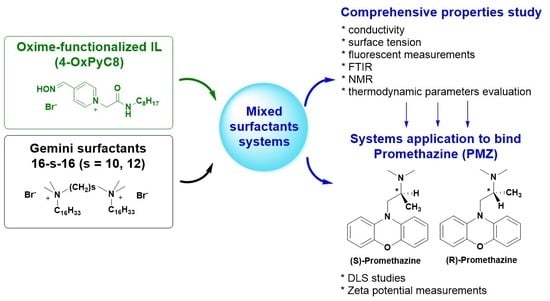
Mixed Oxime-Functionalized IL/16-s-16 Gemini Surfactants System: Physicochemical Study and Structural Transitions in the Presence of Promethazine as a Potential Chiral Pollutant
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
2.2.1. Conductivity
2.2.2. Surface Tension Measurements
2.2.3. Fluorescence Measurements
2.2.4. FTIR Spectroscopy
2.2.5. 1H NMR Measurements
2.2.6. Dynamic Light Scattering Study
3. Results and Discussion
3.1. Determination of the Critical Micelle Concentration
3.1.1. Conductivity
3.1.2. Surface Tension
3.2. Effect of Oxime-Functionalized Ionic Liquid on the Interfacial Properties
3.3. Effect of Oxime-Functionalized Ionic Liquid on Thermodynamic Parameters
3.4. Fluorescence Measurements
3.5. Aggregation Numbers
3.6. FTIR Spectroscopy
3.7. 1H NMR Study
4. Dynamic Light Scattering and Zeta Potential Study of Mixed Gemini:IL System with PMZ
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hembury, G.A.; Borovkov, V.V.; Inoue, Y. Chirality sensing supramolecular systems. Chem. Rev. 2008, 108, 1–73. [Google Scholar] [CrossRef] [PubMed]
- Konrad, N.; Horetski, M.; Sihtmae, M.; Truong, K.-N.; Osadchuk, I.; Burankova, T.; Kielmann, M.; Adamson, J.; Kahru, A.; Rissanen, K.; et al. Thiourea organocatalysts as emerging chiral pollutants: En route to porphyrin-based (chir)optical sensing. Chemosensors 2021, 9, 278. [Google Scholar] [CrossRef]
- Shtykov, S. (Ed.) Nanoanalytics: Nanoobjects and Nanotechnologies in Analytical Chemistry; Walter de Gruyter GmbH: Berlin, Germany, 2018; p. 463. [Google Scholar]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U., Jr.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakharova, L.Y.; Serdyuk, A.A.; Mirgorodskaya, A.B.; Kapitanov, I.V.; Gainanova, G.A.; Karpichev, Y.; Gavrilova, E.L.; Sinyashin, O.G. Amino acid-functionalized calix [4]resorcinarene solubilization by mono-and dicationic surfactants. J. Surfactants Deterg. 2016, 19, 493–499. [Google Scholar] [CrossRef]
- Rasheed, T.; Shafi, S.; Bilal, M.; Hussain, T.; Sherd, F.; Rizwan, K. Surfactants-based remediation as an effective approach for removal of environmental pollutants—A review. J. Mol. Liq. 2020, 318, 113960. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Ranke, J.; Stolte, S.; Stormann, R.; Arning, J.; Jastorff, B. Design of sustainable chemical products—The example of ionic liquids. Chem. Rev. 2007, 107, 2183–2206. [Google Scholar] [CrossRef]
- Clarke, C.J.; Tu, W.-C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green solvents for sustainable organic synthesis: State of the art. Green Chem. 2005, 7, 267–278. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Chong, A.L.; Forsyth, M.; Kar, M.; Vijayaraghavan, R.; Somers, A.; Pringle, J.M. New dimensions in salt-solvent mixtures: A 4th evolution of ionic liquids. Faraday Discuss. 2018, 206, 9–28. [Google Scholar] [CrossRef]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef] [PubMed]
- Aldous, L.; Khan, A.; Hossain, M.M.; Zhao, C.; C Hardacre, V.P. Catalysis in Ionic Liquids: From Catalyst Synthesis to Application; Royal Society of Chemistry: London, UK, 2014; Volume 15, pp. 1–620. [Google Scholar]
