Liquid Chromatography on the Different Methods for the Determination of Lipophilicity: An Essential Analytical Tool in Medicinal Chemistry
Abstract
:1. Lipophilicity and Its Importance in Drug Discovery and Design
2. Methods for Determination of Lipophilicity
2.1. In Silico Determination of Lipophilicity
- Choosing the experimental method;
- Selecting conditions for a LC analysis;
- Examining the plausibility of experimentally obtained values;
- Providing an estimate when experimental methods are not applicable.
2.2. Direct Experimental Determination of Lipophilicity
2.2.1. Shake-Flask Method
2.2.2. Slow-Stirring Method
2.2.3. Water-Plug Aspiration/Injection Method
2.2.4. Flow-Based Method
2.2.5. Miniaturization of Shake-Flask Method
2.2.6. Vortex Liquid–Liquid Microextraction (VALLME) Method
2.2.7. Nano-Absorbent Based Method
2.2.8. Dialysis-Based Method
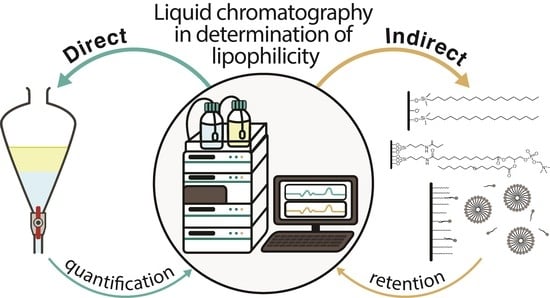
2.3. Indirect Experimental Determination of Lipophilicity
2.3.1. Reversed-Phase Thin-Layer Chromatography
2.3.2. Reversed-Phase High-Performance Liquid Chromatography
- Precision: triplicates should be performed, and the obtained values of the log P values should be within a range of ±0.1 log units [26];
- Sensitivity: HPLC enables log P to be determined over a range of about 0–6 [30];
- Specificity: the presence of impurities may difficult the peak assignment and the interpretation of the obtained values [26];
- Accuracy: the obtained log P values can be within ±1 log unit of the shake-flask value [26].
2.3.3. Ultra-Performance Liquid Chromatography
2.3.4. Counter-Current Chromatography
2.3.5. Immobilized Artificial Membrane Chromatography
2.3.6. Immobilized Liposome Chromatography
2.3.7. Micellar Liquid Chromatography (MLC)
3. Case Study: LC in Lipophilicity Assessment of Xanthone Derivatives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duffus, J.H.; Nordberg, M.; Templeton, D.M. Glossary of terms used in toxicology, 2nd edition (IUPAC recommendations 2007). Pure Appl. Chem. 2007, 79, 1153–1344. [Google Scholar] [CrossRef]
- Arnott, J.A.; Planey, S.L. The influence of lipophilicity in drug discovery and design. Expert Opin. Drug Discov. 2012, 7, 863–875. [Google Scholar] [CrossRef] [PubMed]
- van Balen, G.P.; Martinet, C.; Caron, G.; Bouchard, G.; Reist, M.; Carrupt, P.A.; Fruttero, R.; Gasco, A.; Testa, B. Liposome/water lipophilicity: Methods, information content, and pharmaceutical applications. Med. Res. Rev. 2004, 24, 299–324. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, D.R.P.; Soares, J.X.; Lopes, D.; Macedo, T.; Yordanova, D.; Jakobtorweihen, S.; Nunes, C.; Reis, S.; Pinto, M.M.M.; Afonso, C.M.M. Accessing lipophilicity of drugs with biomimetic models: A comparative study using liposomes and micelles. Eur. J. Pharm. Sci. 2018, 115, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, E.; Pajak, K.; Jozwiak, K. Lipophilicity—methods of determination and its role in medicinal chemistry. Acta Pol. Pharm. 2013, 70, 3–18. [Google Scholar]
- Liu, X.; Testa, B.; Fahr, A. Lipophilicity and its relationship with passive drug permeation. Pharm. Res. 2011, 28, 962–977. [Google Scholar] [CrossRef]
- Young, R.J.; Green, D.V.; Luscombe, C.N.; Hill, A.P. Getting physical in drug discovery II: The impact of chromatographic hydrophobicity measurements and aromaticity. Drug Discov. Today 2011, 16, 822–830. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2012, 64, 4–17. [Google Scholar] [CrossRef]
- Laznicek, M.; Laznickova, A. The effect of lipophilicity on the protein binding and blood cell uptake of some acidic drugs. J. Pharm. Biomed. Anal. 1995, 13, 823–828. [Google Scholar] [CrossRef]
- Cardoso, T.; Almeida, A.S.; Remião, F.; Fernandes, C. Enantioresolution and Binding Affinity Studies on Human Serum Albumin: Recent Applications and Trends. Chemosensors 2021, 9, 304. [Google Scholar] [CrossRef]
- Gleeson, M.P. Generation of a set of simple, interpretable ADMET rules of thumb. J. Med. Chem. 2008, 51, 817–834. [Google Scholar] [CrossRef]
- Hughes, J.D.; Blagg, J.; Price, D.A.; Bailey, S.; DeCrescenzo, G.A.; Devraj, R.V.; Ellsworth, E.; Fobian, Y.M.; Gibbs, M.E.; Gilles, R.W.; et al. Physiochemical drug properties associated with in vivo toxicological outcomes. Bioorg. Med. Chem. Lett. 2008, 18, 4872–4875. [Google Scholar] [CrossRef] [PubMed]
- Waring, M.J.; Johnstone, C. A quantitative assessment of hERG liability as a function of lipophilicity. Bioorg. Med. Chem. Lett. 2007, 17, 1759–1764. [Google Scholar] [CrossRef] [PubMed]
- Giaginis, C.; Tsantili-Kakoulidou, A. Alternative measures of lipophilicity: From octanol-water partitioning to IAM retention. J. Pharm. Sci. 2008, 97, 2984–3004. [Google Scholar] [CrossRef]
- Hann, M.M.; Keseru, G.M. Finding the sweet spot: The role of nature and nurture in medicinal chemistry. Nat. Rev. Drug Discov. 2012, 11, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Leeson, P.D.; Springthorpe, B. The influence of drug-like concepts on decision-making in medicinal chemistry. Nat. Rev. Drug Discov. 2007, 6, 881–890. [Google Scholar] [CrossRef]
- Hitzel, L.; Watt, A.; Locker, K. An Increased Throughput Method for the Determination of Partition Coefficients. Pharm. Res. 2000, 17, 1389–1395. [Google Scholar] [CrossRef]
- Sangster, J. Octanol-Water Partition Coefficients: Fundamentals and Physical Chemistry; John Wiley & Sons: New York, NY, USA, 1997. [Google Scholar]
- Liang, C.; Lian, H.-Z. Recent advances in lipophilicity measurement by reversed-phase high-performance liquid chromatography. TrAC Trends Anal. Chem. 2015, 68, 28–36. [Google Scholar] [CrossRef]
- Bergstrom, C.A.; Norinder, U.; Luthman, K.; Artursson, P. Experimental and computational screening models for prediction of aqueous drug solubility. Pharm. Res. 2002, 19, 182–188. [Google Scholar] [CrossRef]
- Petrauskas, A.A.; Kolovanov, E.A. ACD/Log P method description. Perspect. Drug Discov. Des. 2000, 19, 99–116. [Google Scholar] [CrossRef]
- Ulrich, N.; Goss, K.-U.; Ebert, A. Exploring the octanol–water partition coefficient dataset using deep learning techniques and data augmentation. Commun. Chem. 2021, 4, 90. [Google Scholar] [CrossRef]
- Mannhold, R.; Poda, G.I.; Ostermann, C.; Tetko, I.V. Calculation of molecular lipophilicity: State-of-the-art and comparison of log P methods on more than 96,000 compounds. J. Pharm. Sci. 2009, 98, 861–893. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Yang, X.H.; Liu, H.H. Development of liposome/water partition coefficients predictive models for neutral and ionogenic organic chemicals. Ecotoxicol. Environ. Saf. 2019, 179, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Endo, S.; Escher, B.I.; Goss, K.U. Capacities of Membrane Lipids to Accumulate Neutral Organic Chemicals. Environ. Sci. Technol. 2011, 45, 5912–5921. [Google Scholar] [CrossRef]
- Eadsforth, C.V.; Moser, P. Assessment of reverse-phase chromatographic methods for determining partition coefficients. Chemosphere 1983, 12, 1459–1475. [Google Scholar] [CrossRef]
- Giaginis, C.; Tsantili-Kakoulidou, A. Current State of the Art in HPLC Methodology for Lipophilicity Assessment of Basic Drugs. A Review. J. Liq. Chromatogr. Relat. Technol. 2007, 31, 79–96. [Google Scholar] [CrossRef]
- Lombardo, F.; Shalaeva, M.Y.; Tupper, K.A.; Gao, F.; Abraham, M.H. ElogPoct: A tool for lipophilicity determination in drug discovery. J. Med. Chem. 2000, 43, 2922–2928. [Google Scholar] [CrossRef]
- Pinto, P.; Machado, C.M.; Moreira, J.; Almeida, J.D.P.; Silva, P.M.A.; Henriques, A.C.; Soares, J.X.; Salvador, J.A.R.; Afonso, C.; Pinto, M.; et al. Chalcone derivatives targeting mitosis: Synthesis, evaluation of antitumor activity and lipophilicity. Eur. J. Med. Chem. 2019, 184, 111752. [Google Scholar] [CrossRef]
- OECD. Test No. 117: Partition Coefficient (n-Octanol/Water), HPLC Method; OECD Publishing: Paris, France, 2022. [Google Scholar]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- OECD. Test No. 107: Partition Coefficient (n-Octanol/Water): Shake Flask Method; OECD Publishing: Paris, France, 2022. [Google Scholar]
- Berthod, A.; Carda-Broch, S. Determination of liquid–liquid partition coefficients by separation methods. J. Chromatogr. A 2004, 1037, 3–14. [Google Scholar] [CrossRef]
- Godard, T.; Grushka, E. The use of phospholipid modified column for the determination of lipophilic properties in high performance liquid chromatography. J. Chromatogr. A 2011, 1218, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Roman, I.P.; Mastromichali, A.; Tyrovola, K.; Canals, A.; Psillakis, E. Rapid determination of octanol-water partition coefficient using vortex-assisted liquid-liquid microextraction. J. Chromatogr. A 2014, 1330, 1–5. [Google Scholar] [CrossRef]
- Hartmann, T.; Schmitt, J. Lipophilicity—Beyond octanol/water: A short comparison of modern technologies. Drug Discov. Today Technol. 2004, 1, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Brooke, D.N.; Dobbs, A.J.; Williams, N. Octanol:water partition coefficients (P): Measurement, estimation, and interpretation, particularly for chemicals with P greater than 10(5). Ecotoxicol. Environ. Saf. 1986, 11, 251–260. [Google Scholar] [CrossRef]
- De Bruijn, J.; Busser, F.; Seinen, W.; Hermens, J. Determination of octanol/water partition coefficients for hydrophobic organic chemicals with the “slow-stirring” method. Environ. Toxicol. Chem. 1989, 8, 499–512. [Google Scholar] [CrossRef]
- Jabusch, T.W.; Swackhamer, D.L. Partitioning of polychlorinated biphenyls in octanol/water, triolein/water, and membrane/water systems. Chemosphere 2005, 60, 1270–1278. [Google Scholar] [CrossRef]
- Dohta, Y.; Yamashita, T.; Horiike, S.; Nakamura, T.; Fukami, T. A system for LogD screening of 96-well plates using a water-plug aspiration/injection method combined with high-performance liquid chromatography-mass spectrometry. Anal. Chem. 2007, 79, 8312–8315. [Google Scholar] [CrossRef]
- Nishimura, I.; Hirano, A.; Yamashita, T.; Fukami, T. Improvement of the high-speed logD assay using an injection marker for the water plug aspiration/injection method. J. Chromatogr. A 2009, 1216, 2984–2988. [Google Scholar] [CrossRef]
- Marine, N.A.; Klein, S.A.; Posner, J.D. Partition Coefficient Measurements in Picoliter Drops Using a Segmented Flow Microfluidic Device. Anal. Chem. 2009, 81, 1471–1476. [Google Scholar] [CrossRef]
- Danielsson, L.G.; Zhang, Y.H. Methods for determining n-octanol-water partition constants. TrAC Trends Anal. Chem. 1996, 15, 188–196. [Google Scholar] [CrossRef]
- Chen, Z.; Weber, S.G. High-throughput method for lipophilicity measurement. Anal. Chem. 2007, 79, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Psillakis, E. Vortex-assisted liquid-liquid microextraction revisited. TrAC Trends Anal. Chem. 2019, 113, 332–339. [Google Scholar] [CrossRef]
- Dearden, J.C.; Bresnen, G.M. The Measurement of Partition Coefficients. Quant. Struct.-Act. Relat. 1988, 7, 133–144. [Google Scholar] [CrossRef]
- Bosch Ojeda, C.; Sánchez Rojas, F. Vortex-Assisted Liquid–Liquid Microextraction (VALLME): Applications. Chromatographia 2014, 77, 745–754. [Google Scholar] [CrossRef]
- Gao, X.; Yu, C.H.; Tam, K.Y.; Tsang, S.C. New magnetic nano-absorbent for the determination of n-octanol/water partition coefficients. J. Pharm. Biomed. Anal. 2005, 38, 197–203. [Google Scholar] [CrossRef]
- Yu, C.H.; Tam, K.; Tsang, S.C. A new high-throughput method utilizing porous silica-based nano-composites for the determination of partition coefficients of drug candidates. J. Sep. Sci. 2011, 34, 2505–2512. [Google Scholar] [CrossRef]
- Austin, R.P.; Davis, A.M.; Manners, C.N. Partitioning of Ionizing Molecules between Aqueous Buffers and Phospholipid Vesicles. J. Pharm. Sci. 1995, 84, 1180–1183. [Google Scholar] [CrossRef]
- Park, J.Y.; Rippie, E.G. Micellar distribution equilibria: Ultracentrifugal study of apparent partition coefficients. J. Pharm. Sci. 1977, 66, 858–861. [Google Scholar] [CrossRef]
- Loidl-Stahlhofen, A.; Hartmann, T.; Schöttner, M.; Rhöring, C.; Brodowsky, H.; Schmitt, J.; Keldenich, J. Multilamellar liposomes and solid-supported lipid membranes (TRANSIL): Screening of lipid-water partitioning toward a high-throughput scale. Pharm. Res. 2001, 18, 1782–1788. [Google Scholar] [CrossRef]
- Dolzonek, J.; Cho, C.W.; Stepnowski, P.; Markiewicz, M.; Thoming, J.; Stolte, S. Membrane partitioning of ionic liquid cations, anions and ion pairs—Estimating the bioconcentration potential of organic ions. Environ. Pollut. 2017, 228, 378–389. [Google Scholar] [CrossRef]
- Schrader, W.; Andersson, J.T. Fast and direct method for measuring 1-octanol-water partition coefficients exemplified for six local anesthetics. J. Pharm. Sci. 2001, 90, 1948–1954. [Google Scholar] [CrossRef] [PubMed]
- Pauletti, G.M.; Wunderli-Allenspach, H. Partition coefficients in vitro: Artificial membranes as a standardized distribution model. Eur. J. Pharm. Sci. 1994, 1, 273–282. [Google Scholar] [CrossRef]
- Escher, B.I.; Schwarzenbach, R.P. Partitioning of Substituted Phenols in Liposome–Water, Biomembrane–Water, and Octanol–Water Systems. Environ. Sci. Technol. 1996, 30, 260–270. [Google Scholar] [CrossRef]
- Terada, H. Determination of Log Poct by High-Performance Liquid Chromatography, and its Application in the Study Quantitative Structure-Activity Relationships. Quant. Struct.-Act. Relat. 1986, 5, 81–88. [Google Scholar] [CrossRef]
- Abbas, N.S.; Mohamed, Y.A.S.; Derayea, S.M.; Omar, M.A.; Saleh, G.A. Simple TLC-spectrodensitometric method for studying lipophilicity and quantitative analysis of hypoglycemic drugs in their binary mixture. Biomed. Chromatogr. 2021, 35, e5154. [Google Scholar] [CrossRef] [PubMed]
