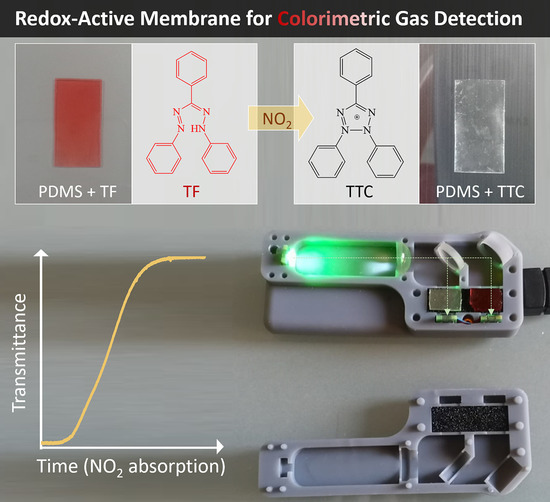
Nitrogen Dioxide Optical Sensor Based on Redox-Active Tetrazolium/Pluronic Nanoparticles Embedded in PDMS Membranes
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Colour-Switchable Nanoparticle (CSN) Preparation
2.3. CSN Characterisation
2.4. Membrane Preparation
2.5. Detection of NO2 Gas
2.6. Interference Gases
2.7. Optical Device Fabrication
3. Results and Discussion
3.1. Synthesis of Colour-Switchable Nanoparticles (CSN)
3.2. Production of Colour-Changing Membranes and NO2 Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, S.; Guo, L.; Zhu, S.; Ma, L.; Tian, Y. Paper-Like Visual Indicator Films for Harmful Hydrophilic Liquids and Vapors. ACS Appl. Polym. Mater. 2021, 3, 4027–4034. [Google Scholar] [CrossRef]
- Barea, E.; Montoro, C.; Navarro, J.A.R. Toxic Gas Removal-Metal-Organic Frameworks for the Capture and Degradation of Toxic Gases and Vapours. Chem. Soc. Rev. 2014, 43, 5419–5430. [Google Scholar] [CrossRef] [Green Version]
- Harrison, R.M.; Shi, J.P. Sources of Nitrogen Dioxide in Winter Smog Episodes. Sci. Total Environ. 1996, 189–190, 391–399. [Google Scholar] [CrossRef]
- Breeze, P. Combustion Plant Emissions: Sulfur Dioxide, Nitrogen Oxides, and Acid Rain. In Electricity Generation and the Environment; ELSEVIER: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Cheremisinoff, P.N. Industry Profile—Fertilizers. In Waste Minimization and Cost Reduction for the Process Industries; William Andrew Publishing: Norwich, NY, USA, 1995. [Google Scholar] [CrossRef]
- Liu, S.K.; Cai, S.; Chen, Y.; Xiao, B.; Chen, P.; Xiang, X.D. The Effect of Pollutional Haze on Pulmonary Function. J. Thorac. Dis. 2016, 8, E41–E56. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Air Quality Guidelines: Global Update 2005: Report on a Working Group Meeting, Bonn, Germany, 18–20 October 2005; World Health Organization, Regional Office for Europe: Geneva, Switzerland, 2006. [Google Scholar]
- Hamra, G.B.; Laden, F.; Cohen, A.J.; Raaschou-Nielsen, O.; Brauer, M.; Loomis, D. Lung Cancer and Exposure to Nitrogen Dioxide and Traffic: A Systematic Review and Meta-Analysis. Environ. Health Perspect. 2015, 123, 1107–1112. [Google Scholar] [CrossRef]
- Kanaparthi, S.; Govind Singh, S. Highly Sensitive and Ultra-Fast Responsive Ammonia Gas Sensor Based on 2D ZnO Nanoflakes. Mater. Sci. Energy Technol. 2019, 3, 91–96. [Google Scholar] [CrossRef]
- Xuan, J.; Zhao, G.; Sun, M.; Jia, F.; Wang, X.; Zhou, T.; Yin, G.; Liu, B. Low-Temperature Operating ZnO-Based NO2 sensors: A Review. RSC Adv. 2020, 10, 39786–39807. [Google Scholar] [CrossRef] [PubMed]
- Saruhan, B.; Lontio Fomekong, R.; Nahirniak, S. Review: Influences of Semiconductor Metal Oxide Properties on Gas Sensing Characteristics. Front. Sens. 2021, 2, 657931. [Google Scholar] [CrossRef]
- Xu, M.; Obodo, D.; Yadavalli, V.K. The Design, Fabrication, and Applications of Flexible Biosensing Devices. Biosens. Bioelectron. 2019, 124–125, 96–114. [Google Scholar] [CrossRef]