- Bica, K.; Gartner, P.; Gritsch, P.J.; Ressmann, A.K.; Schroder, C.; Zirbs, R. Micellar catalysis in aqueous-ionic liquid systems. Chem. Commun. 2012, 48, 5013–5015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinruck, H.P.; Wasserscheid, P. Ionic Liquids in Catalysis. Catal. Lett. 2015, 145, 380–397. [Google Scholar] [CrossRef]
- Han, X.; Armstrong, D.W. Ionic liquids in separations. Acc. Chem. Res. 2007, 40, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Smiglak, M.; Metlen, A.; Rogers, R.D. The second evolution of ionic liquids: From solvents and separations to advanced materi-als-energetic examples from the ionic liquid cookbook. Acc. Chem. Res. 2007, 40, 1182–1192. [Google Scholar] [CrossRef]
- Torimoto, T.; Tsuda, T.; Okazaki, K.; Kuwabata, S. New Frontiers in Materials Science Opened by Ionic Liquids. Adv. Mater. 2010, 22, 1196–1221. [Google Scholar] [CrossRef]
- Jordan, A.; Haib, A.; Spulak, M.; Karpichev, Y.; Kummerer, K.; Gathergood, N. Synthesis of a Series of Amino Acid Derived Ionic Liquids and Tertiary Amines: Green chemistry metrics including microbial toxicity and preliminary biodegradation data analysis. Green Chem. 2016, 18, 4374–4392. [Google Scholar] [CrossRef]
- Garcia, M.T.; Ribosa, I.; Perez, L.; Manresa, A.; Comelles, F. Aggregation Behavior and Antimicrobial Activity of Es-ter-Functionalized Imidazolium- and Pyridinium-Based Ionic Liquids in Aqueous Solution. Langmuir 2013, 29, 2536–2545. [Google Scholar] [CrossRef]
- Teresa Garcia, M.; Ribosa, I.; Perez, L.; Manresa, A.; Comelles, F. Self-assembly and antimicrobial activity of long-chain amide-functionalized ionic liquids in aqueous solution. Colloids Surf. B Biointerfaces 2014, 123, 318–325. [Google Scholar] [CrossRef] [Green Version]
- Haiss, A.; Jordan, A.; Westphal, J.; Logunova, E.; Gathergood, N.; Kuemmerer, K. On the way to greener ionic liquids: Identification of a fully mineralizable phenylalanine-based ionic liquid. Green Chem. 2016, 18, 4361–4373. [Google Scholar] [CrossRef]
- Kusumahastuti, D.K.A.; Sihtmae, M.; Kapitanov, I.V.; Karpichev, Y.; Gathergood, N.; Kahru, A. Toxicity profiling of 24 L-phenylalanine derived ionic liquids based on pyridinium, imidazolium and cholinium cations and varying alkyl chains using rapid screening Vibrio fischeri bioassay. Ecotoxicol. Environ. Saf. 2019, 172, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Kapitanov, I.; Jordan, A.; Karpichev, Y.; Spulak, M.; Perez, L.; Kellett, A.; Kummerer, K.; Gathergood, N. Synthesis, self-assembly, antimicrobial activity, and preliminary biodegradation studies of a series of L- phenylalanine-derived surface active ionic liquids. Green Chem. 2019, 21, 1777–1794. [Google Scholar] [CrossRef]
- Suk, M.; Haiß, A.; Westphal, J.; Jordan, A.; Kellett, A.; Kapitanov, I.; Karpichev, Y.; Gathergood, N.; Kümmerer, K. Design rules for environmental biodegradability of phenylalanine alkyl ester linked ionic liquids for green chemistry. Green Chem. 2020, 22, 4498–4508. [Google Scholar] [CrossRef]
- Pandya, S.J.; Kapitanov, I.V.; Usmani, Z.; Sahu, R.; Sinha, D.; Gathergood, N.; Ghosh, K.K.; Karpichev, Y. An example of green surfactant systems based on inherently biodegradable IL-derived amphiphilic oximes. J. Mol. Liq. 2020, 305, 112857. [Google Scholar] [CrossRef]