- De Mello, H.; Echevarria, A. Hydrophobicity study for some pyrazolo-pyridine derivatives by RP-TLC and RP-HPLC. J. Liq. Chromatogr. Relat. Technol. 2006, 29, 1317–1330. [Google Scholar] [CrossRef]
- Cozma, A.; Vlase, L.; Ignat, A.; Zaharia, V.; Gocan, S.; Grinberg, N. Prediction of the lipophilicity of eight new p-toluenesulfonyl-hydrazinothiazole and hydrazine-bis-thiazole derivatives: A comparison between rp-hptlc and rp-hplc. J. Liq. Chromatogr. Relat. Technol. 2012, 35, 590–601. [Google Scholar] [CrossRef]
- Dross, K.; Rekker, R.F.; de Vries, G.; Mannhold, R. The lipophilic behaviour of organic compounds: 3. The search for interconnections between reversed-phase chromatographic data and log Pfoct values. Quant. Struct.-Act. Relat. 1998, 17, 549–557. [Google Scholar] [CrossRef]
- Komsta, Ł.; Skibiński, R.; Berecka, A.; Gumieniczek, A.; Radkiewicz, B.; Radoń, M. Revisiting thin-layer chromatography as a lipophilicity determination tool—A comparative study on several techniques with a model solute set. J. Pharm. Biomed. Anal. 2010, 53, 911–918. [Google Scholar] [CrossRef]
- Cozma, A.; Zaharia, V.; Ignat, A.; Gocan, S.; Grinberg, N. Prediction of the lipophilicity of nine new synthesized selenazoly and three aroyl-hydrazinoselenazoles derivatives by reversed-phase high performance thin-layer chromatography. J. Chromatogr. Sci. 2012, 50, 157–161. [Google Scholar] [CrossRef]
- Liang, C.; Han, S.-y.; Qiao, J.-q.; Lian, H.-z.; Ge, X. Determination of the n-octanol/water partition coefficients of weakly ionizable basic compounds by reversed-phase high-performance liquid chromatography with neutral model compounds. J. Sep. Sci. 2014, 37, 3226–3234. [Google Scholar] [CrossRef] [PubMed]
- Dacić, M.; Uzunović, A.; Pilipović, S.; Alagić-Džambić, L.; Durić, K. RP-HPLC Determination of Lipophilicity in Series of Corticosteroids. In Proceedings of the CMBEBIH 2021, Cham, Switzerland, 21–24 April 2021; pp. 535–540. [Google Scholar]
- Dobričić, V.; Bošković, J.; Vukadinović, D.; Vladimirov, S.; Čudina, O. Estimation of lipophilicity and design of new 17β-carboxamide glucocorticoids using RP-HPLC and quantitative structure-retention relationships analysis. Acta Chromatogr. 2021, 34, 130–137. [Google Scholar] [CrossRef]
- Krmar, J.; Protic, A.; Dajic, N.; Zecevic, M.; Otasevic, B. Chromatographic and computational lipophilicity assessment of novel antibiofilm agents. J. Liq. Chromatogr. Relat. Technol. 2020, 43, 615–623. [Google Scholar] [CrossRef]
- Jevrić, L.R.; Karadžić, M.Ž.; Mandić, A.I.; Podunavac Kuzmanović, S.O.; Kovačević, S.Z.; Nikolić, A.R.; Oklješa, A.M.; Sakač, M.N.; Penov Gaši, K.M.; Stojanović, S.Z. Lipophilicity estimation and characterization of selected steroid derivatives of biomedical importance applying RP HPLC. J. Pharm. Biomed. Anal. 2017, 134, 27–35. [Google Scholar] [CrossRef]
- Sima, I.A.; Kot-Wasik, A.; Wasik, A.; Namieśnik, J.; Sârbu, C. Assessment of Lipophilicity Indices Derived from Retention Behavior of Antioxidant Compounds in RP-HPLC. Molecules 2017, 22, 550. [Google Scholar] [CrossRef]
- Łączkowski, K.Z.; Biernasiuk, A.; Baranowska-Łączkowska, A.; Zavyalova, O.; Redka, M.; Malm, A. Synthesis, lipophilicity determination, DFT calculation, antifungal and DPPH radical scavenging activities of tetrahydrothiophen-3-one based thiazoles. J. Mol. Struct. 2018, 1171, 717–725. [Google Scholar] [CrossRef]
- Cimpan, G.; Irimie, F.; Gocan, S.; Claessens, H.A. Role of stationary phase and eluent composition on the determination of log P values of N-hydroxyethylamide of aryloxyalkylen and pyridine carboxylic acids by reversed-phase high-performance liquid chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1998, 714, 247–261. [Google Scholar] [CrossRef]
- Giaginis, C.; Tsantili-Kakoulidou, A. The performance of 1-ethyl-3-methylimidazolium tetrafluoroborate ionic liquid as mobile phase additive in HPLC-based lipophilicity assessment. Biomed. Chromatogr. 2011, 25, 606–612. [Google Scholar] [CrossRef]
- El Tayar, N.; Van De Waterbeemd, H.; Gryllaki, M.; Testa, B.; Trager, W.F. The lipophilicity of deuterium atoms a comparison of shake-flask and high performance liquid chromatographic methods. Int. J. Pharm. 1984, 19, 271–282. [Google Scholar] [CrossRef]
- Welerowicz, T.; Buszewski, B. The effect of stationary phase on lipophilicity determination of beta-blockers using reverse-phase chromatographic systems. Biomed. Chromatogr. 2005, 19, 725–736. [Google Scholar] [CrossRef]
- Zagórska, A.; Czopek, A.; Pełka, K.; Bajda, M.; Stanisz-Wallis, K.; Pawłowski, M. Reversed-phase high-performance liquid chromatography study of lipophilicity of imidazo [2,1-f]theophylline derivatives. Acta Pol. Pharm. Drug Res. 2015, 72, 663–669. [Google Scholar]
- Altomare, C.; Carotti, A.; Cellamare, S.; Ferappi, M. Lipophilicity measurements of benzenesulfonamide inhibitors of carbonic-anhydrase by reversed-phase hplc. Int. J. Pharm. 1989, 56, 273–281. [Google Scholar] [CrossRef]
- Griffin, S.; Wyllie, S.G.; Markham, J. Determination of octanol-water partition coefficient for terpenoids using reversed-phase high-performance liquid chromatography. J. Chromatogr. A 1999, 864, 221–228. [Google Scholar] [CrossRef]
- Mrkvickova, Z.; Kovarikova, P.; Balikova, S.; Klimes, J. Determination of lipophilicity of novel potential antituberculotic agents using HPLC on monolithic stationary phase and theoretical calculations. J. Pharm. Biomed. Anal. 2008, 48, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Brychtova, K.; Jampilek, J.; Opatrilova, R.; Raich, I.; Farsa, O.; Csollei, J. Synthesis, physico-chemical properties and penetration activity of alkyl-6-(2,5-dioxopyrrolidin-1-yl)-2-(2-oxopyrrolidin-1-yl)hexanoates as potential transdermal penetration enhancers. Biorg. Med. Chem. 2010, 18, 73–79. [Google Scholar] [CrossRef]
- Ayouni, L.; Cazorla, G.; Chaillou, D.; Herbreteau, B.; Rudaz, S.; Lanteri, P.; Carrupt, P.A. Fast determination of lipophilicity by HPLC. Chromatographia 2005, 62, 251–255. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.G.; Huang, R.Q.; Wang, Q.S. Lipophilicity determination of N-(benzothiazol-2-yl)-alpha-amino alkyl phosphonic diesters by RP-HPLC and RP-HPTLC. Chin. J. Chem. 2000, 18, 872–876. [Google Scholar] [CrossRef]
- Lesniewska, M.A.; Gdaniec, Z.; Muszalska, I. Calculation procedures and HPLC method for analysis of the lipophilicity of acyclovir esters. Drug Dev. Ind. Pharm. 2015, 41, 663–669. [Google Scholar] [CrossRef]
- Du, C.M.; Valko, K.; Bevan, C.; Reynolds, D.; Abraham, M.H. Rapid gradient RP HPLC method for lipophilicity determination: A solvation equation based comparison with isocratic methods. Anal. Chem. 1998, 70, 4228–4234. [Google Scholar] [CrossRef]
- Dolezel, J.; Hirsova, P.; Opletalova, V.; Dohnal, J.; Marcela, V.; Kunes, J.; Jampilek, J. Rhodanineacetic Acid Derivatives as Potential Drugs: Preparation, Hydrophobic Properties and Antifungal Activity of (5-Arylalkylidene-4-oxo-2-thioxo-1,3-thiazolidin-3-yl)acetic Acids. Molecules 2009, 14, 4197–4212. [Google Scholar] [CrossRef]
- Hong, H.; Wang, L.; Zou, G. Retention in RP-HPLC: Lipophilicity Determination of Substituted Biphenyls by Reversed-Phase High Performance Liquid Chromatography. J. Liq. Chromatogr. Relat. Technol. 1997, 20, 3029–3037. [Google Scholar] [CrossRef]
- Giaginis, C.; Theocharis, S.; Tsantili-Kakoulidou, A. Octanol/water partitioning simulation by RP-HPLC for structurally diverse acidic drugs: Comparison of three columns in the presence and absence of n-octanol as the mobile phase additive. J. Sep. Sci. 2013, 36, 3830–3836. [Google Scholar] [CrossRef] [PubMed]
- Musiol, R.; Jampilek, J.; Podeszwa, B.; Finster, J.; Tabak, D.; Dohnal, J.; Polanski, J. RP-HPLC determination of lipophilicity in series of quinoline derivatives. Cent. Eur. J. Chem. 2009, 7, 586–597. [Google Scholar] [CrossRef]
- Vrakas, D.; Panderi, I.; Hadjipavlou-Litina, D.; Tsantili-Kakoulidou, A. Investigation of the relationships between logP and various chromatographic indices for a series of substituted coumarins. Evaluation of their similarity/dissimilarity using multivariate statistics. QSAR Comb. Sci. 2005, 24, 254–260. [Google Scholar] [CrossRef]
- Kostecka, M. Synthesis of a New Group of Aliphatic Hydrazide Derivatives and the Correlations between Their Molecular Structure and Biological Activity. Molecules 2012, 17, 3560–3573. [Google Scholar] [CrossRef]
- Pallicer, J.M.; Sales, J.; Roses, M.; Rafols, C.; Bosch, E. Lipophilicity assessment of basic drugs (log Po/w determination) by a chromatographic method. J. Chromatogr. A 2011, 1218, 6356–6368. [Google Scholar] [CrossRef]
- Cozma, A.; Vlase, L.; Ignap, A.; Zaharia, V.; Gocan, S.; Marutoiu, C.; Fodor, A. Lipophilicity study of new selenazole derivatives by RP-HPLC. Rev. Chim. 2012, 63, 651–655. [Google Scholar]
- Dołowy, M.; Pyka, A. Lipophilicity assessment of spironolactone by means of reversed phase liquid chromatography and by newly developed calculation procedures. Acta Pol. Pharm. Drug Res. 2015, 72, 235–244. [Google Scholar]
- Brychtova, K.; Dvorakova, L.; Opatrilova, R.; Raich, I.; Kacerova, S.; Placek, L.; Kalinowski, D.S.; Richardson, D.R.; Jampilek, J. Investigation of substituted 6-aminohexanoates as skin penetration enhancers. Biorg. Med. Chem. 2012, 20, 86–95. [Google Scholar] [CrossRef]
- Zheng, B.; West, L.M. Estimating the lipophilicity of natural products using a polymeric reversed phase hplc method. J. Liq. Chromatogr. Relat. Technol. 2010, 33, 118–132. [Google Scholar] [CrossRef]
- Zhao, Y.N.; Jona, J.; Chow, D.T.; Rong, H.J.; Semin, D.; Xia, X.Y.; Zanon, R.; Spancake, C.; Maliski, E. High-throughput logP measurement using parallel liquid chromatography/ultraviolet/mass spectrometry and sample-pooling. Rapid Commun. Mass Spectrom. 2002, 16, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Hawrył, A.; Kuśmierz, E.; Hawrył, M.; świeboda, R.; Wujec, M. Determination of lipophilicity of new thiosemicarbazide and 1,2,4-triazole-3-thione derivatives using reversed-phase HPLC method and theoretical calculations. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 430–437. [Google Scholar] [CrossRef]
- Lozowicka, B.; Kaczynski, P.; Nawrot, J. Study of lipophilicity of alpha-asarone derivatives and their deterrent activity against the Colorado potato beetle. Cent. Eur. J. Chem. 2013, 11, 2120–2133. [Google Scholar] [CrossRef]
- Britto, M.M.; Montanari, C.A.; Donnici, C.L.; Cass, Q.B. On the partitioning of some newly synthesized mesoionic 1,3,4-thiadiazolium-2-aminide and precursors evaluated by RP-HPLC. J. Liq. Chromatogr. Relat. Technol. 1999, 22, 357–366. [Google Scholar] [CrossRef]
- Abdou, W.M.; Barghash, R.F.; Sediek, A.A. Design of new arylamino-2-ethane-1,1-diyl- and benzoxazole-2-methylene-bisphosphonates vs. cytotoxicity and chronic inflammation diseases. From hydrophobicity prediction to synthesis and biological evaluation. Eur. J. Med. Chem. 2012, 57, 362–372. [Google Scholar] [CrossRef]
- Teijeiro, S.A.; Moroni, G.N.; Motura, M.I.; Brinon, M.C. Lipophilic character of pyrimidinic nucleoside derivatives: Correlation between shake flask, chromatographic (RP-TLC and RP-HPLC) and theoretical methods. J. Liq. Chromatogr. Relat. Technol. 2000, 23, 855–872. [Google Scholar] [CrossRef]
- Reymond, D.; Chung, G.N.; Mayer, J.M.; Testa, B. Lipophilicity measurement of nicotinates by reversed-phase high-performance liquid-chromatography—Differences in retention behavior, but similarities of log kw values, in methanol-water and acetonitrile-water eluents. J. Chromatogr. A 1987, 391, 97–109. [Google Scholar] [CrossRef]
- Jensen, D.A.; Gary, R.K. Estimation of alkane-water logP for neutral, acidic, and basic compounds using an alkylated polystyrene-divinylbenzene high-performance liquid chromatography column. J. Chromatogr. A 2015, 1417, 21–29. [Google Scholar] [CrossRef]
- Durcekova, T.; Boronova, K.; Mocak, J.; Lehotay, J.; Cizmarik, J. QSRR models for potential local anaesthetic drugs using high performance liquid chromatography. J. Pharm. Biomed. Anal. 2012, 59, 209–216. [Google Scholar] [CrossRef]
- Dimitriou-Christidis, P.; Harris, B.C.; McDonald, T.J.; Reese, E.; Autenrieth, R.L. Estimation of selected physicochemical properties for methylated naphthalene compounds. Chemosphere 2003, 52, 869–881. [Google Scholar] [CrossRef]
- Fikri, K.; Debord, J.; Bollinger, J.C.; Cledat, D.; Penicaut, B.; Robert, J.M.H. RP-HPLC lipophilicity studies for some (hetero)arylamides derived from 2-amino 4,6-dimethyl pyridine: Introduction of an hydrogen-bond descriptor. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 1356–1366. [Google Scholar] [CrossRef]
- Dias, N.C.; Nawas, M.I.; Poole, C.F. Evaluation of a reversed-phase column (Supelcosil LC-ABZ) under isocratic and gradient elution conditions for estimating octanol-water partition coefficients. Analyst 2003, 128, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Paschke, A.; Neitzel, P.L.; Walther, W.; Schuurmann, G. Octanol/water partition coefficient of selected herbicides: Determination using shake-flask method and reversed-phase high-performance liquid chromatography. J. Chem. Eng. Data 2004, 49, 1639–1642. [Google Scholar] [CrossRef]
- Strzemecka, L.; Hawryl, A.; Swieboda, R.; Hawryl, M.; Jagiello-Wojtowicz, E.; Piatkowska-Chmiel, I.; Herbet, M.; Chodkowska, A. Determination of Lipophilicity of Allyl Thiosemicarbazide, N-1-Thiocarbamylamidrazone Derivatives, and their Cyclic Products by RP-HPLC, RP-TLC, and Theoretical Methods: Effects of Selected Compounds on the CNS of Mice. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 1452–1465. [Google Scholar] [CrossRef]
