Synthesis of Graphene-Oxide-Decorated Porous ZnO Nanosheet Composites and Their Gas Sensing Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of GO and Porous Single-Crystal ZnO Nanosheet Composites
2.3. Characterization
2.4. Fabrication of the Gas Sensor and the Gas-Sensing Measurement System
3. Results
3.1. Characterization
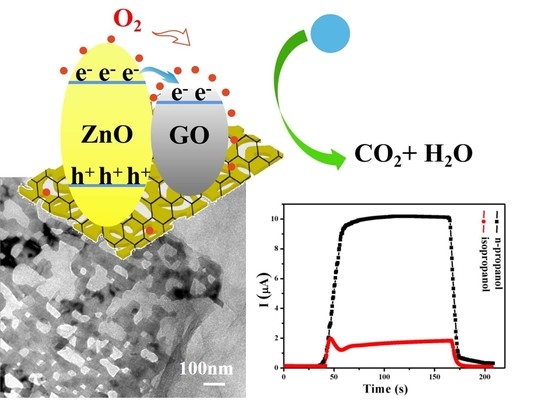
3.2. Sensing Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, B.; Liu, X.; Xing, X.; Chen, N.; Xiao, X.; Liu, S.; Wang, Y. A high response butanol gas sensor based on ZnO hollow spheres. Sensor Actuators B Chem. 2016, 237, 423–430. [Google Scholar] [CrossRef]
- Meng, F.L.; Zheng, H.X.; Chang, Y.L.; Zhao, Y.; Li, M.Q.; Wang, C.; Sun, Y.F.; Liu. J., H. One-step synthesis of Au/SnO2/RGO nanocomposites and their VOC sensing properties. IEEE Trans. Nanotechnol. 2018, 172, 212–219. [Google Scholar] [CrossRef]
- Ma, A.; Park, H.J.; Seo, J.H.; Jang, K.Y.; Lee, H.K.; Kim, D.Y.; Lee, J.E.; Nam, K.M.; Lee, D.S. Phase transition of non-equilibrium wurtzite CoO: Spontaneous deposition of sensing material for ultrasensitive detection of acetone. Sensor Actuators B Chem. 2020, 308, 127698. [Google Scholar] [CrossRef]
- Qin, W.B.; Yuan, Z.Y.; Gao, H.L.; Zhang, R.; Meng, F.L. Perovskite-structured LaCoO3 modified ZnO gas sensor and investigation on its gas sensing mechanism by first principle. Sens. Actuators B Chem. 2021, 341, 130015. [Google Scholar] [CrossRef]
- Ji, H.Y.; Qin, W.B.; Yuan, Z.Y.; Meng, F.L. Qualitative and quantitative recognition method of drug-producing chemicals based on SnO2 gas Sensor with dynamic measurement and PCA weak separation. Sens. Actuators B Chem. 2021, 348, 130698. [Google Scholar] [CrossRef]
- Caicedo, N.; Leturcq, R.; Raskin, J.-P.; Flandre, D.; Lenoble, D. Detection mechanism in highly sensitive ZnO nanowires network gas sensors. Sensor Actuators B Chem. 2019, 297, 126602. [Google Scholar] [CrossRef]
- Bhati, V.S.; Hojamberdiev, M.; Kumar, M. Enhanced sensing performance of ZnO nanostructures-based gas sensors: A review. Energy Rep. 2020, 6, 46–62. [Google Scholar] [CrossRef]
- Wang, C.N.; Li, Y.L.; Gong, F.L.; Zhang, Y.H.; Fang, S.M.; Zhang, H.L. Advances in doped ZnO nanostructures for gas sensor. Chem. Rec. 2020, 20, 1553–1567. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.L.; Shi, X.; Yuan, Z.Y.; Ji, H.Y.; Qin, W.B.; Shen, Y.B.; Xing, C.Y. Detection of Four Alcohol Homologue Gases by ZnO Gas Sensor in Dynamic Interval Temperature Modulation Mode. Sens. Actuators B Chem. 2022, 350, 130867. [Google Scholar] [CrossRef]
- Nundy, S.; Eom, T.Y.; Kang, J.G.; Suh, J.; Cho, M.; Park, J.S.; Lee, H.J. Flower-shaped ZnO nanomaterials for low-temperature operations in NOx gas sensors. Ceram. Int. 2020, 46, 5706–5714. [Google Scholar] [CrossRef]