- Khalifa, M.; Anandhan, S. Highly Sensitive and Wearable NO2 gas Sensor Based on PVDF Nanofabric Containing Embedded Polyaniline/g-C3N4 nanosheet Composites. Nanotechnology 2021, 32, 485504. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, T.; Han, Y.; Lv, W.; Li, B.; Su, C.; Zeng, M.; Yang, J.; Hu, N.; Su, Y.; et al. Wearable NO2 Sensing and Wireless Application Based on ZnS Nanoparticles/Nitrogen-Doped Reduced Graphene Oxide. Sens. Actuators B Chem. 2021, 345, 130423. [Google Scholar] [CrossRef]
- Yu, L.; Guo, F.; Liu, S.; Yang, B.; Jiang, Y.; Qi, L.; Fan, X. Both Oxygen Vacancies Defects and Porosity Facilitated NO2 Gas Sensing Response in 2D ZnO Nanowalls at Room Temperature. J. Alloys Compd. 2016, 682, 352–356. [Google Scholar] [CrossRef]
- Chen, R.; Wang, J.; Xiang, L. Facile Synthesis of Mesoporous ZnO Sheets Assembled by Small Nanoparticles for Enhanced NO2 Sensing Performance at Room Temperature. Sens. Actuators B Chem. 2018, 270, 207–215. [Google Scholar] [CrossRef]
- Novikov, S.; Lebedeva, N.; Satrapinski, A.; Walden, J.; Davydov, V.; Lebedev, A. Graphene Based Sensor for Environmental Monitoring of NO2. Sens. Actuators B Chem. 2016, 236, 1054–1060. [Google Scholar] [CrossRef]
- Singh, A.K.; Uddin, M.A.; Tolson, J.T.; Maire-Afeli, H.; Sbrockey, N.; Tompa, G.S.; Spencer, M.G.; Vogt, T.; Sudarshan, T.S.; Koley, G. Electrically Tunable Molecular Doping of Graphene. Appl. Phys. Lett. 2013, 102, 043101. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, S.; Zhou, P.; Cui, B.; Liu, W.; Wei, D.; Shen, Y. Room-Temperature NO2 Sensing Properties and Mechanism of CuO Nanorods with Au Functionalization. Sens. Actuators B Chem. 2020, 328, 129070. [Google Scholar] [CrossRef]
- Kang, J.Y.; Koo, W.T.; Jang, J.S.; Kim, D.H.; Jeong, Y.J.; Kim, R.; Ahn, J.; Choi, S.J.; Kim, I.D. 2D Layer Assembly of Pt-ZnO Nanoparticles on Reduced Graphene Oxide for Flexible NO2 Sensors. Sens. Actuators B Chem. 2020, 331, 129371. [Google Scholar] [CrossRef]
- Fan, H.; Li, H.; Han, J.; McKeever, N.; Yu, J.; Katz, H.E. A Humid-Air-Operable, NO2-Responsive Polymer Transistor Series Circuit with Improved Signal-to-Drift Ratio Based on Polymer Semiconductor Oxidation. ACS Sens. 2019, 4, 3240–3247. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Choi, H.J.; Koo, W.T.; Huh, D.; Lee, H.; Kim, I.D. Metal-Organic Framework-Templated PdO-Co3O4 Nanocubes Functionalized by SWCNTs: Improved NO2 Reaction Kinetics on Flexible Heating Film. ACS Appl. Mater. Interfaces 2017, 9, 40593–40603. [Google Scholar] [CrossRef]
- Kim, S.H.; Yang, H.; Yang, S.Y.; Hong, K.; Choi, D.; Yang, C.; Chung, D.S.; Park, C.E. Effect of Water in Ambient Air on Hysteresis in Pentacene Field-Effect Transistors Containing Gate Dielectrics Coated with Polymers with Different Functional Groups. Org. Electron. 2008, 9, 673–677. [Google Scholar] [CrossRef]
- Paliwal, A.; Sharma, A.; Tomar, M.; Gupta, V. Room Temperature Detection of NO2 Gas Using Optical Sensor Based on Surface Plasmon Resonance Technique. Sens. Actuators B Chem. 2015, 216, 497–503. [Google Scholar] [CrossRef]