- Takacs-Novak, K.; Szasz, G.; Budvari-Barany, Z.; Jozan, M.; Lore, A. Relationship study between reversed phase HPLC retention and octanol/water partition among amphoteric compounds. J. Liq. Chromatogr. 1995, 18, 807–825. [Google Scholar] [CrossRef]
- Perisic-Janjic, N.; Kaliszan, R.; Wiczling, P.; Milosevic, N.; Uscumlic, G.; Banjac, N. Reversed-Phase TLC and HPLC Retention Data in Correlation Studies with in Silico Molecular Descriptors and Druglikeness Properties of Newly Synthesized Anticonvulsant Succinimide Derivatives. Mol. Pharmaceut. 2011, 8, 555–563. [Google Scholar] [CrossRef]
- Zhao, M.; Li, Z.; Wu, Y.; Tang, Y.-R.; Wang, C.; Zhang, Z.; Peng, S. Studies on log P, retention time and QSAR of 2-substituted phenylnitronyl nitroxides as free radical scavengers. Eur. J. Med. Chem. 2007, 42, 955–965. [Google Scholar] [CrossRef]
- Trezzi, M.M.; Vidal, R.A.; Dick, D.P.; Peralba, M.C.R.; Kruse, N.D. Sorptive behavior of sorgoleone in ultisol in two solvent systems and determination of its lipophilicity. J. Environ. Sci. Health B Pestic. Food Contam. Agric. Wastes 2006, 41, 345–356. [Google Scholar] [CrossRef]
- Liu, X.; Tanaka, H.; Yamauchi, A.; Testa, B.; Chuman, H. Lipophilicity Measurement by Reversed-Phase High-Performance Liquid Chromatography (RP-HPLC): A Comparison of Two Stationary Phases Based on Retention Mechanisms. Helv. Chim. Acta 2004, 87, 2866–2876. [Google Scholar] [CrossRef]
- Fei, X.; Zheng, Q.H. Lipophilicity coefficients of [11C]Me-Halo-CGS 27023A analogs determined by HPLC. J. Liq. Chromatogr. Relat. Technol. 2005, 28, 939–945. [Google Scholar] [CrossRef]
- Pagliara, A.; Khamis, E.; Trinh, A.; Carrupt, P.A.; Tsai, R.S.; Testa, B. Structural Properties Governing Retention Mechanisms on RP-HPLC Stationary Phases Used for Lipophilicity Measurements. J. Liq. Chromatogr. 1995, 18, 1721–1745. [Google Scholar] [CrossRef]
- Kubik, Ł.; Struck-Lewicka, W.; Kaliszan, R.; Wiczling, P. Simultaneous determination of hydrophobicity and dissociation constant for a large set of compounds by gradient reverse phase high performance liquid chromatography-mass spectrometry technique. J. Chromatogr. A 2015, 1416, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Djaković-Sekulić, T.; Ačanski, M.; Perišić-Janjić, N. Evaluation of the predictive power of calculation procedure for molecular hydrophobicity of some estradiol derivates. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2002, 766, 67–75. [Google Scholar] [CrossRef]
- Sarbu, C.; Nascu-Briciu, R.D.; Casoni, D.; Kot-Wasik, A.; Wasik, A.; Namiesnik, J. Chromatographic lipophilicity determination using large volume injections of the solvents non-miscible with the mobile phase. J. Chromatogr. A 2012, 1266, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Chrysanthakopoulos, M.; Nicolaou, I.; Demopoulos, V.J.; Tsantili-Kakoulidou, A. HPLC-based lipophilicity of pyrrolyl-acetic acid ARIs: Relationships with biological activity. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 61–67. [Google Scholar] [CrossRef]
- Eltayar, N.; Vandewaterbeemd, H.; Testa, B. Lipophilicity measurements of protonated basic compounds by reversed-phase high-performance liquid chromatography: II. Procedure for the determination of a lipophilic index measured by reversed-phase high-performance liquid chromatography. J. Chromatogr. A 1985, 320, 305–312. [Google Scholar] [CrossRef]
- Liu, X.; Tanaka, H.; Yamauchi, A.; Testa, B.; Chuman, H. Determination of lipophilicity by reversed-phase high-performance liquid chromatography. Influence of 1-octanol in the mobile phase. J. Chromatogr. A 2005, 1091, 51–59. [Google Scholar] [CrossRef]
- Caiali, E.; Casoni, D.; Ionita, P.; David, V.; Sarbu, C. Parabens lipophilicity determination with mobile phases containing low and medium hydrophobic alcohols. J. Liq. Chromatogr. Relat. Technol. 2014, 37, 2287–2301. [Google Scholar] [CrossRef]
- Han, S.-y.; Qiao, J.-q.; Zhang, Y.-y.; Yang, L.-l.; Lian, H.-z.; Ge, X.; Chen, H.-y. Determination of n-octanol/water partition coefficient for DDT-related compounds by RP-HPLC with a novel dual-point retention time correction. Chemosphere 2011, 83, 131–136. [Google Scholar] [CrossRef]
- Brychtova, K.; Opatrilova, R.; Raich, I.; Kalinowski, D.S.; Dvorakova, L.; Placek, L.; Csollei, J.; Richardson, D.R.; Jampilek, J. Investigating the activity of 2-substituted alkyl-6-(2,5-dioxopyrrolidin-1-yl)hexanoates as skin penetration enhancers. Biorg. Med. Chem. 2010, 18, 8556–8565. [Google Scholar] [CrossRef]
- Yamana, T.; Tsuji, A.; Miyamoto, E.; Kubo, O. Novel method for determination of partition coefficients of penicillins and cephalosporins by high-pressure liquid chromatography. J. Pharm. Sci. 1977, 66, 747–749. [Google Scholar] [CrossRef] [PubMed]
- Nowotnik, D.P.; Feld, T.; Nunn, A.D. Examination of some reversed-phase high-performance liquid chromatography systems for the determination of lipophilicity. J. Chromatogr. A 1993, 630, 105–115. [Google Scholar] [CrossRef]
- Koba, M.; Belka, M.; Ciesielski, T.; Baczek, T. Determination of lipophilicity for antitumor acridinone derivatives supported by gradient high-performance liquid chromatography method. Cent. Eur. J. Chem. 2012, 10, 216–223. [Google Scholar] [CrossRef]
- Valko, K.; Bevan, C.; Reynolds, D. Chromatographic Hydrophobicity Index by Fast-Gradient RP-HPLC: A High-Throughput Alternative to log P/log D. Anal. Chem. 1997, 69, 2022–2029. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hefesha, H.; Tanaka, H.; Scriba, G.; Fahr, A. Lipophilicity measurement of drugs by reversed phase HPLC over wide pH range using an alkaline-resistant silica-based stationary phase, XBridge(TM) Shield RP18. Chem. Pharm. Bull. 2008, 56, 1417–1422. [Google Scholar] [CrossRef] [PubMed]
- Huszar, M.; Varga, A.; Horvath, A.; Lorand, T.; Agocs, A.; Idei, M.; Mandl, J.; Vantus, T.; Keri, G. Comparative Characterization of Experimental and Calculated Lipophilicity and Anti-Tumour Activity of Isochromanone Derivatives. Curr. Med. Chem. 2010, 17, 321–333. [Google Scholar] [CrossRef]
- Chen, F.-y.; Cao, X.-w.; Han, S.-y.; Lian, H.-z.; Mao, L. Relationship between hydrophobicity and rplc retention behavior of amphoteric compounds. J. Liq. Chromatogr. Relat. Technol. 2014, 37, 2711–2724. [Google Scholar] [CrossRef]
- Bajda, M.; Bucki, A.; Szlek, J.; Szwaczkiewicz, M.; Swierczek, M.; Malawska, B. Determination of lipophilicity of alpha-(4-phenylpiperazine) derivatives of N-benzylamides using chromatographic and computational methods. Biomed. Chromatogr. 2008, 22, 428–432. [Google Scholar] [CrossRef]
- Harnisch, M.; Möckel, H.J.; Schulze, G. VII. International symposium on column liquid chromatographyRelationship between log Pow, shake-flask values and capacity factors derived from reversed-phase high-performance liquid chromatography for n-alkylbenzenes and some oecd reference substances. J. Chromatogr. A 1983, 282, 315–332. [Google Scholar] [CrossRef]
- Epifanio, I.; Genovese, S.; Carlucci, G.; Epifano, F.; Locatelli, M. Secondary plant metabolites LogP determination: The case of boropinic and geraniloxyferulic acids. Curr. Bioact. Compd. 2015, 11, 131–141. [Google Scholar] [CrossRef]
- Pachuta-Stec, A.; Hawrył, A.M.; Wróbel, A.; Hawrył, M.A.; Pitucha, M. Chromatographic Evaluation of the Lipophilic Properties of Some 1,2,4-Triazole with Potential Antitumour Activity. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 1199–1206. [Google Scholar] [CrossRef]
- Bartalis, J.; Halaweish, F.T. Relationship between cucurbitacins reversed-phase high-performance liquid chromatography hydrophobicity index and basal cytotoxicity on HepG2 cells. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2005, 818, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Podunavac-Kuzmanovic, S.O.; Jevric, L.R.; Tepic, A.N.; Sumic, Z. Reversed-phase HPLC retention data in correlation studies with lipophilicity molecular descriptors of carotenoids. Hem. Ind. 2013, 67, 933–940. [Google Scholar] [CrossRef]
- Barghash, R.F.; Ganoub, N.A.; Abdou, W.M. Development of a general and efficient route to highly lipophilic benzoxazole-2ylphosphonates and their antineoplastic properties. Monatsh. Chem. 2014, 145, 1621–1630. [Google Scholar] [CrossRef]
- Musilek, K.; Jampilek, J.; Dohnal, J.; Jun, D.; Gunn-Moore, F.; Dolezal, M.; Kuca, K. RP-HPLC determination of the lipophilicity of bispyridinium reactivators of acetylcholinesterase bearing a but-2-ene connecting linker. Anal. Bioanal. Chem. 2008, 391, 367–372. [Google Scholar] [CrossRef]
- Flieger, J.; Czajkowska-Zelazko, A.; Rzadkowska, M.; Szacon, E.; Matosiuk, D. Usefulness of reversed-phase HPLC enriched with room temperature imidazolium based ionic liquids for lipophilicity determination of the newly synthesized analgesic active urea derivatives. J. Pharm. Biomed. Anal. 2012, 66, 58–67. [Google Scholar] [CrossRef]
- Mornar, A.; Damic, M.; Nigovic, B. Pharmacokinetic Parameters of Statin Drugs Characterized by Reversed Phase High-Performance Liquid Chromatography. Anal. Lett. 2011, 44, 1009–1020. [Google Scholar] [CrossRef]
- Wiczling, P.; Kawczak, P.; Nasal, A.; Kaliszan, R. Simultaneous determination of pKa and lipophilicity by gradient RP HPLC. Anal. Chem. 2006, 78, 239–249. [Google Scholar] [CrossRef]
- Kobarfard, F.; Khalaj, A.; Daryaee, F.; Ardeshir, L.Z.; Rezaee, S. Correlation between different lipophilicity parameters and antimycobacterial activities of 2-hydroxyacetamides. DARU J. Pharm. Sci. 2008, 16, 55–59. [Google Scholar]
- Sztanke, M.; Tuzimski, T.; Janicka, M.; Sztanke, K. Structure-retention behaviour of biologically active fused 1,2,4-triazinones—Correlation with in silico molecular properties. Eur. J. Pharm. Sci. 2015, 68, 114–126. [Google Scholar] [CrossRef]
- Wiczling, P.; Waszczuk-Jankowska, M.; Markuszewski, M.J.; Kaliszan, R. The application of gradient reversed-phase high-performance liquid chromatography to the pK(a) and log k(w) determination of polyprotic analytes. J. Chromatogr. A 2008, 1214, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Yuksek, H.; Akyildirim, O.; Yola, M.L.; Gursoy-Kol, O.; Celebier, M.; Kart, D. Synthesis, In Vitro Antimicrobial and Antioxidant Activities of Some New 4,5-Dihydro-1H-1,2,4-triazol-5-one Derivatives. Arch. Pharm. 2013, 346, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, S.; Khalaj, A.; Adibpour, N.; Saffary, M. Correlation between lipophilicity and antimicrobial activity of some 2-(4-substituted phenyl)-3(2H)-isothiazolones. DARU J. Pharm. Sci. 2009, 17, 256–263. [Google Scholar]
- Posa, M.; Pilipovic, A.; Lalic, M.; Popovic, J. Determination and importance of temperature dependence of retention coefficient (RPHPLC) in QSAR model of nitrazepams’ partition coefficient in bile acid micelles. Talanta 2011, 83, 1634–1642. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, A.M.; McQuitty, R.J.; Mackay, F.S.; Zhao, Y.; Woods, J.A.; Sadler, P.J. Cellular Accumulation, Lipophilicity and Photocytotoxicity of Diazido Platinum(IV) Anticancer Complexes. ChemMedChem 2014, 9, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Pallicer, J.M.; Pous-Torres, S.; Sales, J.; Roses, M.; Rafols, C.; Bosch, E. Determination of the hydrophobicity of organic compounds measured as log P(o/w) through a new chromatographic method. J. Chromatogr. A 2010, 1217, 3026–3037. [Google Scholar] [CrossRef]
- Flieger, J. Application of perfluorinated acids as ion-pairing reagents for reversed-phase chromatography and retention-hydrophobicity relationships studies of selected beta-blockers. J. Chromatogr. A 2010, 1217, 540–549. [Google Scholar] [CrossRef]
- Rudraraju, A.V.; Amoyaw, P.N.A.; Hubin, T.J.; Khan, M.O.F. Determination of log P values of new cyclen based antimalarial drug leads using RP-HPLC. Pharmazie 2014, 69, 655–662. [Google Scholar]
- Roda, A.; Minutello, A.; Angellotti, M.A.; Fini, A. Bile acid structure-activity relationship: Evaluation of bile acid lipophilicity using 1-octanol/water partition coefficient and reverse phase HPLC. J. Lipid Res. 1990, 31, 1433–1443. [Google Scholar] [CrossRef]
- Flieger, J.; Tatarczak-Michalewska, M.; Wujec, M.; Pitucha, M.; Swieboda, R. RP-HPLC analysis and in vitro identification of antimycobacterial activity of novel thiosemicarbazides and 1,2,4-triazole derivatives. J. Pharm. Biomed. Anal. 2015, 107, 501–511. [Google Scholar] [CrossRef]
- Komsta, L.; Pietras, R.; Bartuzi, E.; Skibinski, R.; Gumieniczek, A. Chemometric Comparison of Thin-Layer Chromatography, Gradient High-Performance Liquid Chromatography, and Computational Methods for Lipophilicity Assessment of Model Compounds. JPC J. Planar Chromatogr. Mod. TLC 2015, 28, 115–118. [Google Scholar] [CrossRef]
- Pietrogrande, M.C.; Borea, P.A.; Biagi, G.L. Lipophilicity measurement of benzodiazepine-receptor ligands by reversed-phase liquid chromatography. Comparison between high-performance liquid and thin-layer chromatography. J. Chromatogr. A 1988, 447, 404–409. [Google Scholar] [CrossRef]
- Han, S.Y.; Liang, C.; Qiao, J.Q.; Lian, H.Z.; Ge, X.; Chen, H.Y. A novel evaluation method for extrapolated retention factor in determination of n-octanol/water partition coefficient of halogenated organic pollutants by reversed-phase high performance liquid chromatography. Anal. Chim. Acta 2012, 713, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Hawryl, A.M.; Popiolek, L.P.; Hawryl, M.A.; Swieboda, R.S.; Niejedli, M.A. Chromatographic and Calculation Methods for Analysis of the Lipophilicity of Newly Synthesized Thiosemicarbazides and their Cyclic Analogues 1,2,4-Triazol-3-thiones. J. Braz. Chem. Soc. 2015, 26, 1617–1624. [Google Scholar] [CrossRef]