- Alev, O.; Sarıca, N.; Özdemir, O.; Arslan, L.C.; Büyükköse, S.; Öztürk, Z.Z. Cu-doped ZnO nanorods based QCM sensor for hazardous gases. J. Alloy. Compd. 2020, 826, 154177. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, M.; Qiu, J.; Qiu, T.; Chen, Y.; Qi, S.; Wei, X.; Tian, X.; Xu, D. Mesoporous ZnO nanosheet as gas sensor for sensitive triethylamine detection. Anal. Bioanal. Chem. 2022, 414, 2181–2188. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.C.; Paes, S.C.; Correa, B.S.; Cabrera-Pasca, G.A.; Costa, M.S.; Costa, C.S.; Otubo, L.; Carbonari, A.W. Growth of long ZnO nanowires with high density on the ZnO surface for gas sensors. ACS Appl. Nano Mater. 2019, 3, 175–185. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.W.; Porte, Y.; Ko, K.Y.; Kim, H.; Myoung, J.M. Micropatternable double-faced ZnO nanoflowers for flexible gas sensor. ACS Appl. Mater. Inter. 2017, 9, 32876–32886. [Google Scholar] [CrossRef]
- Choi, S.M.; Kim, Y.M.; Mirzaei, A.; Kim, H.S.; Kim, S.I.; Baek, S.H.; Chun, D.W.; Jin, C.; Lee, H.K. Selective, sensitive, and stable NO2 gas sensor based on porous ZnO nanosheets. Appl. Surf. Sci. 2021, 568, 150910. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Fan, J.; Zhu, B.; Yu, J. Triethylamine gas sensor based on Pt-functionalized hierarchical ZnO microspheres. Sensor Actuators B Chem. 2021, 331, 129425. [Google Scholar] [CrossRef]
- Young, S.J.; Chu, Y.L. Platinum nanoparticle-decorated ZnO nanorods improved the performance of methanol gas sensor. J. Electrochem. Soc. 2020, 167, 147508. [Google Scholar] [CrossRef]
- Aubekerov, K.K.; Punegova, N.; Sergeenko, R.; Kuznetsov, A.; Kondratev, V.M.; Kadinskaya, S.A.; Nalimova, S.S.; Moshnikov, V.A. Synthesis and study of gas sensitive ZnFe2O4-modified ZnO nanowires. J. Phys. 2022, 2227, 012014. [Google Scholar] [CrossRef]
- Xu, X.-Y.; Yan, B. Eu(iii)-functionalized ZnO@MOF heterostructures: Integration of pre-concentration and efficient charge transfer for the fabrication of a ppb-level sensing platform for volatile aldehyde gases in vehicles. J. Mater. Chem. A 2017, 5, 2215–2223. [Google Scholar] [CrossRef]
- Ugale, A.D.; Umarji, G.G.; Jung, S.H.; Deshpande, N.G.; Lee, W.; Cho, H.K.; Yoo, J.B. ZnO decorated flexible and strong graphene fibers for sensing NO2 and H2S at room temperature. Sensor Actuators B Chem. 2020, 308, 127690. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, X.; Liu, T.; Yang, J.; Wang, S.; Li, Y.; Shao, D.; Feng, L.; Song, H. Facile strategy to synthesize porous GO/ZnO heterostructure for enhanced acetone gas sensing properties. Sensor Actuators B Chem. 2022, 359, 131601. [Google Scholar] [CrossRef]