- Pal, B.K.; Moulik, S.P. Ionic Liquid-Based Surfactant Science: Formulation, Characterization, and Applications; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2015; p. 576. [Google Scholar]
- Chen, L.G.; Strassburg, S.H.; Bermudez, H. Micelle co-assembly in surfactant/ionic liquid mixtures. J. Colloid Interface Sci. 2016, 477, 40–45. [Google Scholar] [CrossRef]
- Kumar, A.; Banjare, M.K.; Sinha, S.; Yadav, T.; Sahu, R.; Satnami, M.L.; Ghosh, K.K. Imidazolium-Based Ionic Liquid as Mod-ulator of Physicochemical Properties of Cationic, Anionic, Nonionic, and Gemini Surfactants. J. Surfactants Deterg. 2018, 21, 355–366. [Google Scholar] [CrossRef]
- Shang, Y.Z.; Wang, T.F.; Han, X.; Peng, C.J.; Liu, H.L. Effect of Ionic Liquids C(n)mimBr on Properties of Gemini Surfactant 12-3-12 Aqueous Solution. Ind. Eng. Chem. Res. 2010, 49, 8852–8857. [Google Scholar] [CrossRef]
- El Seoud, O.; Keppeler, N.; Malek, N.I.; Galdano, P.D. Ionic Liquid-Based Surfactants: Recent Advances in Their Syntheses, Solution Properties, and Applications. Polymers 2021, 13, 1100. [Google Scholar] [CrossRef]
- Menger, F.M.; Keiper, J.S. Gemini surfactants. Angew. Chem. Int. Ed. 2000, 39, 1907–1920. [Google Scholar] [CrossRef]
- Zana, R. Dimeric and oligomeric surfactants. Behavior at interfaces and in aqueous solution: A review. Adv. Colloid Interface Sci. 2002, 97, 205–253. [Google Scholar] [CrossRef]
- Zana, R. Gemini (dimeric) surfactants. Curr. Opin. Colloid Interface Sci. 1996, 1, 566–571. [Google Scholar] [CrossRef]
- Akram, M.; Anwar, S. Biophysical investigation of promethazine hydrochloride binding with micelles of biocompatible gemini surfactants: Combination of spectroscopic and electrochemical analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 215, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, R.; Branco, L.C.; Prudencio, C.; Noronha, J.P.; Petrovski, Z. Ionic Liquids as Active Pharmaceutical Ingredients. ChemMedChem 2011, 6, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Ozan, M.; Gokturk, S. Effect of ionic liquids as active pharmaceutical ingredients on the micellar binding of an amphiphilic drug trifluopromazine hydrochloride. J. Dispers. Sci. Technol. 2019, 14, 214–222. [Google Scholar] [CrossRef]
- Mahajan, S.; Sharma, R.; Mahajan, R.K. An investigation of drug binding ability of a surface active ionic liquid: Micellization, electrochemical, and spectroscopic studies. Langmuir 2012, 28, 17238–17246. [Google Scholar] [CrossRef]
- Mahajan, S.; Sharma, R.; Mahajan, R.K. Interactions of new 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) based surface active ionic liquids with amitriptyline hydrochloride: Micellization and interfacial studies. Colloids Surf. A Physicochem. Eng. Asp. 2013, 424, 96–104. [Google Scholar] [CrossRef]
- Banjare, M.K.; Behera, K.; Banjare, R.K.; Pandey, S.; Ghosh, K.K.; Karpichev, Y. Molecular interactions between novel synthesized biodegradable ionic liquids with antidepressant drug. Chem. Thermodyn. Therm. Anal. 2021, 3, 100012. [Google Scholar] [CrossRef]