- Reavill, C.; Walther, B.; Stolerman, I.P.; Testa, B. Behavioral and pharmacokinetic studies on nicotine, cytisine and lobeline. Neuropharmacology 1990, 29, 619–624. [Google Scholar] [CrossRef]
- Casoni, D.; Kot-Wasik, A.; Namiesnik, J.; Sarbu, C. Lipophilicity data for some preservatives estimated by reversed-phase liquid chromatography and different computation methods. J. Chromatogr. A 2009, 1216, 2456–2465. [Google Scholar] [CrossRef]
- Sztanke, K.; Markowski, W.; Swieboda, R.; Polak, B. Lipophilicity of novel antitumour and analgesic active 8-aryl-2,6,7,8-tetrahydroimidazo 2,1-c 1,2,4 triazine-3,4-dione derivatives determined by reversed-phase HPLC and computational methods. Eur. J. Med. Chem. 2010, 45, 2644–2649. [Google Scholar] [CrossRef]
- Almási, J.; Takács-Novák, K.; Kökösi, J.; Vámos, J. Characterization of potential NMDA and cholecystokinin antagonists II. Lipophilicity studies on 2-methyl-4-oxo-3H-quinazoline-3-alkyl-carboxylic acid derivatives. Int. J. Pharm. 1999, 180, 13–22. [Google Scholar] [CrossRef]
- Giaginis, C.; Theocharis, S.; Tsantili-Kakoulidou, A. Contribution to the standardization of the chromatographic conditions for the lipophilicity assessment of neutral and basic drugs. Anal. Chim. Acta 2006, 573, 311–318. [Google Scholar] [CrossRef]
- Benhaim, D.; Grushka, E. Effect of n-octanol in the mobile phase on lipophilicity determination by reversed-phase high-performance liquid chromatography on a modified silica column. J. Chromatogr. A 2008, 1209, 111–119. [Google Scholar] [CrossRef]
- Kovačević, S.; Banjac, M.K.; Podunavac-Kuzmanović, S.; Milošević, N.; Ćurčić, J.; Vulić, J.; Šeregelj, V.; Banjac, N.; Ušćumlić, G. Chromatographic and computational screening of anisotropic lipophilicity and pharmacokinetics of newly synthesized 1-aryl-3-ethyl-3-methylsuccinimides. Comput. Biol. Chem. 2020, 84, 107161. [Google Scholar] [CrossRef] [PubMed]
- Henchoz, Y.; Guillarme, D.; Martel, S.; Rudaz, S.; Veuthey, J.-L.; Carrupt, P.-A. Fast log P determination by ultra-high-pressure liquid chromatography coupled with UV and mass spectrometry detections. Anal. Bioanal. Chem. 2009, 394, 1919–1930. [Google Scholar] [CrossRef] [PubMed]
- Pallicer, J.M.; Calvet, C.; Port, A.; Rosés, M.; Ràfols, C.; Bosch, E. Extension of the liquid chromatography/quantitative structure-property relationship method to assess the lipophilicity of neutral, acidic, basic and amphotheric drugs. J. Chromatogr. A 2012, 1240, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Henchoz, Y.; Guillarme, D.; Rudaz, S.; Veuthey, J.L.; Carrupt, P.A. High-throughput log P determination by ultraperformance liquid chromatography: A convenient tool for medicinal chemists. J. Med. Chem. 2008, 51, 396–399. [Google Scholar] [CrossRef]
- Kovačević, S.; Karadžić Banjac, M.; Anojčić, J.; Podunavac-Kuzmanović, S.; Jevrić, L.; Nikolić, A.; Savić, M.; Kuzminac, I. Chemometrics of anisotropic lipophilicity of anticancer androstane derivatives determined by reversed-phase ultra high performance liquid chromatography with polar aprotic and protic modifiers. J. Chromatogr. A 2022, 1673, 463197. [Google Scholar] [CrossRef]
- Rybka, S.; Obniska, J.; Żmudzki, P.; Koczurkiewicz, P.; Wójcik-Pszczoła, K.; Pękala, E.; Bryła, A.; Rapacz, A. Synthesis and Determination of Lipophilicity, Anticonvulsant Activity, and Preliminary Safety of 3-Substituted and 3-Unsubstituted N-[(4-Arylpiperazin-1-yl)alkyl]pyrrolidine-2,5-dione Derivatives. ChemMedChem 2017, 12, 1848–1856. [Google Scholar] [CrossRef]
- Guillot, A.; Henchoz, Y.; Moccand, C.; Guillarme, D.; Veuthey, J.-L.; Carrupt, P.-A.; Martel, S. Lipophilicity Determination of Highly Lipophilic Compounds by Liquid Chromatography. Chem. Biodivers. 2009, 6, 1828–1836. [Google Scholar] [CrossRef]
- Darrouzain, F.; Dallet, P.; Dubost, J.P.; Ismaili, L.; Pehourcq, F.; Bannwarth, B.; Matoga, M.; Guillaume, Y.C. Molecular lipophilicity determination of a huperzine series by HPLC: Comparison of C18 and IAM stationary phases. J. Pharm. Biomed. Anal. 2006, 41, 228–232. [Google Scholar] [CrossRef]
- Bajda, M.; Boryczka, S.; Wietrzyk, J.; Malawska, B. Investigation of lipophilicity of anticancer-active thioquinoline derivatives. Biomed. Chromatogr. 2007, 21, 123–131. [Google Scholar] [CrossRef]
- Giaginis, C.; Theocharis, S.; Tsantili-Kakoulidou, A. Investigation of the lipophilic behaviour of some thiazolidinediones Relationships with PPAR-gamma activity. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 857, 181–187. [Google Scholar] [CrossRef]
- Matysiak, J.; Juszczak, M.; Karpinska, M.M.; Langner, E.; Walczak, K.; Lemieszek, M.K.; Skrzypek, A.; Niewiadomy, A.; Rzeski, W. Synthesis of 2-(2,4-dihydroxyphenyl)thieno-1,3-thiazin-4-ones, their lipophilicity and anticancer activity in vitro. Mol. Divers. 2015, 19, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Malawska, B.; Kulig, K.; Bucki, A.; Zbek, P.; Wieckowska, A. The study of the lipophilicity of alpha-(4-phenylpiperazin-1-yl)-gamma-phthalimidobutyramides using chromatographic and computational methods. Biomed. Chromatogr. 2008, 22, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Lazaro, E.; Rafols, C.; Abraham, M.H.; Roses, M. Chromatographic estimation of drug disposition properties by means of immobilized artificial membranes (IAM) and C18 columns. J. Med. Chem. 2006, 49, 4861–4870. [Google Scholar] [CrossRef] [PubMed]
- Essaid, D.; Chaminade, P.; Maillard, P.; Kasselouri, A. Lipophilicity of porphyrins and their retention in IAM, C8-C18 and HILIC chromatographic systems. J. Pharm. Biomed. Anal. 2015, 114, 227–240. [Google Scholar] [CrossRef]
- Flieger, J.; Pizon, M.; Plech, T. Chromatographic behavior of new antiepileptic active compounds on different reversed-phase materials. J. Chromatogr. A 2014, 1338, 188–196. [Google Scholar] [CrossRef]
- Bocian, S.; Buszewski, B. Comparison of retention properties of stationary phases imitated cell membrane in RP HPLC. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 990, 198–202. [Google Scholar] [CrossRef]
- Dąbrowska, M.; Starek, M.; Komsta, Ł.; Szafrański, P.; Stasiewicz-Urban, A.; Opoka, W. Assessment of the chromatographic lipophilicity of eight cephalosporins on different stationary phases. Eur. J. Pharm. Sci. 2017, 101, 115–124. [Google Scholar] [CrossRef]
- Vrakas, D.; Giaginis, C.; Tsantili-Kakoulidou, A. Different retention behavior of structurally diverse basic and neutral drugs in immobilized artificial membrane and reversed-phase high performance liquid chromatography: Comparison with octanol–water partitioning. J. Chromatogr. A 2006, 1116, 158–164. [Google Scholar] [CrossRef]
- Grumetto, L.; Russo, G.; Barbato, F. Relationships between human intestinal absorption and polar interactions drug/phospholipids estimated by IAM-HPLC. Int. J. Pharm. 2015, 489, 186–194. [Google Scholar] [CrossRef]
- Valkó, K.L.; Nunhuck, S.B.; Hill, A.P. Estimating Unbound Volume of Distribution and Tissue Binding by In Vitro HPLC-Based Human Serum Albumin and Immobilised Artificial Membrane-Binding Measurements. J. Pharm. Sci. 2011, 100, 849–862. [Google Scholar] [CrossRef]
- Barbato, F.; Cirocco, V.; Grumetto, L.; La Rotonda, M.I. Comparison between immobilized artificial membrane (IAM) HPLC data and lipophilicity in n-octanol for quinolone antibacterial agents. Eur. J. Pharm. Sci. 2007, 31, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, X.; Xu, D.; Lai, L.; Crommen, J.; Guo, J.; Jiang, Z. Development of acidic phospholipid containing immobilized artificial membrane column to predict drug-induced phospholipidosis potency. J. Chromatogr. A 2021, 1647, 462147. [Google Scholar] [CrossRef] [PubMed]
- Grumetto, L.; Russo, G.; Barbato, F. Indexes of polar interactions between ionizable drugs and membrane phospholipids measured by IAM-HPLC: Their relationships with data of Blood-Brain Barrier passage. Eur. J. Pharm. Sci. 2014, 65, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Ijzerman, A.P.; Heitman, L.H. K(v)11.1 (hERG)-induced cardiotoxicity: A molecular insight from a binding kinetics study of prototypical K(v)11.1 (hERG) inhibitors. Br. J. Pharmacol. 2015, 172, 940–955. [Google Scholar] [CrossRef]
- Vrakas, D.; Giaginis, C.; Tsantili-Kakoulidou, A. Electrostatic interactions and ionization effect in immobilized artificial membrane retention A comparative study with octanol-water partitioning. J. Chromatogr. A 2008, 1187, 67–78. [Google Scholar] [CrossRef]
- Taillardat-Bertschinger, A.; Martinet, C.A.M.; Carrupt, P.-A.; Reist, M.; Caron, G.; Fruttero, R.; Testa, B. Molecular Factors Influencing Retention on Immobilized Artificial Membranes (IAM) Compared to Partitioning in Liposomes and n-Octanol. Pharm. Res. 2002, 19, 729–737. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Yang, Q.; Hara, M.; Nakamura, C.; Miyake, J. A novel chromatographic solid support with immobilized unilamellar liposomes for model analysis of solute–membrane interaction: Comparison with analysis using immobilized artificial membranes and free liposomal membranes. Mater. Sci. Eng. C 2001, 17, 119–126. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Nakamura, C.; Yang, Q.; Kamo, N.; Miyake, J. Immobilized liposome chromatography to study drug–membrane interactions: Correlation with drug absorption in humans. J. Chromatogr. A 2002, 961, 113–118. [Google Scholar] [CrossRef]
- Janicka, M.; Mycka, A.; Sztanke, M.; Sztanke, K. Predicting Pharmacokinetic Properties of Potential Anticancer Agents via Their Chromatographic Behavior on Different Reversed Phase Materials. Int. J. Mol. Sci. 2021, 22, 4257. [Google Scholar] [CrossRef]
- Waters, L.J.; Shahzad, Y.; Mitchell, J.C. pH effects in micellar liquid chromatographic analysis for determining partition coefficients for a series of pharmaceutically related compounds. Curr. Pharm. Anal. 2012, 8, 272–277. [Google Scholar] [CrossRef]
- Koneva, A.S.; Ritter, E.; Anufrikov, Y.A.; Lezov, A.A.; Klestova, A.O.; Smirnova, N.A.; Safonova, E.A.; Smirnova, I. Mixed aqueous solutions of nonionic surfactants Brij 35/Triton X-100: Micellar properties, solutes’ partitioning from micellar liquid chromatography and modelling with COSMOmic. Colloids Surf. A 2018, 538, 45–55. [Google Scholar] [CrossRef]
- De Vrieze, M.; Janssens, P.; Szucs, R.; Van der Eycken, J.; Lynen, F. In vitro prediction of human intestinal absorption and blood–brain barrier partitioning: Development of a lipid analog for micellar liquid chromatography. Anal. Bioanal. Chem. 2015, 407, 7453–7466. [Google Scholar] [CrossRef] [PubMed]
- Molero-Monfort, M.; Escuder-Gilabert, L.; Villanueva-Camañas, R.M.; Sagrado, S.; Medina-Hernández, M.J. Biopartitioning micellar chromatography: An in vitro technique for predicting human drug absorption. J. Chromatogr. B Biomed. Sci. Appl. 2001, 753, 225–236. [Google Scholar] [CrossRef]
- Waters, L.J.; Shokry, D.S.; Parkes, G.M.B. Predicting human intestinal absorption in the presence of bile salt with micellar liquid chromatography. Biomed. Chromatogr. 2016, 30, 1618–1624. [Google Scholar] [CrossRef] [PubMed]
- Molero-Monfort, M.; Martín-Biosca, Y.; Sagrado, S.; Villanueva-Camañas, R.M.; Medina-Hernández, M.J. Micellar liquid chromatography for prediction of drug transport. J. Chromatogr. A 2000, 870, 1–11. [Google Scholar] [CrossRef]
- Martínez-Pla, J.J.; Sagrado, S.; Villanueva-Camañas, R.M.; Medina-Hernández, M.J. Retention–property relationships of anticonvulsant drugs by biopartitioning micellar chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2001, 757, 89–99. [Google Scholar] [CrossRef]
- Wu, L.-P.; Chen, Y.; Wang, S.-R.; Chen, C.; Ye, L.-M. Quantitative retention–activity relationship models for quinolones using biopartitioning micellar chromatography. Biomed. Chromatogr. 2008, 22, 106–114. [Google Scholar] [CrossRef]
- Dobričić, V.; Savić, J.; Nikolic, K.; Vladimirov, S.; Vujić, Z.; Brborić, J. Application of biopartitioning micellar chromatography and QSRR modeling for prediction of gastrointestinal absorption and design of novel β-hydroxy-β-arylalkanoic acids. Eur. J. Pharm. Sci. 2017, 100, 280–284. [Google Scholar] [CrossRef]
- Tsopelas, F.; Danias, P.; Pappa, A.; Tsantili-Kakoulidou, A. Biopartitioning micellar chromatography under different conditions: Insight into the retention mechanism and the potential to model biological processes. J. Chromatogr. A 2020, 1621, 461027. [Google Scholar] [CrossRef]
- Enami, T.; Nagae, N.; Doshi, S. The retention behaviour of reversed-phase HPLC columns when used under 100% aqueous conditions. LC-GC Europe 2003, 16, 418–425. [Google Scholar]
- Khan, B.M.; Liu, Y. High speed counter current chromatography: Overview of solvent-system and elution-mode. J. Liq. Chromatogr. Relat. Technol. 2018, 41, 629–636. [Google Scholar] [CrossRef]
- Valko, K. Application of high-performance liquid chromatography based measurements of lipophilicity to model biological distribution. J. Chromatogr. A 2004, 1037, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Gluck, S.J.; Martin, E.J. Extended Octanol-Water Partition Coefficient Determination by Dual-Mode Centrifugal Partition Chromatography. J. Liq. Chromatogr. 1990, 13, 3559–3570. [Google Scholar] [CrossRef]
- Tsopelas, F.; Vallianatou, T.; Tsantili-Kakoulidou, A. Advances in immobilized artificial membrane (IAM) chromatography for novel drug discovery. Expert Opin. Drug Discov. 2016, 11, 473–488. [Google Scholar] [CrossRef]
- Technologies, R. Available online: https://www.registech.com/immobilized-artificial-membrane-iam-chromatography/iam-pc-dd2/iam-pc-dd2/ (accessed on 12 July 2022).