- Huang, W.C.; Tsai, H.J.; Lin, T.C.; Weng, W.C.; Chang, Y.C.; Chiu, J.L.; Lin, J.-J.; Lin, C.F.; Lin, Y.-S.; Chen, H. Incorporation of carbon nanotube and graphene in ZnO nanorods-based hydrogen gas sensor. Ceram. Int. 2018, 44, 12308–12314. [Google Scholar] [CrossRef]
- Wu, J.; Shen, X.; Jiang, L.; Wang, K.; Chen, K. Solvothermal synthesis and characterization of sandwich-like graphene/ZnO nanocomposites. Appl. Surf. Sci. 2010, 256, 2826–2830. [Google Scholar] [CrossRef]
- Khosravi, Y.; Sasar, M.; Abdi, Y. Light-induced oxygen sensing using ZnO/GO based gas sensor. Mat. Sci. Semicon. Proc. 2018, 85, 9–14. [Google Scholar] [CrossRef]
- Galstyan, V.; Comini, E.; Kholmanov, I.; Faglia, G.; Sberveglieri, G. Reduced graphene oxide/ZnO nanocomposite for application in chemical gas sensors. RSC Adv. 2016, 6, 34225–34232. [Google Scholar] [CrossRef]
- Kamble, C.; Narwade, S.; Mane, R. Detection of acetylene (C2H2) gas using Ag-modified ZnO/GO nanorods prepared by a hydrothermal synthesis. Mat. Sci. Semicon. Proc. 2023, 153, 107145. [Google Scholar] [CrossRef]
- Vessalli, B.A.; Zito, C.A.; Perfecto, T.M.; Volanti, D.P.; Mazon, T. ZnO nanorods/graphene oxide sheets prepared by chemical bath deposition for volatile organic compounds detection. J. Alloys Compd. 2017, 696, 996–1003. [Google Scholar] [CrossRef] [Green Version]
- Singh, G.; Choudhary, A.; Haranath, D.; Joshi, A.G.; Singh, N.; Singh, S.; Pasricha, R. ZnO decorated luminescent graphene as a potential gas sensor at room temperature. Carbon 2012, 50, 385–394. [Google Scholar] [CrossRef]
- Yin, Y.; Shen, Y.; Zhou, P.; Lu, R.; Li, A.; Zhao, S.; Liu, W.; Wei, D.; Wei, K. Fabrication, characterization and n-propanol sensing properties of perovskite-type ZnSnO3 nanospheres based gas sensor. Appl. Surf. Sci. 2020, 509, 145335. [Google Scholar] [CrossRef]
- Mokoena, T.P.; Hillie, K.T.; Swart, H.C.; Leshabane, N.; Tshilongo, J.; Motaung, D.E. Fabrication of a propanol gas sensor using p-type nickel oxide nanostructures: The effect of ramping rate towards luminescence and gas sensing characteristics. Mater. Chem. Phys. 2020, 253, 123316. [Google Scholar] [CrossRef]
- Dai, K.; Lu, L.H.; Liang, C.H.; Dai, J.M.; Zhu, G.P.; Liu, Z.L.; Liu, Q.Z.; Zhang, Y.X. Graphene oxide modified ZnO nanorods hybrid with high reusable photocatalytic activity under UV-LED irradiation. Mater. Chem. Phys. 2014, 143, 1410–1416. [Google Scholar] [CrossRef]
- Jin, Z.; Zhang, Y.X.; Meng, F.L.; Jia, Y.; Luo, T.; Yu, X.Y.; Wang, J.; Liu, J.H.; Huang, X.J. Facile synthesis of porous single crystalline ZnO nanoplates and their application in photocatalytic reduction of Cr(VI) in the presence of phenol. J. Hazard. Mater. 2014, 276, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.R.; Ren, H.B.; Sun, P.P.; Gu, C.P.; Sun, Y.F.; Liu, J.H. Facile synthesis of porous ZnO nanowires consisting of ordered nanocrystallites and their enhanced gas-sensing. Sensor Actuators B Chem. 2013, 188, 249–256. [Google Scholar] [CrossRef]