- Pal, M.; Rai, R.; Yadav, A.; Khanna, R.; Baker, G.A.; Pandey, S. Self-aggregation of sodium dodecyl sulfate within (choline chlo-ride+urea) deep eutectic solvent. Langmuir 2014, 30, 13191–13198. [Google Scholar] [CrossRef]
- Sun, T.; Gao, S.; Chen, Q.; Shen, X. Investigation on the interactions between hydrophobic anions of ionic liquids and Triton X-114 micelles in aqueous solutions. Colloids Surf. A Physicochem. Eng. Asp. 2014, 456, 18–25. [Google Scholar] [CrossRef]
- Abdul Rub, M. Aggregation and interfacial phenomenon of amphiphilic drug under the influence of pharmaceutical excipients (green/biocompatible gemini surfactant). PLoS ONE 2019, 14, e0211077. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.A.; Mohammad, R.; Alam, M.S.; Kabir-ud-Din. Effect of alkylamine chain length on the critical micelle concentration of cationic Gemini butanediyl-a,x-bis(dimethylcetylammonium bromide) surfactant. J. Dispers. Sci. Technol. 2009, 30, 1486–1493. [Google Scholar] [CrossRef]
- Markiewicz, R.; Klimaszyk, A.; Jarek, M.; Taube, M.; Florczak, P.; Kempka, M.; Fojud, Z.; Jurga, S. Influence of Alkyl Chain Length on Thermal Properties, Structure, and Self-Diffusion Coefficients of Alkyltriethylammonium-Based Ionic Liquids. Int. J. Mol. Sci. 2021, 22, 5935. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Siddiqui, U.S.; Khan, I.A. Physicochemical study of cationic Gemini surfactant butanediyl-1,4- bis(dimethyldodecylammonium bromide) with various counterions in aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2012, 394, 46–56. [Google Scholar] [CrossRef]
- Wattebled, L.; Laschewsky, A. Effects of organic salt additives on the behavior of dimeric (‘‘Gemini’’) surfactants in aqueous solution. Langmuir 2007, 23, 10044–10052. [Google Scholar] [CrossRef] [PubMed]
- Huddleston, J.G.; Visser, A.E.; Reichert, W.M.; Willauer, H.D.; Broker, G.A.; Rogers, R.D. Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem. 2001, 3, 156–164. [Google Scholar] [CrossRef]
- Tikariha, D.; Singh, N.; Satnami, M.L.; Ghosh, K.K.; Barbero, N.; Quagliotto, P. Physicochemical characterization of cationic Gemini surfactants and their effect on reaction kinetics in ethylene glycol–water medium. Colloids Surf. A Physicochem. Eng. Asp. 2012, 411, 1–11. [Google Scholar] [CrossRef]
- Comelles, F.; Ribosa, I.; Gonzalez, J.J.; Garcia, M.T. Micellization of sodium laurylethoxy sulfate (SLES) and short chain imidazo-lium ionic liquids in aqueous solution. J. Colloid Interface Sci. 2014, 425, 44–45. [Google Scholar] [CrossRef]
- Wang, X.; Yan, F.; Li, Z.; Zhang, L.; Zhaoa, S.; An, J.; Jiayong, Y. Synthesis and surface properties of several nonionic–anionic surfactants with straight chain alkyl-benzyl hydrophobic group. Colloids Surf. A Physicochem. Eng. Asp. 2007, 302, 532–539. [Google Scholar] [CrossRef]
- Li, N.; Gao, Y.A.; Zheng, L.; Zhang, J.; Yu, L.; Li, X. Studies on the micro polarities of bmimBF4/TX-100/toluene ionic liquid microemulsions and their behaviors characterized by UV–visible spectroscopy. Langmuir 2007, 23, 1091–1097. [Google Scholar] [CrossRef]
- Bhaisare, M.L.; Pandy, S.; Khan, M.S.; Talib, A.; Wu, H.F. Fluorophotometric determination of critical micelle concentration (CMC) of ionic and non-ionic surfactants with carbon dots via stokes shift. Talanta 2015, 132, 572–578. [Google Scholar] [CrossRef]