- Stepanić, V.; Žiher, D.; Gabelica-Marković, V.; Jelić, D.; Nunhuck, S.; Valko, K.; Koštrun, S. Physicochemical profile of macrolides and their comparison with small molecules. Eur. J. Med. Chem. 2012, 47, 462–472. [Google Scholar] [CrossRef]
- Lundahl, P.; Yang, Q. Liposome chromatography: Liposomes immobilized in gel beads as a stationary phase for aqueous column chromatography. J. Chromatogr. A 1991, 544, 283–304. [Google Scholar] [CrossRef]
- Eeman, M.; Deleu, M. From biological membranes to biomimetic model membranes. Biotechnol. Agron. Soc. Environ. 2010, 14, 719–736. [Google Scholar]
- Janicka, M.; Stępnik, K.; Pachuta-Stec, A. Quantification of Lipophilicity of 1,2,4-Triazoles Using Micellar Chromatography. Chromatographia 2012, 75, 449–456. [Google Scholar] [CrossRef]
- Escuder-Gilabert, L.; Martinez-Pla, J.J.; Sagrado, S.; Villanueva-Camanas, R.M.; Medina-Hernandez, M.J. Biopartitioning micellar separation methods: Modelling drug absorption. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003, 797, 21–35. [Google Scholar] [CrossRef]
- Quiñones-Torrelo, C.; Sagrado, S.; Villanueva-Camañas, R.M.; Medina-Hernández, M.J. Development of Predictive Retention−Activity Relationship Models of Tricyclic Antidepressants by Micellar Liquid Chromatography. J. Med. Chem. 1999, 42, 3154–3162. [Google Scholar] [CrossRef]
- Khaledi, M.G.; Strasters, J.K.; Rodgers, A.H.; Breyer, E.D. Simultaneous enhancement of separation selectivity and solvent strength in reversed-phase liquid chromatography using micelles in hydro-organic solvents. Anal. Chem. 1990, 62, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Carraro, M.L.; Ribeiro, J.; Araújo, J.; Tiritan, M.E.; Pinto, M.M.M. Synthetic Chiral Derivatives of Xanthones: Biological Activities and Enantioselectivity Studies. Molecules 2019, 24, 791. [Google Scholar] [CrossRef]
- Soares, J.X.; Loureiro, D.R.P.; Dias, A.L.; Reis, S.; Pinto, M.M.M.; Afonso, C.M.M. Bioactive Marine Xanthones: A Review. Marine Drugs 2022, 20, 58. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.M.M.; Sousa, E.M.; Nascimento, S.J.M. Xanthone Derivatives: New Insights in Biological Activities. Curr. Med. Chem. 2005, 12, 2517–2538. [Google Scholar] [CrossRef]
- Takashima, I.; Kawagoe, R.; Hamachi, I.; Ojida, A. Development of an AND Logic-Gate-Type Fluorescent Probe for Ratiometric Imaging of Autolysosome in Cell Autophagy. Chem. Eur. J. 2015, 21, 2038–2044. [Google Scholar] [CrossRef] [PubMed]
- Sathyadevi, P.; Chen, Y.-J.; Wu, S.-C.; Chen, Y.-H.; Wang, Y.-M. Reaction-based epoxide fluorescent probe for in vivo visualization of hydrogen sulfide. Biosens. Bioelectron. 2015, 68, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Phyo, Y.Z.; Teixeira, J.; Tiritan, M.E.; Cravo, S.; Palmeira, A.; Gales, L.; Silva, A.M.S.; Pinto, M.M.M.; Kijjoa, A.; Fernandes, C. New chiral stationary phases for liquid chromatography based on small molecules: Development, enantioresolution evaluation and chiral recognition mechanisms. Chirality 2020, 32, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Tiritan, M.E.; Cravo, S.; Phyo, Y.Z.; Kijjoa, A.; Silva, A.M.S.; Cass, Q.B.; Pinto, M.M.M. New chiral stationary phases based on xanthone derivatives for liquid chromatography. Chirality 2017, 29, 430–442. [Google Scholar] [CrossRef]
- Pinto, M.M.M.; Palmeira, A.; Fernandes, C.; Resende, D.I.S.P.; Sousa, E.; Cidade, H.; Tiritan, M.E.; Correia-da-Silva, M.; Cravo, S. From Natural Products to New Synthetic Small Molecules: A Journey through the World of Xanthones. Molecules 2021, 26, 431. [Google Scholar] [CrossRef]
- Santos, Á.; Soares, J.X.; Cravo, S.; Tiritan, M.E.; Reis, S.; Afonso, C.; Fernandes, C.; Pinto, M.M.M. Lipophilicity assessement in drug discovery: Experimental and theoretical methods applied to xanthone derivatives. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1072, 182–192. [Google Scholar] [CrossRef]
- Fernandes, C.; Masawang, K.; Tiritan, M.E.; Sousa, E.; de Lima, V.; Afonso, C.; Bousbaa, H.; Sudprasert, W.; Pedro, M.; Pinto, M.M. New chiral derivatives of xanthones: Synthesis and investigation of enantioselectivity as inhibitors of growth of human tumor cell lines. Biorg. Med. Chem. 2014, 22, 1049–1062. [Google Scholar] [CrossRef]
- Fernandes, C.; Oliveira, L.; Tiritan, M.E.; Leitao, L.; Pozzi, A.; Noronha-Matos, J.B.; Correia-de-Sá, P.; Pinto, M.M. Synthesis of new chiral xanthone derivatives acting as nerve conduction blockers in the rat sciatic nerve. Eur. J. Med. Chem. 2012, 55, 1–11. [Google Scholar] [CrossRef]
- Fernandes, C.; Palmeira, A.; Ramos, I.I.; Carneiro, C.; Afonso, C.; Tiritan, M.E.; Cidade, H.; Pinto, P.C.A.G.; Saraiva, M.L.M.F.S.; Reis, S.; et al. Chiral Derivatives of Xanthones: Investigation of the Effect of Enantioselectivity on Inhibition of Cyclooxygenases (COX-1 and COX-2) and Binding Interaction with Human Serum Albumin. Pharmaceuticals 2017, 10, 50. [Google Scholar] [CrossRef]






| Analytes | Aim | Chromatographic Method | Chromatographic Conditions | Ref. |
|---|---|---|---|---|
| Series of corticosteroids | Estimate lipophilicity | RP-HPLC (254 nm) | Columns: Zorbax Eclipse XDB-C18 (150 × 4.6 mm, 3.5 µm) and Zorbax Phenyl (250 × 4.6 mm, 5 µm); MP: mixtures of ACN (5–70%) and water with HCOOH 0.1%; FR: 1.0 mL/min | [65] |
| 17β-Carboxamide glucocorticoids | Estimate lipophilicity | RP-HPLC | Columns: Zorbax Eclipse XDB-C8 (150 × 4.6 mm, 5 µm), Zorbax Eclipse XDB-phenyl (150 × 4.6 mm, 5 µm), and Zorbax SB-CN (150 × 4.6 mm, 5 µm); MP: mixtures of MeOH or ACN (50–90%) and water; FR: not specified | [66] |
| Antibiofilm agents | Estimate lipophilicity | RP-HPLC | Columns: end-capped RP BDS Hypersil C18 (30 × 4.0 mm, 3 µm) and end-capped Kinetex C8 (150 × 4.6 mm, 5 µm); MP: mixtures of ACN (45–55%) and water with ammonium acetate; FR: 1.0 mL/min | [67] |
| Steroid derivatives | Estimate lipophilicity | RP-HPLC (210 nm) | Column: Zorbax SB-C18 (250 × 3.0 mm, 5 µm); MP: mixtures of MeOH or ACN (70–80%) and water; FR: 0.6 mL/min | [68] |
| Antioxidant compounds | Determine the effects of stationary phase on the retention in terms of hydrophobicity | RP-HPLC (254 nm) | Columns: Purosphere RP-18e (125 × 3.0 mm, 5 µm), Zorbax Eclipse XDBC8 (150 × 4.6 mm, 5 µm), Discovery RP-Amide C16 (150 × 4.6 mm, 5 µm), CN100 Lichrosphere (250 × 4.0 mm, 5 µm), and pentafluorophenyl Kinetex (150 × 2.1 mm, 2.6 µm); MP: mixtures of MeOH (55–55%) and water with 0.1% formic acid; FR: 0.7 or 0.2 mL/min | [69] |
| Tetrahydrothiophen-3-one based thiazoles | Estimate lipophilicity | RP-HPLC (254 nm) | Column: Superspher 100 RP-18; MP: mixtures of MeOH (60–95%) and water; FR: 0.7 mL/min | [70] |
| N-Hydroxyethylamides of aryloxyalkyllen and pyridine carboxylic acids | Study the role of the stationary and mobile phases on the determination of log P | RP-HPLC/ UV-Vis (254 nm) | Columns: LiChrosorb RP-18 (125 × 4.0 mm, 5.0 μm), LiChrospher 60 RP-Select B (125 × 4.0 mm, 5.0 μm), Zorbax (5 μm, L = 150 × 4.6 mm; 5.0 μm), Zorbax-Eclipse XDB-C18 (150 × 4.6 mm; 5.0 μm); MP: mixtures of MeOH (0–60%) or ACN (0–50%) and water or 20 mM phosphate buffer at pH 7.0 or 20 mM tricine buffer at pH 7.0; FR: 1.0 mL/min | [71] |
| Basic and neutral drugs | Evaluate the performance of [EMIM][BF4] in producing extrapolated log kw indices | RP-HPLC/ UV-Vis (220–254–268 nm) | Columns: Hypersil end-capped BDS C18 column (250 × 4.6 mm, 5.0 μm) and ABZ+ (150 × 4.6 mm, 5.0 μm); MP: mixtures of MeOH (20–80%) and 20 mM MOPS at pH 7.4 with [EMIM][BF4] (1%) and with octan-1-ol (0–0.2%); FR: not specified | [72] |
| Deuterated benzene, toluene, p-xylene, pyridine, and aniline, and their isotopomers, | Compare partition coefficients of deuterium atoms by shake-flask and HPLC methods | RP-HPLC/ UV-Vis (254 nm) | Column: LiChrosorb RP-18 (300 × 4 mm, 10 μm); MP: mixtures of MeOH and water; FR: not specified | [73] |
| β-Blockers and anti-arrhythmic drugs | Determine the effects of stationary phase on the retention in terms of hydrophobicity | RP-HPLC/DAD (220, 230, 254 and 280 nm) | Columns: SG-CHOL (250 × 4.6 mm) cholesterolic; SG-AP (250 × 4.6 mm); End-capped Supelcosil C18-DB (250 × 4.6 mm, 5.0 μm); Merck Chromolith Performance RP-18e (100 × 4.6 mm, 5.0 μm); MP: mixtures of ACN or MeOH (20–100%) (gradient mode); FR: 1.0 mL/min | [74] |
| Arylpiperazines and tetrahydroisoquinoline derivatives with imidazo [2,1-f]purine-2,4-dione moiety | Estimate lipophilicity | RP-HPLC/DAD (230 nm) | Column: LiChrospher 100 R C18 (100 × 4.6 mm, 5.0 μm); MP: mixtures of ACN (35–65%) and water with 0.01% TFA; FR: 1.0 mL/min | [75] |
| meta and para Substituted benzenesulfonamides | Estimate lipophilicity | RP-HPLC/ UV-Vis (254 nm) | Column: μ-bondapak C18 (150 × 3.9 mm,10 μm); MP: mixtures of MeOH or ACN and water; FR: 1.0 mL/min | [76] |
| Terpenoids | Determine lipophilicity by RP-HPLC and compare with shake-flask and predicted values | RP-HPLC/DAD (215 nm); RP-HPLC/RI | Column: Alltech Altima C18 (150 × 4.6 mm, 5 μm); MP: mixtures of MeOH (30–75%) and 0.02 M MOPS buffer at pH 7.2; FR: 1.0 mL/min | [77] |
| Anilides of pyrazine- 2-carboxylic acid and 4-benzylsulfanylpyridine derivatives | Determine lipophilicity by RP-HPLC and compare with predicted values | RP-HPLC/ UV-Vis (254 nm) | Column: Chromolith RP18e; (100 × 4.6 mm); MP: mixture of MeOH (37–42%) and 0.05 M phosphate buffer at pH 7.4; FR: 4.0 mL/min | [78] |
| Esters of substituted 6-aminohexanoic acid | Determine lipophilicity by RP-HPLC and compare with predicted values | RP-HPLC/DAD (204 nm) | Column: Zorbax Eclipse XDB C18 (150 mm × 4.6 mm, 5 μm); MP: mixture of MeOH (85%) and water; FR: 0.4 mL/min | [79] |
| Set of compounds representing various functional groups such as ketones, acids, or alcohols | Determine lipophilicity by RP-HPLC and compare it with reported values | RP-HPLC/ UV-Vis (220 and 254 nm) | Columns: Discovery RP Amide C16 (150 × 4.0 mm, 5.0 μm) and Discovery RP Amide C16 (20 × 4.0 mm, 5.0 μm); MP: mixtures of MeOH and phosphate buffer at pH 3 with octanol (0.25%); FR: 1.0 or 2.0 mL/min | [80] |
| N-(Bemothiazol-2-yl)- α-amino alkyl phosphonic diesters | Compare lipophilicity determination between RP-HPLC and RP-HPTLC | RP-HPLC/ UV-Vis (230 nm) | Column: ODS (250 × 4.6 mm, 5 μm); MP: mixtures of MeOH (70–90%) and water, FR: 1.0 mL/min | [81] |
| Acyclovir esters | Determine lipophilicity by RP-HPLC and compare with predicted values | RP-HPLC/ UV-Vis (240 nm) | Column: LiChrospher RP-18 (250 × 4.0 mm, 5.0 μm); MP: mixtures ACN or MeOH and 20 mM phosphate buffer pH 6.7; FR: 1.5 mL/min | [82] |
| Diverse set of compounds | Compare the solvation equations obtained for different RP chromatographic retention parameters | RP-HPLC | Column: ODS2-IK5 Inertsil (150 × 4.6 mm); MP: mixtures of ACN and 0.1% phosphoric acid or ammonium acetate buffer at pH 9.5 (gradient mode); FR: 1.0 mL/min | [83] |
| [(5Z)-(5-Arylalkylidene-4-oxo-2-thioxo-1,3-thiazolidin-3-yl)]acetic acids derivatives | Determine lipophilicity by RP-HPLC and compare it with predicted values | RP-HPLC/DAD (210 nm) | Column: end-capped Symmetry C18 (250 × 4.6 mm, 5 μm); MP: mixture of MeOH (70%) and water; FR: 0.9 mL/min | [84] |
| Weakly ionizable basic compounds and neutral compounds | Determine lipophilicity by RP-HPLC | RP-HPLC/DAD | Column: Phenomenex Gemini C18 (150 × 4.6 mm, 5 μm); MP: mixtures of MeOH and 0.02 M ammonium chloride buffer at pH 7.4 and 9.0; FR: 1.0 mL/min | [64] |
| 4-Alkyl or alkoxy-4′-cyanobiphenyl derivatives | Determine lipophilicity by RP-HPLC | RP-HPLC/ UV-VIS | Column:C18 Nucleosil 7 (15 × 4.6 mm); MP: mixtures of MeOH (75–100%) and water; FR: 1.0 mL/min | [85] |
| Structurally diverse acidic drugs both in the neutral and ionized form | Determine lipophilicity by RP-HPLC and compare three columns in the presence and absence of octan-1-ol as additive | RP-HPLC/ UV-Vis (220, 254, 268 nm) | Columns: Supelcosil ABZ+ Plus (150 × 4.6 mm, 5 μm) and Supelcosil Aquasil (150 × 4.6 mm, 5 μm); MP: mixtures of MeOH (10–85%) and 20 m M MOPS buffer at pH 2.5 and 7.4 in the presence or absence of octan-1-ol; FR: 1.0 mL/min | [86] |