- Dong, C.J.; Xing, X.X.; Chen, N.; Liu, X.; Wang, Y.D. Biomorphic synthesis of hollow CuO fibers for low-ppm-level n-propanol detection via a facile solution combustion method. Sensor Actuat. B Chem. 2016, 230, 1–8. [Google Scholar]
- Shen, Y.B.; Fan, A.F.; Wei, D.Z.; Gao, S.L.; Liu, W.G.; Han, C.; Cui, B.Y. A low-temperature n-propanol gas sensor based on TeO2 nanowires as the sensing layer. RSC Adv. 2015, 5, 29126. [Google Scholar] [CrossRef]
- Alali, K.T.; Lu, Z.T.; Zhang, H.S.; Liu, J.Y.; Liu, Q.; Li, R.M.; Aljebawi, K.; Wang, J. P-p heterojunction CuO/CuCo2O4 nanotubes synthesized via electrospinning technology for detecting n-propanol gas at room temperature. Inorg. Chem. Front. 2017, 4, 1219–1230. [Google Scholar] [CrossRef]
- Wang, N.; Zhou, Y.; Chen, K.; Wang, T.S.; Sun, P.; Wang, C.G.; Chuai, X.H.; Zhang, S.M.; Liu, X.M.; Lu, G.Y. Double shell Cu2O hollow microspheres as sensing material for high performance n-propanol sensor. Sensor Actuators B Chem. 2021, 333, 129540. [Google Scholar] [CrossRef]














| Sensing Materials | Temperature (°C) | Response @ Concentration (ppm) | Selectivity | Response Time (s) | References |
|---|---|---|---|---|---|
| ZnO nanowires | 300 | 30.1@50 (2-propanol) | General | 5 | [33] |
| CuO nanofiber | 200 | 4.66@100 | General | 4.66 | [34] |
| TeO2 nanowires | 50 | 3.15@500 | Poor | 20 | [35] |
| CuO/CuCo2O4 nanotubes | Room temperature | 14@100 | General | 6.3 | [36] |
| Cu2O hollow microspheres | 187 | 11@100 | General | 50 | [37] |
| GO/ZnO nanosheet composites | 300 | 17.2@50 | Good | 45 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Jin, Z.; Chao, Y.; Wang, A.; Wang, D.; Chen, S.; Qian, Q. Synthesis of Graphene-Oxide-Decorated Porous ZnO Nanosheet Composites and Their Gas Sensing Properties. Chemosensors 2023, 11, 65. https://doi.org/10.3390/chemosensors11010065
Li J, Jin Z, Chao Y, Wang A, Wang D, Chen S, Qian Q. Synthesis of Graphene-Oxide-Decorated Porous ZnO Nanosheet Composites and Their Gas Sensing Properties. Chemosensors. 2023; 11(1):65. https://doi.org/10.3390/chemosensors11010065
Chicago/Turabian StyleLi, Jie, Zhen Jin, Yang Chao, Aijing Wang, Decai Wang, Shaohua Chen, and Quan Qian. 2023. "Synthesis of Graphene-Oxide-Decorated Porous ZnO Nanosheet Composites and Their Gas Sensing Properties" Chemosensors 11, no. 1: 65. https://doi.org/10.3390/chemosensors11010065
APA StyleLi, J., Jin, Z., Chao, Y., Wang, A., Wang, D., Chen, S., & Qian, Q. (2023). Synthesis of Graphene-Oxide-Decorated Porous ZnO Nanosheet Composites and Their Gas Sensing Properties. Chemosensors, 11(1), 65. https://doi.org/10.3390/chemosensors11010065