- More, U.; Kumari, P.; Vaid, Z.; Behra, K.; Malek, N.I. Interaction between ionic liquids and Gemini surfactant: A detailed investigation into the role of ionic liquids in modifying properties of aqueous Gemini surfactant. J. Surfactants Deterg. 2016, 19, 75–89. [Google Scholar] [CrossRef]
- Palchowdhury, S.; Bhargava, B.L. Effect of cation asymmetry on the aggregation in aqueous 1-alkyl, 3-decylimidazolium bromide solutions: Molecular dynamics studies. J. Phys. Chem. B 2014, 118, 6241–6249. [Google Scholar] [CrossRef] [PubMed]
- Banjare, M.K.; Kurrey, R.; Yadav, T.; Sinha, S.; Satnami, M.L.; Ghosh, K.K. A comparative study on the effect of imidazoli-um-based ionic liquid on self-aggregation of cationic, anionic and nonionic surfactants studied by surface tension, conductivity, fluorescence and FTIR spectroscopy. J. Mol. Liq. 2017, 241, 622–632. [Google Scholar] [CrossRef]
- Ba-Salem, A.O.; Duhamel, J. Determination of the Aggregation Number of Pyrene-Labeled Gemini Surfactant Micelles by Pyrene Fluorescence Quenching Measurements. Langmuir 2021, 37, 6069–6079. [Google Scholar] [CrossRef] [PubMed]
- Gargi, B.R.; Indranil, C.; Satya, P.M. Pyrene absorption can be a convenient method for probing critical micellar concentration (CMC) and indexing micellar polarity. J. Colloid Interface Sci. 2006, 294, 248–254. [Google Scholar]
- Karpovich, D.S.; Blanchard, G.J. Relating the polarity-dependent fluorescence response of pyrene to vibronic coupling. Achieving a fundamental understanding of the pyrene polarity scale. J. Phys. Chem. 1995, 99, 3951–3958. [Google Scholar] [CrossRef]
- Dong, D.C.; Winnik, M.A. The py scale of solvent polarities. Can. J. Chem. 1984, 62, 2560–2565. [Google Scholar] [CrossRef]
- Derman, O.S.; Stilbs, P.; Price, W.S. NMR Studies of Surfactants. Concepts Magn. Reson. Part A Educ. J. 2004, 23, 121–135. [Google Scholar] [CrossRef]
- Savsunenko, O.; Matondo, H.; Messant, S.F.; Perez, E.; Popov, A.F.; Lattes, I.R.; Lattes, A.; Karpichev, Y. Functionalized vesicles based on amphiphilic boronic acids: A system for recognizing biologically important polyols. Langmuir 2013, 29, 3207–3213. [Google Scholar] [CrossRef]
- Serdyuk, A.A.; Mirgorodskaya, A.B.; Kapitanov, I.V.; Gathergood, N.; Zakharova, L.Y.; Sinyashin, O.G.; Karpichev, Y. Effect of structure of polycyclic aromatic substrates on solubilization capacity and size of cationic monomeric and gemini 14-s-14 surfactant aggregates. Colloids Surf. A Physicochem. Eng. Asp. 2016, 509, 613–622. [Google Scholar] [CrossRef]
- Desnoyers, J.E.; Perron, G. Temperature dependence of the free energy of micellization from calorimetric data. Langmuir 1996, 12, 4044–4045. [Google Scholar] [CrossRef]
- Devinsky, F.; Masarova, L.; Lacko, I. Surface activity and micelle formation of some new bisquaternary ammonium salts. J. Colloid Interface Sci. 1985, 105, 235–239. [Google Scholar] [CrossRef]
- Shafi, M.; Bhat, P.A.; Dar, A.A. Solubilization capabilities of mixtures of cationic Gemini surfactant with conventional cationic, nonionic and anionic surfactants towards polycyclic aromatic hydrocarbons. J. Hazard. Mater. 2009, 167, 575–581. [Google Scholar]