| Quinoline derivatives | Determine lipophilicity by RP-HPLC and compare with predicted values | RP-HPLC/DAD (210 nm) | Column: Symmetry C18 (250 × 4.6 mm, 5 μm); MP: mixtures of MeOH and water; FR: 0.9 mL/min | [87] |
| Amidoximes or substituted heterocyclic coumarin derivatives | Study the relationship between log P and various chromatographic indices, including the extrapolated capacity factors derived by HPLC and reversed-phase TLC | RP-HPLC/ UV-Vis (254 nm) | Column: BDS C18 (250 × 4.6 mm, 5 μm); MP: mixtures of MeOH (40–90%) and water; FR: not specified | [88] |
| Aliphatic hydrazide derivatives | Determine lipophilicity | RP-HPLC/ UV-Vis (320 nm) | Column: Hypersil BDS C18 (150 × 4.6 mm, 5 μm); MP: mixtures of MeOH (45–95%) with acetate buffer at pH 4.0; FR: 0.5 mL/min | [89] |
| Diverse set of compounds | Determine lipophilicity | RP-HPLC/DAD | Columns: Phenomenex Gemini NX (150 × 4.6 mm, 5 μm), Waters XTerra RP-18 (150 × 4.6 mm, 5 μm), Waters XTerra MS C18 (150 × 4.6 mm, 5 μm); MP: mixtures of ACN (40–50%) and pyrrolidine and ammonium hydrogenocarbonate buffer at pH 11.0; FR: 1.0 mL/min | [90] |
| Selenazole derivatives | Determine lipophilicity by RP-HPLC and compare with predicted values | RP-HPLC/ UV-Vis (210 nm) | Column: Zorbax SB-C18 (100 × 3.0 mm, 3.5 μm); MP: mixtures of MeOH (45–90%) and water; FR: not specified | [91] |
| Spironolactone | Determine lipophilicity by RP-HPLC and compare with predicted values | RP-HPLC/ UV-Vis (238 nm) | Column: C18 Eurospher 100–5 (250 × 4.0 mm, 5 μm), MP: mixtures of MeOH or dioxane (55–95%) and water; FR: 1.0 mL/min | [92] |
| 6-Aminohexanoates derivatives | Determine lipophilicity by RP-HPLC and compare with predicted values | RP-HPLC/DAD (204 nm) | Column: C18 Zorbax Eclipse XDB (150 × 4.0 mm, 5 μm); MP: mixture of MeOH (85%) and water; FR: 0.4 mL/min | [93] |
| Marine natural products | Determine lipophilicity by RP-HPLC and compare with predicted values | RP-HPLC/DAD | Column: Hamilton polystyrene-divinylbenzene PRP-1 column (150 × 4.6 mm, 5 μm); MP: mixtures of ACN and 25 mM AcONH4 at pH 4.5, 7.2, 9.8 in the range 0–100%; FR: 1.0 mL/min | [94] |
| Wide range of commercially available compounds | High-throughput log P measurement using parallel liquid chromatography/ultraviolet/mass spectrometry and sample-pooling | RP-HPLC/UV/MS | Columns: LUNA C18 (100 × 3.0 mm, 5 μm) for use on single channel LC/MS and LUNA C18 (50 × 3.0 mm, 5 μm) for high-throughput analysis on parallel LC/MS; MP: mixtures of MeOH (45–100%) with 20 mM ammonium carbonate at pH 8.0 or 20 mM ammonium formate at pH 1.0; FR: 0.45 mL/min | [95] |
| Thiosemicarbazide and 1,2,4-triazole-3-thione derivatives | Determine lipophilicity by RP-HPLC and compare it with predicted values | RP-HPLC/ UV-Vis (254 nm) | Column: RP-18 Waters Symmetry (150 × 4.6 mm, 5 μm); MP: mixtures of MeOH (55–80%) or ACN (40–80%) and water; FR: 1.0 mL/min | [96] |
| α-Asarone derivatives | Determine lipophilicity by RP-HPLC | RP-HPLC/DAD (254 nm) | Columns: RP-18e Purospher STAR C18 (150 × 4.0 mm, 5 μm); RP-8e Purospher STAR C8 (150 × 4.6 mm, 5 μm); Zorbax Eclipse XDB-C (150 × 4.6 mm, 5 μm); Nucleosil Phenyl (250 × 4.6 mm, 7 μm); MP: mixtures of MeOH or ACN and water; FR: 1.0 mL/min | [97] |
| Mesoionic 1,3,4-thuadiazolium-2-aminide derivatives | Determine lipophilicity by RP-HPLC | RP-HPLC/ UV-Vis | Column: C18 ODS-Shim-Pack (18.0 × 6.0 mm); MP: a mixture of MeOH (25–85%) and 0.005 M of phosphoric and glacial acetic acid buffer at pH 4.6; FR: not specified | [98] |
| Arylamino-2-ethane-1,1-diyl- and benzoxazole-2-methylenebisphosphonates | Determine lipophilicity by RP-HPLC and compare with predicted values | RP-HPLC/DAD (210 nm) | Column: Symmetry C18 (250 × 4.6 mm, 5μm); MP: mixture of MeOH (90%) and water; FR: 1.0 mL/min | [99] |
| Pyrimidinic nucleoside derivatives | Determine lipophilicity by shake-flask, RP-TLC, RP-HPLC, and in silico methods | RP-HPLC/ UV-Vis (265 nm) | Column: CLC-ODS(N)PN 228-17873-91(15 cm) QTY:2, packed with a C18 chemically bonded non-polar stationary phase; MP: mixtures of MeOH (30–80%) and water; FR: 1.0 mL/min | [100] |
| Nicotinates esters | Determine lipophilicity by RP-HPLC | RP-HPLC/ UV-Vis (262 nm) | Column: LiChrosorb RP-18 (250 × 4 mm, 10 μm); MP: mixtures of MeOH or ACN and 0.02 M MOPS buffer at pH 7.0 wit N-decylamine (0.2%); FR: 1.5 mL/min | [101] |
| Structurally diverse set of neutral, acidic, and basic compounds | Determine lipophilicity by RP-HPLC | RP-HPLC/variable wavelength detector (220–300 nm) | Column: PRP-C18 (50 × 4.6 mm, 5 μm) or (33 × 2.1 mm, 5 μm); MP: mixtures of ACN and water with 0.2% phosphoric acid or 30 mM of diethylamine; FR: 2.0 mL/min | [102] |
| Esters of alkoxyphenylcarbamic acid | Study the QSRR models for potential local anaesthetic drugs | RP-HPLC/DAD (210–290 nm) | Column: Separon SGX C18 (150 × 3.2 mm, 5 μm) or Separon SGX Phenyl (150 × 3.2 mm, 5 μm); MP: mixtures of MeOH or ACN (80%) and water; FR: 1.0 mL/min | [103] |
| Methylated naphthalene derivatives | Determine lipophilicity by RP-HPLC | RP-HPLC/DAD (254 nm) | Column: octadecylsilyl SUPELCOSIL LC-PAH (250 × 4.6 mm, 5 μm); MP: mixture of ACN (40–100%) and water; FR: 2.0 mL/min | [104] |
| (Hetero)arylamides of 2-amino 4,6-dimethyl pyridine | Determine lipophilicity by RP-HPLC | RP-HPLC/ UV-Vis (254 or 285 nm) | Column: porous polystyrene/divinylbenzene copolymer PLRP-S (250 × 4.6 mm, 8 μm); MP: mixture of ACN (60%) and 0.02 M Na2HPO4 buffer at pH 9.4; FR: 1.5 mL/min | [105] |
| Neutral compounds of varied structure | Evaluate a RP column under isocratic and gradient elution conditions for estimating lipophilicity | RP-HPLC/ UV-Vis | Column: Supelcosil LC-ABZ (150 × 4.6 mm, 5 μm); MP: mixtures of MeOH and water; FR: 1.5 mL min | [106] |
| Herbicides including triazines and phenylurea derivatives | Determine lipophilicity by shake-flask, RP-HPLC, and in silico methods | RP-HPLC/DAD (218, 230, 245 or 270 nm) | Column: Spherisorb ODS 2 (125 × 4 mm, 5 μm); MP: mixtures of ACN (10–45%) and 1 mM ammonium acetate buffer at pH 6.7–7.3; FR: 0.8 mL/min | [107] |
| Allyl thiosemicarbazide, N1-thiocarbamylamidrazone derivatives | Determine lipophilicity by RP-TLC, RP-HPLC and in silico method | RP-HPLC/ UV-Vis (254 nm) | Column: RP-18 Waters Symmetry (150 × 4.6 mm, 5 μm); MP: mixtures of MeOH (50–80%) and water; FR: 1.0 mL/min | [108] |
| Amphoteric compounds | Determine lipophilicity RP-HPLC | RP-HPLC/ UV-Vis | Column: C18 Hypersil 5 ODS (250 × 4.6 mm, 5 μm); MP: mixture of MeOH (50%) and phosphate buffer at pH range of 3.0 to 8.0; FR: 1.0 mL/min | [109] |
| Succinimide derivatives | Determine lipophilicity by RP-TLC, RP-HPLC and in silico method | RP-HPLC/DAD | Column: XTerra MS C-18 (150 × 4.6 mm, 5 μm); MP: mixtures of MeOH (5–100%) and water; FR: 1.0 mL/min | [110] |
| 2-Substituted phenylnitronyl nitroxides | Determine lipophilicity RP-HPLC | RP-HPLC/ UV-Vis (275 nm) | Column: Rainbow C18 (150 × 4.6 mm); MP: mixture of MeOH (40%) and 0.001 M phosphate buffer; FR: 0.25 mL/min | [111] |
| Sorgoleone | Determine lipophilicity RP-HPLC | RP-HPLC/ UV-Vis (280 nm) | Column: C18 (250 × 4.6 mm); MP: mixture of ACN (70%) and water; FR: 1.8 mL/min | [112] |
| Solutes and drugs with well-defined solvatochromic parameters | Compare two Stationary Phases Based on Retention Mechanisms | RP-HPLC/ UV-Vis | Columns: Supelcosil Silica-based Discovery-RP-Amide-C16 (50 × 4.6 mm, 5 μm) and Asahipak Polymer-based ODP-50-4B (50 × 4.6 mm, 5 μm); MP: mixtures of MeOH (10–80%) and 0.02 M phosphate buffer at pH 3.0, 4.0, or 7.0 with octan-1-ol (0.25%); FR: 0.5 or 1 mL/min | [113] |
| [11C]Me-Halo-CGS 27023A analogues | Determine lipophilicity RP-HPLC | RP-HPLC/UV (240 nm) and γ-ray (NaI) | Column: Prodigy C18 (250 × 4.6 mm, 5 μm); MP: mixture of ACN, MeOH, and 20 mM KHPO4 buffer at pH 6.7; FR: 1.5 mL/min | [114] |
| Set of compounds covering a wide and regular range of structural parameters | Understand the structural properties governing retention mechanisms on RP-HPLC stationary phases | RP-HPLC/ UV-Vis and RI | Column: ODS Supelcosil LC-ABZ (150 × 4.6 mm, 5 μm) pre-treated with electrostatic coating; MP: mixtures of MeOH (10–50%) and 0.02 M MOPS buffer at pH 7.4; FR: 1.0 mL/min | [115] |
| Local anesthetics | Compare a fast and direct method of determining lipophilicity against an indirect HPLC determination | RP-HPLC/UV | Column: C18 LiChroCART Purospher (125 × 3.0 mm, 5 μm); MP: not specified; FR: 0.5 mL min | [54] |
| Large and diverse group of drugs | Determine lipophilicity and dissociation constant | RP HPLC–ESI-TOF–MS | Column: XBridge-C18 (50 × 3.0 mm, 2.5 μm); MP: series of pH and organic modifier gradients; FR: 0.5 mL/min | [116] |
| Estradiol derivatives | Evaluate the predictive power of the calculation procedure for molecular hydrophobicity | RP-HPLC/ UV-Vis (254 nm) | Columns: LiChrosorb® RP-18 (250 × 4.0 mm, 5 μm) and LiChrosphert RP-8 (150 × 4.6 mm, 5 μm); MP: mixtures of MeOH (60–95%) or ACN (70–90%) and water; FR: 1.0 mL/min | [117] |
| Natural compounds (mycotoxins and alkaloids, and amines) | Determine lipophilicity RP-HPLC | RP HPLC/DAD/MS | Columns: LiChroCART, Purospher RP-18e (125 × 3.0 mm; 5 μm) or LiChro-CART, LiChrospher RP-18e (250 × 4.0 mm; 5 μm) or Zorbax, Eclipse XDB-C8 (150 × 4.6 mm; 5 μm); MP: mixtures of MeOH or ACN and water at pH of 9.6 or 2.8; FR: 0.5 or 0.8 mL/min | [118] |
| Pyrrolyl-acetic acid derivatives | Determine lipophilicity RP-HPLC at low pH and at pH 7.4 | RP-HPLC/ UV-Vis (254 nm) | Column: Supelcosil ABZ+ (15 × 4.6 mm, 5 μm); MP: different mixtures of MeOH (10–70%) and MOPS buffer at pH 3.0 and 7.4 with octan-1-ol (0–0.25%); FR: not specified | [119] |
| Protonated basic compounds | Determine lipophilicity by RP-HPLC | RP-HPLC/ UV-Vis (254 nm) | Column: LiChrosorb RP-18 (250 × 4 mm, 5 μm); MP: mixtures of MeOH and 0.02 M MOPS buffer with N-decylamine (0.2%); FR: 1.5 mL/min | [120] |
| Drugs and flavonoids | Study the influence of 1-octanol in the determination of lipophilicity by RP-HPLC | RP-HPLC/ UV-Vis (254 nm) | Column: Supelcosil Discovery-RP-Amide-C16 (50 × 4.6 mm, 5 μm); MP: mixtures of MeOH (10–70%) an 0.02 M phosphate buffer at pH 3.0 with octan-1-ol (0–0.25%); FR: 1.0 mL/min | [121] |
| Parabens | Lipophilicity determination with mobile phases containing low and medium hydrophobic alcohols | RP-HPLC/MWD (254 nm) | Column: double end-capped Zorbax Eclipse XDB-C18 (150 × 4.6 mm, 5 μm); MP: mixtures of MeOH with 1% of different alcohols and water with 0.1% H3PO4; FR: 1.0 mL/min | [122] |
| DDT and related compounds | Determine lipophilicity by RP-HPLC | RP-HPLC/ UV-Vis | Column: Kromasil C18 (150 × 4.6 mm, 5 μm); MP: mixtures of MeOH and water; FR: 1.0 mL/min | [123] |
| 2-Substituted alkyl-6-(2,5-dioxopyrrolidin-1-yl)hexanoates | Investigate the activity as skin penetration enhancers | RP-HPLC/DAD (204 nm) | Column: Zorbax Eclipse XDB (150 × 4.6 mm, 5 μm); MP: mixture of MeOH and water; FR: 0.4 mL/min | [124] |
| Penicillins and cephalosporins | Determine lipophilicity by RP-HPLC | RP-HPLC/ UV-Vis (254 nm) | Column: porous silica gel bonded chemically with octadecyl chains (250 mm); MP: mixture of MeOH and 0.035 M ammonium chloride buffer at pH 7.4; FR: not specified | [125] |
| Set of diverse compounds | Determine lipophilicity by RP-HPLC | RP-HPLC/ UV-Vis (210, 230 and 254 nm) | Column: Interaction ACT-l (150 × 4.6 mm, 10 μm), Nucleosil Cs (150 × 4.6 mm, 5 μm), Hamilton divinylbenzene-styrene copolymer (PRP-1) (150 × 4.1 mm, 10 μm); MP: mixtures of ACN (60–70%) and 0.1 M ammonium acetate buffer at pH 4.6; FR: 0.75 or 2.0 mL/min | [126] |
| Acridinone derivatives | Determine the lipophilicity by RP-HPLC | RP-HPLC/DAD (254 nm) | Columns: Luna 5u C18(2) (150 × 4.6 mm, 5 μm), Candeza CD-C18 (150 × 4.6 mm, 3 μm), TSK-gel ODS-80TS (150 × 4.6 mm, 5 μm), Ascentis C18 (150 × 4.6 mm, 5 μm), Unison UK-C18 (150 × 4.6 mm, 3 μm), Zorbax SB-C8 column (75 × 4.6 mm, 3.5 μm); MP: mixtures of ACN or MeOH and water with 0.1% of formic acid; FR: 1.0 mL/min | [127] |