- Qin, X.; Yu, J.; Jiao, M.; Shan, X.; Xian, X.; Wang, D.; Tao, N. Integrating Electrochemical and Colorimetric Sensors with a Webcam Readout for Multiple Gas Detection. Anal. Chem. 2019, 92, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Park, J.; Kim, B.; Na, K.; Cho, I.; Rho, J.; Yang, D.; Lee, J.Y.; Park, I. Self-Powered Gas Sensor Based on a Photovoltaic Cell and a Colorimetric Film with Hierarchical Micro/Nanostructures. ACS Appl. Mater. Interfaces 2020, 12, 39024–39032. [Google Scholar] [CrossRef] [PubMed]
- Flores, M.E.; Garcés-Jerez, P.; Fernández, D.; Aros-Perez, G.; González-Cabrera, D.; Álvarez, E.; Cañas, I.; Oyarzun-Ampuero, F.; Moreno-Villoslada, I. Facile Formation of Redox-Active Totally Organic Nanoparticles in Water by In Situ Reduction of Organic Precursors Stabilized through Aromatic–Aromatic Interactions by Aromatic Polyelectrolytes. Macromol. Rapid Commun. 2016, 37, 1729–1734. [Google Scholar] [CrossRef] [PubMed]
- Sabaeifard, P.; Abdi-Ali, A.; Soudi, M.R.; Dinarvand, R. Optimization of Tetrazolium Salt Assay for Pseudomonas Aeruginosa Biofilm Using Microtiter Plate Method. J. Microbiol. Methods 2014, 105, 134–140. [Google Scholar] [CrossRef]
- Strlič, M.; Pihlar, B. Determination of Reducing Carbonyl Groups in Cellulose in the Solvent System LiCl/N,N-Dimethylacetamide. Fresenius J. Anal. Chem. 1997, 357, 670–675. [Google Scholar] [CrossRef]
- Otero, A.J.; Rodríguez, I.; Falero, G. 2,3,5-Triphenyl Tetrazolium Chloride (TTC) Reduction as Exponential Growth Phase Marker for Mammalian Cells in Culture and for Myeloma Hybridization Experiments. Cytotechnology 1991, 6, 137–142. [Google Scholar] [CrossRef]
- Nagaraja, P.; Hemantha Kumar, M.S.; Yathirajan, H.S. Silver-Enhanced Reduction of 2,3,5-Triphenyl-2H-Tetrazolium by Semicarbazide for the Spectrophotometric Determination of Traces of Silver(I). Anal. Sci. 2002, 18, 815–817. [Google Scholar] [CrossRef] [Green Version]
- Araya-Hermosilla, E.; Catalán-Toledo, J.; Muñoz-Suescun, F.; Oyarzun-Ampuero, F.; Raffa, P.; Polgar, L.M.; Picchioni, F.; Moreno-Villoslada, I. Totally Organic Redox-Active PH-Sensitive Nanoparticles Stabilized by Amphiphilic Aromatic Polyketones. J. Phys. Chem. B 2018, 122, 1747–1755. [Google Scholar] [CrossRef]
- Araya-Hermosilla, E.; Moreno-Villoslada, I.; Araya-Hermosilla, R.; Flores, M.E.; Raffa, P.; Biver, T.; Pucci, A.; Picchioni, F.; Mattoli, V. Ph-Responsive Polyketone/5,10,15,20-Tetrakis-(Sulfonatophenyl)Porphyrin Supramolecular Submicron Colloidal Structures. Polymers 2020, 12, 2017. [Google Scholar] [CrossRef]
- Moreno-Villoslada, I.; Soto, M.; González, F.; Montero-Silva, F.; Hess, S.; Takemura, I.; Oyaizu, K.; Nishide, H. Reduction of 2,3,5-Triphenyl-2H-Tetrazolium Chloride in the Presence of Polyelectrolytes Containing 4-Styrenesulfonate Moieties. J. Phys. Chem. B 2008, 112, 5350–5354. [Google Scholar] [CrossRef]
- Moreno-Villoslada, I.; Torres, C.; González, F.; Soto, M.; Nishide, H. Stacking of 2,3,5-Triphenyl-2H-Tetrazolium Chloride onto Polyelectrolytes Containing 4-Styrenesulfonate Groups. J. Phys. Chem. B 2008, 112, 11244–11249. [Google Scholar] [CrossRef]
