training program enhances dynamic balance and functional performance in Parkinsonian nonfallers: A randomized controlled trial with six-month follow-up. Arch. Phys. Med. Rehabil. 2015, 96, 2103–2111. [Google Scholar] [CrossRef] [PubMed]
- Eils, E.; Rosenbaum, D. A multi-station proprioceptive exercise program in patients with ankle instability. Med. Sci. Sports Exerc. 2001, 33, 1991–1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alizadehsaravi, L.; Bruijn, S.M.; Maas, H.; van Dieën, J.H. Modulation of soleus muscle H-reflexes and ankle muscle co-contraction with surface compliance during unipedal balancing in young and older adults. Exp. Brain Res. 2020, 238, 1371–1383. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-S.; Zhou, S. Soleus H-reflex and its relation to static postural control. Gait Posture 2011, 33, 169–178. [Google Scholar] [CrossRef]
- Gruber, M.; Taube, W.; Gollhofer, A.; Beck, S.; Amtage, F.; Schubert, M. Training-specific adaptations of h-and stretch reflexes in human soleus muscle. J. Mot. Behav. 2007, 39, 68–78. [Google Scholar] [CrossRef]
- Taube, W. Neurophysiological adaptations in response to balance training. Dtsch. Z. Sportmed. 2012, 2012, 273–277. [Google Scholar] [CrossRef]



| Week 1: | Week 2: |
|
|
| Week 3: | Week 4: |
|
|
| Week 5: | Week 6: |
|
|
| Variables | SMT Alone (n = 16) | SMT Combined with SFE (n = 16) | p |
|---|---|---|---|
| Age (years) | 21.81 ± 3.60 | 21.56 ± 1.83 | 0.806 |
| Gender (male/female) | 7/9 | 7/9 | - |
| Height (cm) | 165.69 ± 7.19 | 165.69 ± 6.05 | 1.000 |
| Body mass (kg) | 58.69 ± 8.91 | 61.81 ± 13.99 | 0.613 |
| Leg length (cm) | 82.78 ± 4.31 | 83.34 ± 3.98 | 0.704 |
| Navicular drop (mm) | 13.19 ± 1.48 | 13.03 ± 1.71 | 0.785 |
| Variables (Unit) | SMT Alone (n = 16) | SMT Combined with SFE (n = 16) | Effect | F | p | ES | |||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||||||
| Static balance | A-P COPE (cm) | 99.20 ± 24.47 | 97.19 ± 19.40 | 95.85 ± 7.42 | 92.42 ± 8.83 | Group | 0.506 | 0.482 | 0.316 |
| Time | 3.653 | 0.066 | |||||||
| Group × Time | 0.250 | 0.621 | |||||||
| M-L COPE (cm) | 112.40 ± 15.73 | 114.09 ± 14.74 | 116.31 ± 11.89 | 107.01 ± 12.93 | Group | 0.117 | 0.734 | 0.510 | |
| Time | 5.388 | 0.027 * | |||||||
| Group × Time | 11.234 | 0.002 * | |||||||
| Dynamic balance | ANT reach distance (%) | 65.72 ± 7.30 | 74.28 ± 7.03 | 63.57 ± 6.20 | 71.40 ± 8.53 | Group | 1.256 | 0.271 | 0.368 |
| Time | 40.329 | <0.001 * | |||||||
| Group × Time | 0.080 | 0.780 | |||||||
| PM reach distance (%) | 96.96 ± 10.22 | 98.24 ± 8.14 | 95.31 ± 10.01 | 113.15 ± 9.04 | Group | 4.644 | 0.039 * | 1.733 | |
| Time | 59.005 | <0.001 * | |||||||
| Group × Time | 44.187 | <0.001 * | |||||||
| PL reach distance (%) | 91.89 ± 11.96 | 93.50 ± 13.09 | 91.61 ± 8.75 | 112.88 ± 9.96 | Group | 6.483 | 0.016 * | 1.666 | |
| Time | 105.042 | <0.001 * | |||||||
| Group × Time | 77.459 | <0.001 * | |||||||
| Hmax/Mmax ratio | 0.38 ± 0.18 | 0.30 ± 0.13 | 0.37 ± 0.15 | 0.29 ± 0.14 | Group | 0.038 | 0.848 | 0.074 | |
| Time | 21.478 | <0.001 * | |||||||
| Group × Time | 0.097 | 0.757 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, D.; Jung, J. Effect of Incorporating Short-Foot Exercises in the Balance Rehabilitation of Flat Foot: A Randomized Controlled Trial. Healthcare 2021, 9, 1358. https://doi.org/10.3390/healthcare9101358
Moon D, Jung J. Effect of Incorporating Short-Foot Exercises in the Balance Rehabilitation of Flat Foot: A Randomized Controlled Trial. Healthcare. 2021; 9(10):1358. https://doi.org/10.3390/healthcare9101358
Chicago/Turabian StyleMoon, Dongchul, and Juhyeon Jung. 2021. "Effect of Incorporating Short-Foot Exercises in the Balance Rehabilitation of Flat Foot: A Randomized Controlled Trial" Healthcare 9, no. 10: 1358. https://doi.org/10.3390/healthcare9101358
APA StyleMoon, D., & Jung, J. (2021). Effect of Incorporating Short-Foot Exercises in the Balance Rehabilitation of Flat Foot: A Randomized Controlled Trial. Healthcare, 9(10), 1358. https://doi.org/10.3390/healthcare9101358