| Neutral drugs | Development of a method to determine the lipophilicity | RP-HPLC/DAD (235, 255, 265 and 275 nm) | Column: Supelcosil LC-ABZ (50 × 4.6 mm, 5 μm); MP: mixture of MeOH (15–70%) and 20 mM MOPS buffer at pH 7.4 with 0.25% of octanol; FR: 0.5, 1.0, or 2.0 mL/min | [28] |
| Structurally unrelated compounds | Determine the lipophilicity by RP-HPLC with a fast generic gradient | RP-HPLC | Column: ODS2-IK5 Inertsil (150 × 4.6 mm); MP: mixture of ACN (0–100%) and 50 mM ammonium acetate (pH ranging from 7.0 to 7.3); FR: 1.0 mL/min | [128] |
| Drugs with diverse chemical nature | Determine the lipophilicity by RP-HPLC | RP-HPLC/ UV-Vis | Column: XBridgeTM Shield RP18 (50 × 4.6 mm, 5 μm); MP: mixtures of MeOH (70 to 10%) and 0.02 M phosphate buffer at pH 7.0 with 1-octanol/buffer partitioning a 0.25%; FR: 1.0 or 0.5 mL/min | [129] |
| 1H-Pyrazolo[3,4-b]pyridine derivatives | Determine the lipophilicity by RP-TLC and RP-HPLC | RP-HPLC/ UV-Vis (254 nm) | Column: LC8 SUPELCO (250 × 4.6 mm × 5μm); MP: mixtures of ACN (40–65%) and 0.1 M phosphate buffer at pH 7.4; FR: 1.5 mL/min | [59] |
| Isochromanone derivatives | Determine the lipophilicity by RP-HPLC | RP-HPLC/DAD | Column: Hypersil 5 MOS (250 × 4.6 mm, 5 μm); MP: mixture of ACN (40%) and triethyl-ammonium phosphate buffer; FR: 1.0 mL/min | [130] |
| Amphoteric compounds, amino acids, and small peptides | Determine the lipophilicity by RP-HPLC in a broad pH range | RP-HPLC/ UV-Vis | Column: Kromasil C18 (150 × 4.6 mm, 5 μm); MP: mixtures of MeOH (10–90%) and ammonium acetate buffer at different pH; FR: 1.0 mL/min | [131] |
| α-(4-Phenylpiperazine) derivatives of N-benzylamides | Determine lipophilicity by RP-HPLC and in silico methods | RP-HPLC/ UV-Vis (215 nm) | Column: Lichrospher RP18, (125 × 4.0 mm, 5 μm); MP: mixtures of ACN (45–65%) and water with addition of 0.1%TFA; FR: 1.0 mL/min | [132] |
| N-Alkylbenzenes and some OECD reference substances | Correlate log P shake-flask values and capacity factors derived from RP-HPLC | RP-HPLC/ UV-Vis | Column: C18-SIL-X-5 (250 × 4.0 mm, 5 μm); MP: mixtures of MeOH (60–95%) and water; FR: 1.0 mL/min | [133] |
| Natural secondary plant metabolites | Determine lipophilicity by RP-HPLC and in silico methods | RP-HPLC/DAD | Column: GraceSmart RP18 (150 × 4.6 mm, 5 μm); MP: mixtures of MeOH (90–30%) and 0.01 M phosphate buffer at pH 2.0; FR: 1.0 mL/min | [134] |
| Derivatives of N-substituted amides of 3-(3-ethylthio-1,2,4-triazol-5-yl)propenoic acid) | Determine the lipophilicity by RP-HPLC | RP-HPLC/ UV-Vis (254 nm) | Column: RP-18 Waters Symmetry (150 × 4.6 mm 5 μm); MP: mixtures of MeOH (50–75%) and water with or without ammonium buffer; FR: 1.0 mL/min | [135] |
| Cucurbitacins | Correlate the hydrophobicity index and the basal cytotoxicity on HepG2 cells | RP-HPLC/DAD | Column: Alltima C18 (250 × 4.6 mm, 5 μm) or Econosil C18 (150 × 4.6 mm, 5 μm); MP: mixtures of ACN and ammonium acetate buffer; FR: 1.0 mL/min | [136] |
| Carotenoids | Determine the lipophilicity by RP-HPLC | RP-HPLC/DAD | Column: Zorbax SB-C18 (250 × 3.0 mm, 5μm); MP: mixture of acetone (0–75%) and water (gradient mode); FR: 0.5 mL/min | [137] |
| Highly lipophilic benzoxazole-2ylphosphonates | Determine the lipophilicity by RP-HPLC | RP-HPLC/DAD (210 nm) | Column: Symmetry C18 (250 × 4.6 mm, 5 μm); MP: mixture of ethanol (90%) and water; FR: 1.0 mL/min | [138] |
| Bispyridinium derivatives | Determine lipophilicity by RP-HPLC | RP-HPLC/DAD (210 nm) | Column: Symmetry C18 (250 × 4.6 mm, 5μm); MP: mixture of MeOH (15%) and water; FR: 0.9 mL/min | [139] |
| 1-(1-Arylimidazolidyn-2-ylidyn)-3-arylalkyl urea derivatives | Compare the conventional RP-HPLC and RP-HPLC enriched with room temperature imidazolium-based ionic liquids | RP-HPLC/DAD | Column: Zorbax Extend-C18 (150 × 4.6 mm, 5 μm); MP: mixtures of MeOH or ACN and water with the addition of butyl-methyl imidazolium-based ionic liquids; FR: 1.0 mL/min | [140] |
| Statin drugs | Determine lipophilicity by RP-HPLC | RP-HPLC/DAD | Columns: Zorbax SB-C18 (250 × 4.6 mm, 5 μm), LiChrospher RP-C8 (250 × 4.0 mm, 5 μm); MP: mixtures of ACN (20–80%) and water; FR: 1.0 mL/min | [141] |
| Basic and acidic analytes | Determine simultaneously pKa and lipophilicity by RP-HPLC | RP-HPLC/DAD | Column: XTerra MS C-18 (150 × 4.6 mm, 5 μm); MP: mixtures of MeOH with buffers at different pH (gradient mode); FR: 1.0 mL/min | [142] |
| Di-substituted 2-hydroxyacetamides | Correlate different lipophilicity parameters and antimycobacterial activities | RP-HPLC/ UV-Vis (205 nm) | Column: RP-C18 (250 × 4.0 mm, 5 μm); MP: mixtures of MeOH (10–50%) and water; FR: 1.0 mL/min | [143] |
| Fused 1,2,4-triazinones derivatives | Determine lipophilicity by RP-HPLC and in silico methods | RP-HPLC/ UV-Vis (245 and 366 nm) | Column: Supelcosil LC-18 (150 × 4.6 mm, 5 μm); MP: mixtures of ACN (10–70%) or dioxane (5–80%) or MeOH (10–80%) and water; FR: 1.0 mL/min | [144] |
| Polyprotic analytes | Determine pKa and lipophilicity by RP-HPLC | RP-HPLC/DAD | Column: XTerra MSC-18 (150 × 4.6 mm, 5 μm); MP: mixtures of MeOH and different buffers; FR: 1.0 mL/min | [145] |
| 4,5-Dihydro-1H-1,2,4-triazol-5-one derivatives | Determine lipophilicity by RP-HPLC | RP-HPLC/ UV-Vis (254 nm) | Column: Luna C18 (250 × 4.6 mm, 5 μm); MP: mixtures of MeOH (25–100%) and water; FR: 1.0 mL/min | [146] |
| 2-(4-Substituted phenyl)-3(2H)-isothiazolones derivatives | Determine lipophilicity by RP-HPLC and correlate it with biological activity | RP-HPLC/ UV-Vis (260 and 285 nm) | Column: octadecyl–poly(vinyl alcohol) (20 × 4.0 mm, 5 μm); MP: mixtures of MeOH and buffers at pH 2.0, 7.0 and 10.0 (gradient elution); FR: 1.5 mL/min | [147] |
| Nitrazepam in bile acid micelles | Evaluate the importance of temperature dependence of retention coefficient in QSAR model | RP-HPLC/DAD (210 nm) | Column: Eclipse Plus C18 (250 × 3.0 mm, 5 μm); MP: mixture of MeOH and 0.01 M phosphate buffer at pH 7.0; FR: 1.0 mL/min | [148] |
| Diazido Platinum(IV) complexes | Study the cellular accumulation, lipophilicity, and photocytotoxicity | RP-HPLC/UV | Column: Agilent Zorbax eclipse plus C18 (250 × 4.6 mm, 5 μm); MP: mixtures of MeOH and water with 0.1% TFA; FR: 1.0 mL/min | [149] |
| Organic compounds | Determine lipophilicity by RP-HPLC | RP-HPLC/DAD | Columns: Luna C18 (150 × 4.6 mm, 5 μm), Chromolith Performance RP-18e monolithic (100 × 4.6 mm); MP: mixtures of ACN or MeOH and buffers at different pH; FR: 1.0 or 2.0 mL/min | [150] |
| β-Blockers | Apply perfluorinated acids as ion-pairing reagents for RP chromatography and retention-hydrophobicity relationships | RP-HPLC/DAD (220 nm) | Column: Zorbax Extend-C18 (150 × 4.6 mm, 5 μm); MP: mixtures of MeOH and water with perfluorinated acids; FR: 1.0 mL/min | [151] |
| Cyclen derivatives | Determine lipophilicity by RP-HPLC | RP-HPLC/DAD (250 and 280 nm) | Column: Waters X-Bridge C-18 (150 × 4.6 mm, 5 μm); MP: mixture of ACN, MeOH and water (gradient mode); FR: 1.0 mL/min | [152] |
| Naturally occurring bile acids | Evaluate the bile acid lipophilicity | RP-HPLC/ UV-Vis (200 nm) | Column: C-18 (100 mm, 5 μm); MP: mixture of MeOH (35–65%) and 0.01 M phosphate buffer at pH 7.0; FR: 0.2 mL/min | [153] |
| Thiosemicarbazides and 1,2,4-triazole derivatives | Determine lipophilicity by RP-HPLC | RP-HPLC/DAD | Column: Zorbax 100 Extend-C18 (150 × 4.6 mm, 5 μm); MP: mixtures of can or MeOH and water; FR: 1.0 mL/min | [154] |
| Simple model compounds | Assess the lipophilicity by different techniques | RP-HPLC/DAD | Columns: C18 (125 × 4.0 mm); CN (125 × 4.0 mm); C18e (125 × 4.0 mm); DIOL (125 × 4.0 mm); MP: mixtures of ACN or MeOH and water (gradient mode); FR: 1.0 mL/min | [155] |
| Benzodiazepine-receptor ligands | Measure the lipophilicity by RP-HPLC and RP-TLC | RP-HPLC/ UV-Vis (254 nm) | Column: μBondapak Cl8 (30 × 3.9 mm); MP: mixtures of ACN (30–70%) and phosphate buffer at pH 7.0; FR: not specified | [156] |
| Halogenated organic pollutants | Determine lipophilicity by RP-HPLC | RP-HPLC/DAD | Columns: Venusil XBP C8 (150 × 2.1 mm, 5 μm); Venusil XBP C18 (150 × 2.1 mm, 5 μm); MP: mixtures of MeOH and water; FR: not specified | [157] |
| Thiosemicarbazides and analogues | Determine lipophilicity by chromatographic and in silico methods | RP-HPLC/ UV-Vis (254 nm) | Column: RP-18 Waters Symmetry (150 × 4.6 mm, 5 μm); MP: mixtures of MeOH (40–65%) and water; FR: 1.0 mL/min | [158] |
| Nicotine, cytisine and lobeline | Correlate the activity of cytisine and lobeline in ligand-binding and behavioural experiments and determine their lipophilicity | RP-HPLC/ UV-Vis (254 nm) | Column: Lichrosorb RP-18 (250 × 4.0 mm, 10 μm); MP: mixtures of MeOH and 0.02 M MOPS buffer at pH 7.4 with N-decylamine (0.2%); FR: 1.5 mL/min | [159] |
| Preservatives | Determine lipophilicity by chromatographic and in silico methods | RP-HPLC/DAD/MS (230, 254 and 366 nm) | Columns: endcapped C18 LiChroCART, Purosphere RP-18e (125 × 3.0 mm, 5 μm), double endcapped C8 Zorbax, Eclipse XDBC8, (150 × 4.6 mm, 5 μm), CN100 Lichrosphere, (250 × 4.0 mm, 5 μm), endcapped Supelcosil LC-NH2 (150 × 3.0 mm, 3 μm); MP: mixture of MeOH and water with 0.1% formic acid; FR: 1.0 and 0.6 mL/min | [160] |
| 8-Aryl-2,6,7,8-tetrahydroimidazo[2,1-c][1,2,4]triazine-3,4-dione derivatives | Determine lipophilicity by chromatographic and in silico methods | RP-HPLC/ UV-Vis (254 nm) | Column: Zorbax Eclipse XDB C-18 (150 × 4.6 mm, 7 μm); MP: mixtures of MeOH and water; FR: 1.0 mL/min | [161] |
| 2-Methyl-4-oxo-3H-quinazoline-3-alkyl-carboxylic acid derivatives | Determine the lipophilicity | RP-HPLC/ UV-Vis (270 nm) | Column: Ultrasphere C8 (250 × 4.0 mm, 5 μm); MP: mixtures of MeOH and water; FR: 1.0 mL/min | [162] |
| Neutral and basic drugs | Determine the lipophilicity | RP-HPLC/ UV-Vis | Column: Hypersil BDS C-18 (250 × 4.6 mm, 5 μm); MP: mixtures of MeOH (85%) and 20 mM MOPS at pH 7.4 with N-decylamine (0.15–20%) or octan-1-ol (0.25%); FR: not specified | [163] |
| Acidic and basic drugs | Evaluate the effect of octan-1-ol in the mobile phase | RP-HPLC/DAD | Column: Gemini C18 (150 × 4.6 mm, 5 μm), Gemini C18 (50 × 4.6 mm, 5 μm); MP: mixtures of MeOH with 0.25% of octan-1-ol (40–55%) and buffer saturated with octan-1-ol; FR: 1.0 mL/min | [164] |
| p-Toluenesulfonyl-hydrazinothiazole and hydrazine-bis-thiazole derivatives | Compare between RP-HPLC and RP-HPTLC | RP-HPLC/ UV-Vis (210 nm) | Column: Zorbax SB-C18 (100 × 3.0 mm, 3.5 µm); MP: mixtures of MeOH (45–90%) and water; FR: not specified | [60] |
| 1-Aryl-3-ethyl-3-methylsuccinimides | Compare between RP-HPLC and RP-HPTLC | RP-HPLC (254 nm) | Column: Luna C18 (250 × 4.6 mm, 5 µm); MP: mixtures of MeOH (55–55%) and water; FR: 1.0 mL/min | [165] |
| Basic compounds such as β-blockers, local anesthetics, and piperazines | Determine lipophilicity | RP-UPLC/ UV-Vis or RP-UPLC/MS | Columns: Acquity BEH Shield RP18, Acquity BEH C18, Acquity BEH C8, Acquity BEH Phenyl (30 mm × 2.1 mm, 1.7 μm); MP: mixtures of MeOH or ACN and aqueous buffer saturated in 1-octanol; FR: 0.5 mL/min | [166] |
| Neutral, acidic, basic, and amphoteric drugs | Determine lipophilicity | RP-UPLC/DAD and RP-HPLC/UV-Vis | Columns: Gemini NX, (150 × 4.6 mm, 5 μm), Kinetex C18 (100 × 4.6 mm, 2.6 μm), Waters Acquity BEH C18 (50 × 2.1 mm, 1.7 μm); MP: mixtures of ACN (50%) and buffers at different pH values; FR: 0.5 and 1.0 mL/min | [167] |
| Diverse set of compounds | Determine the lipophilicity | RP-UPLC/ UV-Vis (215 or 220 nm) | Columns: Acquity BEH Shield RP18, Acquity BEH C18, Acquity BEH C8, AcquityBEH phenyl (30 × 2.1 mm, 1.7 μm); MP: mixtures of MeOH or ACN and aqueous buffer; FR: 0.5 mL/min | [168] |
| Androstane derivatives | Determine the lipophilicity | RP-UPLC/ UV-Vis (210 and 230 nm) | Column: ZORBAX Eclipse C18 (2.1 × 50 mm, 1.8 µm); MP: mixtures of MeOH (30–60%) or ACN (30–60%) and water); FR: 0.3 mL/min | [169] |
| N-[(4-Arylpiperazin-1-yl)alkyl]pyrrolidine-2,5-dione derivatives | Determine the lipophilicity | RP-UPLC–MS | Column: Acquity UPLC BEH C18 (2.1 × 100 mm, 1.7 µm); MP: mixtures of methanol (30–90%) and 0.01 ammonium acetate at pH 7.4); FR: 0.3 mL/min | [170] |