- Li, Q.; Wang, X.; Yue, X.; Chen, X. Wormlike micelles formed using Gemini surfactants with quaternary hydroxyethyl methylammonium headgroups. Soft Matter 2013, 9, 9667–9674. [Google Scholar] [CrossRef]
- Mahajan, S.; Mahajan, R.K. Interactions of phenothiazine drugs with bile salts: Micellization and binding studies. J. Colloid Interface Sci. 2012, 387, 194–204. [Google Scholar] [CrossRef]
- Pisarcik, M.; Polakovicova, M.; Markuliak, M.; Lukac, M.; Devinsky, F. Self-Assembly Properties of Cationic Gemini Surfactants with Biodegradable Groups in the Spacer. Molecules 2019, 24, 1481. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.; Huang, X.; Deng, M.; Lin, Y.; Jiang, L.; Huang, J.; Wang, Y.; Zana, R. Effects of Inorganic and Organic Salts on Aggregation Behavior of Cationic Gemini Surfactants. J. Phys. Chem. B 2010, 114, 14955–14964. [Google Scholar] [CrossRef]
- Oliver, R.C.; Lipfert, J.; Fox, D.A.; Lo, R.H.; Doniach, S.; Columbus, L. Dependence of Micelle Size and Shape on Detergent Alkyl Chain Length and Head Group. PLoS ONE 2013, 8, 62488–62497. [Google Scholar] [CrossRef]
- Ghosh, S.; Roy, A.; Banik, D.; Kundu, N.; Kuchlyan, J.; Dhir, A.; Sarkar, N. How Does the Surface Charge of Ionic Surfactant and Cholesterol Forming Vesicles Control Rotational and Translational Motion of Rhodamine 6G Perchlorate (R6G ClO4)? Langmuir 2015, 31, 2310–2320. [Google Scholar] [CrossRef]
- Geng, Y.; Romsted, L.S.; Menger, F. Specific ion pairing and interfacial hydration as controlling factors in gemini micelle mor-phology. Chemical trapping studies. J. Am. Chem. Soc. 2006, 128, 492–501. [Google Scholar] [CrossRef]
- Zana, R. Dimeric (gemini) surfactants: Effect of the spacer group on the association behavior in aqueous solution. J. Colloid Interface Sci. 2002, 248, 203–220. [Google Scholar] [CrossRef] [PubMed]












| IL 4-PyC8 (wt%) | CMC (mM) | ||
|---|---|---|---|
| Conductivity | Surface Tension | α | |
| 16-10-16 | |||
| Water | 0.028 a | 0.029 | 0.60 |
| 0.2 | 0.023 | 0.024 | 0.51 |
| 0.5 | 0.023 | 0.021 | 0.31 |
| 0.7 | 0.016 | 0.016 | 0.23 |
| 1.0 | 0.013 | 0.013 | 0.23 |
| 16-12-16 | |||
| Water | 0.021 a | 0.022 | 0.67 |
| 0.2 | 0.017 | 0.016 | 0.44 |
| 0.5 | 0.011 | 0.011 | 0.39 |
| 0.7 | 0.009 | 0.009 | 0.29 |
| 1.0 | 0.005 | 0.006 | 0.25 |
| IL 4-PyC8 (wt%) | γCMC (mN∙m−1) | Γmax (106 mol∙m−2) | Amin 1020 (m2 mol−1) | πCMC (mN∙m−1) |
|---|---|---|---|---|
| 16-10-16 | ||||
| Water | 38 | 2.10 | 79.18 | 34.1 |
| 0.2 | 41 | 1.30 | 128.36 | 31.4 |
| 0.5 | 45 | 1.22 | 135.75 | 27.1 |
| 0.7 | 46 | 1.19 | 139.04 | 25.6 |
| 1.0 | 49 | 0.99 | 167.09 | 22.9 |
| 16-12-16 | ||||
| Water | 40 | 1.99 | 83.88 | 31.0 |
| 0.2 | 45 | 1.27 | 131.45 | 26.5 |
| 0.5 | 50 | 1.26 | 132.04 | 21.5 |
| 0.7 | 53 | 1.20 | 138.21 | 18.5 |
| 1.0 | 55 | 0.93 | 178.72 | 16.5 |
| IL 4-PyC8 (wt%) | ΔG(s)min (kJ/mol) | ΔG°m (kJ/mol) | ΔG°m,tail (kJ/mol) | ΔG°ads (kJ/mol) | ΔG°trans (kJ/mol) |
|---|---|---|---|---|---|
| 16-10-16 | |||||
| Water | 18.12 | −14.69 | −7.34 | −30.95 | – |
| 0.2 | 31.48 | −16.55 | −8.27 | −40.82 | −1.86 |
| 0.5 | 36.79 | −20.23 | −10.11 | −42.40 | −5.54 |
| 0.7 | 38.93 | −22.10 | −11.05 | −43.55 | −7.41 |
| 1.0 | 49.51 | −22.68 | −11.34 | −45.74 | −7.99 |