- Santander-Ortega, M.J.; Jódar-Reyes, A.B.; Csaba, N.; Bastos-González, D.; Ortega-Vinuesa, J.L. Colloidal Stability of Pluronic F68-Coated PLGA Nanoparticles: A Variety of Stabilisation Mechanisms. J. Colloid Interface Sci. 2006, 302, 522–529. [Google Scholar] [CrossRef]
- Raval, N.; Maheshwari, R.; Kalyane, D.; Youngren-Ortiz, S.R.; Chougule, M.B.; Tekade, R.K. Importance of Physicochemical Characterization of Nanoparticles in Pharmaceutical Product Development. In Basic Fundamentals of Drug Delivery; ACADEMIC PRESS: Cambridge, MA, USA, 2018. [Google Scholar] [CrossRef]
- Rahme, K.; Oberdisse, J.; Schweins, R.; Gaillard, C.; Marty, J.D.; Mingotaud, C.; Gauffre, F. Pluronics-Stabilized Gold Nanoparticles: Investigation of the Structure of the Polymer-Particle Hybrid. ChemPhysChem 2008, 9, 2230–2236. [Google Scholar] [CrossRef]
- Karolewicz, B.; Gajda, M.; Pluta, J.; Górniak, A. Dissolution Study and Thermal Analysis of Fenofibrate–Pluronic F127 Solid Dispersions. J. Therm. Anal. Calorim. 2015, 125, 751–757. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Shen, Y.; Zhong, X.; Li, T.; Zhao, S.; Zhou, P.; Han, C.; Wei, D.; Shen, Y. Synthesis of ZnO Nanowires/Au Nanoparticles Hybrid by a Facile One-Pot Method and Their Enhanced NO2 Sensing Properties. J. Alloys Compd. 2019, 783, 503–512. [Google Scholar] [CrossRef]
- Ponnuvelu, D.V.; Dhakshinamoorthy, J.; Prasad, A.K.; Dhara, S.; Kamruddin, M.; Pullithadathil, B. Geometrically Controlled Au-Decorated ZnO Heterojunction Nanostructures for NO2 Detection. ACS Appl. Nano Mater. 2020, 3, 5898–5909. [Google Scholar] [CrossRef]
- Shingange, K.; Swart, H.C.; Mhlongo, G.H. Au Functionalized ZnO Rose-like Hierarchical Structures and Their Enhanced NO2 Sensing Performance. Phys. B Condens. Matter 2018, 535, 216–220. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araya-Hermosilla, E.; Araya-Hermosilla, R.; Visentin, F.; Picchioni, F.; Pucci, A.; Mattoli, V. Nitrogen Dioxide Optical Sensor Based on Redox-Active Tetrazolium/Pluronic Nanoparticles Embedded in PDMS Membranes. Chemosensors 2022, 10, 213. https://doi.org/10.3390/chemosensors10060213
Araya-Hermosilla E, Araya-Hermosilla R, Visentin F, Picchioni F, Pucci A, Mattoli V. Nitrogen Dioxide Optical Sensor Based on Redox-Active Tetrazolium/Pluronic Nanoparticles Embedded in PDMS Membranes. Chemosensors. 2022; 10(6):213. https://doi.org/10.3390/chemosensors10060213
Chicago/Turabian StyleAraya-Hermosilla, Esteban, Rodrigo Araya-Hermosilla, Francesco Visentin, Francesco Picchioni, Andrea Pucci, and Virgilio Mattoli. 2022. "Nitrogen Dioxide Optical Sensor Based on Redox-Active Tetrazolium/Pluronic Nanoparticles Embedded in PDMS Membranes" Chemosensors 10, no. 6: 213. https://doi.org/10.3390/chemosensors10060213
APA StyleAraya-Hermosilla, E., Araya-Hermosilla, R., Visentin, F., Picchioni, F., Pucci, A., & Mattoli, V. (2022). Nitrogen Dioxide Optical Sensor Based on Redox-Active Tetrazolium/Pluronic Nanoparticles Embedded in PDMS Membranes. Chemosensors, 10(6), 213. https://doi.org/10.3390/chemosensors10060213