| Highly lipophilic compounds | Determine lipophilicity | RP-HPLC/ UV-Vis and UHPLC/UV-Vis | Columns: Discovery RPAmide C16 (20 × 4.0 mm, 5 μm) or Hypersil GOLD Javelin HTS (10 × 2.1 mm, 1.9 μm) or Acquity BEH Shield RP18 (30 × 2.1 mm, 1.7 μm); MP: mixtures of MeOH or ACN and water; FR: 0.5 or 1.0 mL/min | [171] |
| Huperzine A derivatives | Determine the lipophilicity | RP-HPLC/DAD and IAM-HPLC/DAD | Columns: ODS μBondapak C18 (300 × 3.9 mm) and IAM.PC.MG (150 × 4.6 mm); MP: mixtures of water and ACN; FR: 1.0 mL/min | [172] |
| Thioquinoline derivatives | Determine lipophilicity by RP-HPLC and IAM | RP-HPLC/UV-Vis and IAM-HPLC/UV-Vis (238, 215, 230, and 234 nm) | Column: LiChrospher RP18 (125 × 4.0 mm, 5 μm), IAM. PC DD2 Regis (100 × 4.6 mm, 12 μm); MP: mixtures of ACN (55–90%) and water or mixtures of ACN (35–60%) and phosphate buffer at pH 5.4; FR: 1.0 mL/min | [173] |
| Thiazolidinediones | Study the lipophilic behaviour and the relationships with PPAR-γ activity | RP-HPLC/UV-Vis and IAM-HPLC/UV-Vis (228, 267, or 254 nm) | Columns: Hypersil BDS C-18 (250 × 4.6 mm, 5 μm), Supelcosil ABZ+ Plus (150 × 4.6 mm, 5 μm), Supelcosil Aquasil (150 × 4.6 mm, 5 μm), IAM.PC.DD2; MP: mixtures of MeOH (35–80%) and 20 mM MOPS buffer at 7.4 or mixtures of ACN (10 to 35%) and 0.01 M PBS buffer in the pH range 2.5–7.4; FR: 1.0, 2.0, or 3.0 mL/min | [174] |
| 2-(2,4-Dihydroxyphenyl)thieno-1,3-thiazin-4-one derivatives | Determine lipophilicity by RP-HPLC and IAM | IAM-HPLC/ UV-Vis (320 nm) and RP-HPLC/UV-Vis (380 nm) | Column: HypersilGold C18 (100 × 3 mm, 3 μm) and IAM.PC.DD2 (100 × 4.6 mm, 12 μm); MP: mixtures of MeOH (30–95%) and 20 mM phosphate buffer at pH 4.0 or mixtures of ACN (10–50%) and 20 mM phosphate buffer at pH 7.4; FR: 0.5 or 1.0 mL/min | [175] |
| Sα-(4-Phenylpiperazin-1-yl)-γ-phthalimido-butyramides derivatives | Determine lipophilicity by chromatographic and in silico methods | IAM-HPLC/UV-Vis and RP-HPLC/UV-Vis (210 nm) | Column: LiChirospher 100 RP18 (100 × 4.6 mm, 5 μm) and IAM.PC.DD2 (100 × 4.6 mm); MP: mixtures of ACN (35–55%), water with 0.01% TFA; FR: 1.0 mL/min | [176] |
| Solutes with sufficiently varied properties | Characterize an IAM column and study its similarity with common C18 chromatographic and biological systems | IAM-HPLC and RP-HPLC/ UV-Vis (254 or 214 nm) | Columns: IAM.PC.DD2 (100 × 4.6 mm; 12 μm), XTerra MS C18 (150 × 4.6 mm, 5 μm), XTerra RP18 (150 × 4.6 mm, 5 μm); MP: mixtures of ACN and 0.01 M phosphate aqueous buffer adjusted to pH 7.0; FR: 1.0 mL/min | [177] |
| Porphyrins | Study the lipophilicity of and retention in IAM, C8-C18, and HILIC chromatographic systems | IAM-HPLC and RP-HPLC/DAD (254 and 417 nm) | Columns: Gravity C18 (125 × 4.0 mm, 5 μm); Gravity C8 (125 × 4.0 mm, 5 μm); PolarTec (125 × 4.0 mm, 5 μm); HILIC (125 × 4.0 mm, 5 μm); IAM.PC.DD2 (30 × 4.6 mm, 10 μm); MP: mixtures of ACN (5–100%) and aqueous 20 mM ammonium acetate at pH 7.0 (gradient mode); FR: 1.0 mL/min | [178] |
| 4,5-Disubstituted-2,4-dihydro-3H- 1,2,4-triazole-3-thiones derivatives | Study the chromatographic behaviour on different reversed-phase materials | IAM-HPLC and RP-HPLC/DAD | Columns: Zorbax Extend-C18 (150 × 4.6 mm, 5 μm); Cogent UDC Cholesterol (150 mm × 4.6 mm, 4 μm); Regis IAM.PC. DD2 (150 × 4.6 mm, 10 μm); MP: mixtures of MeOH (75–90%) or ACN (55–70%) and water; FR: 1.0 mL/min | [179] |
| Alkyl benzene derivatives and PAHs, flavonoids, nucleosides, and nucleic bases | Compare retention properties of stationary phases imitated cell membrane in RP HPLC | IAM-HPLC and RP-HPLC/DAD | Column: IAM.PC.DD2 (150 × 4.6 mm, 12 μm); Amino-P-C18 (125 × 4.6 mm, 5 μm); MP: mixtures of MeOH or ACN and water; FR: 1.0 mL/min | [180] |
| Macrolides | Investigate the relationships between retention factors and cellular permeation | RP-HPLC/MS IAM-HPLC/MS | Columns: Luna C18 (50 × 3 mm), IAM PC DD; MP: mixture of ACN (0–100%) and 0.05 M ammonium acetate at pH 7.4; FR: 1.0 mL/min | |
| Cephalosporins | Estimate lipophilicity | IAM-HPLC | Columns: IAM.PC.DD 2 (100 × 4.6 mm, 12 µm); MP: phosphate buffer at pH 6.9; FR: 1.0 mL/min | [181] |
| Structurally diverse basic and neutral drugs | Compare different IAM and reversed-phase retention factors and their relationships with lipophilicity data | IAM-HPLC | Column: IAM.PC.DD2, BDS C-18 (250 × 4.6 mm, 5 μm); MP: mixtures of ACN and 0.01 M PBS buffer or 0.02 M MOPS buffer at pH 7; FR: 1.0, 2.0, or 3.0 mL/min | [182] |
| Structurally non-related orally administered drugs | Investigate possible relationships between pharmacokinetic parameters and intestinal permeation data. | IAM-HPLC | Columns: IAM.PC.MG (150 × 4.6 mm; 12 μm), IAM.PC.DD2 (100 × 4.6 mm; 10 μm); MP: mixtures of ACN and 0.1 M phosphate buffer at pH 7.0; FR: not specified | [183] |
| Structurally diverse drugs | Investigate the relationships, retention factors, and volume of distribution | IAM-HPLC | Column: IAM PC.DD2 (150 × 3.0 mm); MP: Mobile phase A: mixtures of ACN (0–70%) and 0.05 M ammonium acetate at pH 7.4; FR: 1.0 mL/min | [184] |
| Quinolone drugs | Investigate possible relationships between IAM retention data and various lipophilicity scales | IAM-HPLC | Column: IAM.PC.MG (150 × 4.6 mm) and IAM.PC.DD2 (100 × 4.6 mm); MP: mixtures of ACN and 0.1 M phosphate buffer at pH 7.0; FR: 0.9 or 1.0 mL/min | [185] |
| Structurally diverse drugs | Characterize column and study the relationships between IAM retention data and DIPL | IAM-nano-HPLC | Monolith column prepared via copolymerization of MDPC and MDPA; MP: 0.05 M ammonium acetate at pH 7.4 or 4.5; FR: 0.6 µL/min | [186] |
| BBB permeant drugs | Investigate the relationships between BBB permeation data and lipophilicity | IAM-HPLC | Columns: IAM.PC.MG (150 × 4.6 mm; 12 μm); IAM.PC.DD2 (100 × 4.6 mm; 10 μm); MP: mixtures of ACN and 0.1 M phosphate buffer at pH 7.0; FR: 1.0, 2.0 or 3.0 mL/min | [187] |
| Kv11.1 inhibitors | Determine the lipophilicity by RP-HPLC and IAM | IAM-HPLC | Column: Supelcosil LC-ABZ (50 × 4.6 mm, 5 μm), IAM.PC-DD2 (100 × 4.6 mm, 10 μm); MP: mixtures of water and MeOH or ACN; FR: not specified | [188] |
| Set of basic, neutral, acidic and ampholytic drugs | Explore the effect of electrostatic interactions of anionic species | IAM-HPLC | Column: IAM.PC.DD2; MP: mixture of ACN (0–35%) and MOPS buffer pH: 7.4, 0.01 M PBS buffer at pH: 7.4, 0.01 M PBS buffer at pH: 5.0; FR: 1.2 or 3.0 mL/min | [189] |
| Structurally diverse drugs | Compare between IAM and equilibrium dialysis | IAM-HPLC (220 or 254 nm) | Column: IAM.PC.DD2 (100 × 4.6 m, 12 µm); MP: mixtures of methanol and 0.02 M phosphate buffer at pH 7.0; FR: 1.5 or 2.0 mL/min | [190] |
| Neutral, acidic, and basic drugs, including steroid hormones, local anesthetics, β-blockers, and NSAIDs | Characterize a reversed-phase amide column coated with phosphatidylcholine-based liposomes | ILC/DAD (230 or 254 nm) | Column: Ascentis® RP-amide HPLC column (50 × 4.6 mm, 5.0 μm) previously coated by immobilizing the PC liposomes on the column; MP: mixture of MeOH (20–80%) and phosphate buffer; FR: 1.0 and 2.0 mL/min | [34] |
| β-blockers, imidazoline derivatives, and p-alkylphenols | Characterize column coated with phosphatidylethanolamine-based liposomes | ILC/UV-Vis (220 nm) | Column: 5 mm glass column containing PE liposomes bounded to Sephacryl S-1000 gel by avidin–biotin binding; MP: 0.01 M HEPES with NaCl (0.15 M,) at pH 7.4; FR: 0.3 mL/min | [191] |
| Structurally diverse drugs | Estimate lipophilicity and correlate it with absorption | ILC/UV-Vis (220 nm) | Column: 5–5.5 cm×5 mm glass column containing EPC, PS–chol, and EPC–PS–PE liposomes CNBr-Sepharose gel by avidin–biotin binding; MP: 0.01 M HEPES with NaCl (0.15 M,) at pH 7.4; FR: 0.3 mL/min | [192] |
| Fused azaisocytosine analogues | Compare between RP-HPLC, IAM, and MLC | IAM-HPLC MLC/UV-Vis | Columns: Spherisorb ODS-2 (125 × 4 mm, 5 μm), IAM.PC.DD2 (100 × 4.6 mm, 10 μm), Purosphere RP-18e column (125 × 4 mm, 5 μm); MP: 0.01 M phosphate citrate buffer at pH 7.4 with Brij 35 (0.15–0.075 M) and with isopropanol (7%); mixtures of ACN (20–50%) and 0.01 M phosphate citrate buffer at pH 7.4; FR: 0.1, 1 and 1.3 mL/min | [193] |
| Series of pharmaceutically related compounds | Study the effects of the variations in pH in MLC determination | MLC/UV-Vis (221 or 285 nm) | Column: RP cyanopropyl Spherisorb (150 × 4.6 mm, 5μm); MP: 0.1 M phosphate buffer with SDS at pH 3.0, 4.0, 5.0, 5.8, 6.4 and 7.0; FR: 1.35 mL/min | [194] |
| Benzene derivatives | Study the influence of mixtures of Triton X-100 and Brij 35 | MLC/DAD | Column: Nucleodur C18 Gravity (2 × 125 mm, 5 μm); MP: HCl solution at pH 2 with different Brij 35/Triton X-100–ratios (0.05–0.30%); FR: 0.3 mL/min | [195] |
| Structurally diverse drugs | Estimate lipophilicity and correlate it with intestinal absorption and BBB | MLC/UV-Vis (210 or 300 nm) | Column: Grace GraceSmart C18 (150 × 2.1 mm, 3 μm); MP: 0.05 M phosphate buffer at pH 7.4 with 0.01 M miltefosine; FR: 0.2 mL/min | [196] |
| Structurally diverse drugs | Estimate lipophilicity and correlate it with absorption | MLC/UV-Vis | Column: Kromasil octadecyl-silane C18 (150 × 4.6 mm, 5 μm); MP: 0.05 M phosphate buffer at pH 6.5 and 7.4 with 0.04 M Brij35; FR: 0.5 mL/min | [197] |
| Structurally diverse drugs | Estimate lipophilicity and correlate it with absorption | MLC/UV-Vis (221 or 360 nm) | Column: Spherisorb (15 cm × 4.6 mm, 5 μm); MP: water with sodium deoxycholate (5–20 mM); FR: 1.34 mL/min | [198] |
| Structurally diverse drugs | Estimate lipophilicity and correlate it with absorption | MLC/UV-Vis (240 or 254 nm) | Columns: Spherisorb octadecyl-silane ODS-2 C18 (120 × 4 mm, 5 μm), Kromasil octadecyl-silane ODS-2 C18 (50×4.6 mm, 5 μm), Kromasil octadecyl-silane C18 (150 × 4.6 mm, 5 μm); MP: 0.05 M phosphate buffer at pH 7.4 with 0.02 M Brij35; FR: 1 and 1.5 mL/min | [199] |
| Anticonvulsant drugs | Estimate lipophilicity and correlate it with pharmacokinetic parameters | MLC/UV-Vis (220 or 240 nm) | Column: Kromasil C18 column (150 × 4.6 mm, 5 μm); MP: 0.05 M phosphate buffer at pH 7.4 with 0.04 M Brij35; FR: 1 mL/min | [200] |
| Quinolone drugs | Estimate lipophilicity and correlate it with pharmacokinetic parameters | MLC/UV-Vis (270 nm) | Column: Kromasil C18 column (150 × 4.6 mm, 5 μm); MP: 0.05 M phosphate buffer at pH 7.4 with Brij35 and with NaCl (9.20 g/L); FR: 1 mL/min | [201] |
| β-Hydroxy-β-arylalkanoic acids analogues | Estimate lipophilicity and correlate it with absorption | MLC/DAD | Column: Zorbax Extend-C18 (150 mm × 4.6 mm, 5 μm); MP: 40 mM solution of Brij35 in a mixture of ACN (20%) and 0.07 M phosphate buffer at pH 5.50 with0.04 M Brij35; FR: 1 mL/min | [202] |
| Structurally diverse drugs | Study the influence of pH, temperature, and ionic strength in MLC and compare it with IAM | MLC/UV-Vis (220 nm) | Column: High Stability C18 Supelco (150 × 4.6 mm, 5 μm); MP: 0.05 M phosphate buffer at pH 5.5 and 7.4 with NaCl (0–9.2 g/L) with 0.04 M Brij35; FR: 1 mL/min | [203] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, J.X.; Santos, Á.; Fernandes, C.; Pinto, M.M.M. Liquid Chromatography on the Different Methods for the Determination of Lipophilicity: An Essential Analytical Tool in Medicinal Chemistry. Chemosensors 2022, 10, 340. https://doi.org/10.3390/chemosensors10080340
Soares JX, Santos Á, Fernandes C, Pinto MMM. Liquid Chromatography on the Different Methods for the Determination of Lipophilicity: An Essential Analytical Tool in Medicinal Chemistry. Chemosensors. 2022; 10(8):340. https://doi.org/10.3390/chemosensors10080340
Chicago/Turabian StyleSoares, José X., Álvaro Santos, Carla Fernandes, and Madalena M. M. Pinto. 2022. "Liquid Chromatography on the Different Methods for the Determination of Lipophilicity: An Essential Analytical Tool in Medicinal Chemistry" Chemosensors 10, no. 8: 340. https://doi.org/10.3390/chemosensors10080340
APA StyleSoares, J. X., Santos, Á., Fernandes, C., & Pinto, M. M. M. (2022). Liquid Chromatography on the Different Methods for the Determination of Lipophilicity: An Essential Analytical Tool in Medicinal Chemistry. Chemosensors, 10(8), 340. https://doi.org/10.3390/chemosensors10080340