| 16-12-16 | |||||
| Water | 20.46 | −14.11 | −7.57 | −29.69 | – |
| 0.2 | 35.62 | −18.49 | −9.24 | −39.4 | −4.38 |
| 0.5 | 39.76 | −20.39 | −10.10 | −37.49 | −6.28 |
| 0.7 | 44.12 | −22.75 | −11.30 | −38.15 | −8.64 |
| 1.0 | 59.19 | −25.08 | −12.50 | −42.83 | −10.97 |
| IL 4-PyC8 (wt%) | CMC a (mM) | Nagg | KSV |
|---|---|---|---|
| 16-10-16 | |||
| Water | 0.030 b | 57 | 6.99 |
| 0.2 | 0.025 | 53 | 6.50 |
| 0.5 | 0.021 | 51 | 6.17 |
| 0.7 | 0.017 | 49 | 5.88 |
| 1.0 | 0.013 | 45 | 5.45 |
| 16-12-16 | |||
| Water | 0.023 b | 51 | 6.25 |
| 0.2 | 0.016 | 50 | 6.12 |
| 0.5 | 0.011 | 48 | 5.85 |
| 0.7 | 0.009 | 46 | 5.55 |
| 1.0 | 0.006 | 43 | 5.18 |
| Assignment | Band Position (cm−1) | |||
|---|---|---|---|---|
| 16-10-16 | 16-10-16 + IL 4-PyC8 | 16-12-16 | 16-12-16 + IL 4-PyC8 | |
| Symmetric and asymmetric stretching of the C-H stretching vibration of the alkyl chains | 2917.27, 2850.08 | 2917.72, 2849.32 | 2917.32, 2848.35 | 2921.55, 2852.26 |
| Symmetric and asymmetric stretching of the C-H scissoring vibration of the CH3-N+ moiety | 1467.97 | 1466.07 | 1468.53 | 1464.31 |
| C-N+ stretching bands’ rocking mode of the methylene chain | 889.09 | 889.75 | 889.07 | 889.60 |
| Rocking mode of the methylene chain | 721.45 | 720.80 | 721.96 | 720.98 |
| System Composition | Aggregate Characteristics | |
|---|---|---|
| d (nm) | Zeta Potential, ξ (mV) | |
| 16-10-16 (1 mM) | 124 (105–164) | 22.6 |
| 16-10-16 + PMZ (0.01 mM) | 270 (220–395) | 24.8 |
| 16-10-16 + PMZ + 0.1 wt% IL4-PyC8 | 210 (165-255), 825 (615–1105) | 24.1 |
| 16-10-16 + PMZ + 0.5 wt% IL4-PyC8 | 182 (142–220) | 20.6 |
| 16-10-16 + PMZ + 1.0 wt% IL4-PyC8 | 1.4 (1.1–1.8) | 23.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandya, S.J.; Kapitanov, I.V.; Banjare, M.K.; Behera, K.; Borovkov, V.; Ghosh, K.K.; Karpichev, Y. Mixed Oxime-Functionalized IL/16-s-16 Gemini Surfactants System: Physicochemical Study and Structural Transitions in the Presence of Promethazine as a Potential Chiral Pollutant. Chemosensors 2022, 10, 46. https://doi.org/10.3390/chemosensors10020046
Pandya SJ, Kapitanov IV, Banjare MK, Behera K, Borovkov V, Ghosh KK, Karpichev Y. Mixed Oxime-Functionalized IL/16-s-16 Gemini Surfactants System: Physicochemical Study and Structural Transitions in the Presence of Promethazine as a Potential Chiral Pollutant. Chemosensors. 2022; 10(2):46. https://doi.org/10.3390/chemosensors10020046
Chicago/Turabian StylePandya, Subhashree Jayesh, Illia V. Kapitanov, Manoj Kumar Banjare, Kamalakanta Behera, Victor Borovkov, Kallol K. Ghosh, and Yevgen Karpichev. 2022. "Mixed Oxime-Functionalized IL/16-s-16 Gemini Surfactants System: Physicochemical Study and Structural Transitions in the Presence of Promethazine as a Potential Chiral Pollutant" Chemosensors 10, no. 2: 46. https://doi.org/10.3390/chemosensors10020046
APA StylePandya, S. J., Kapitanov, I. V., Banjare, M. K., Behera, K., Borovkov, V., Ghosh, K. K., & Karpichev, Y. (2022). Mixed Oxime-Functionalized IL/16-s-16 Gemini Surfactants System: Physicochemical Study and Structural Transitions in the Presence of Promethazine as a Potential Chiral Pollutant. Chemosensors, 10(2), 46. https://doi.org/10.3390/chemosensors10020046